A Century of Bacteriophages: Insights, Applications, and Current Utilization
Abstract
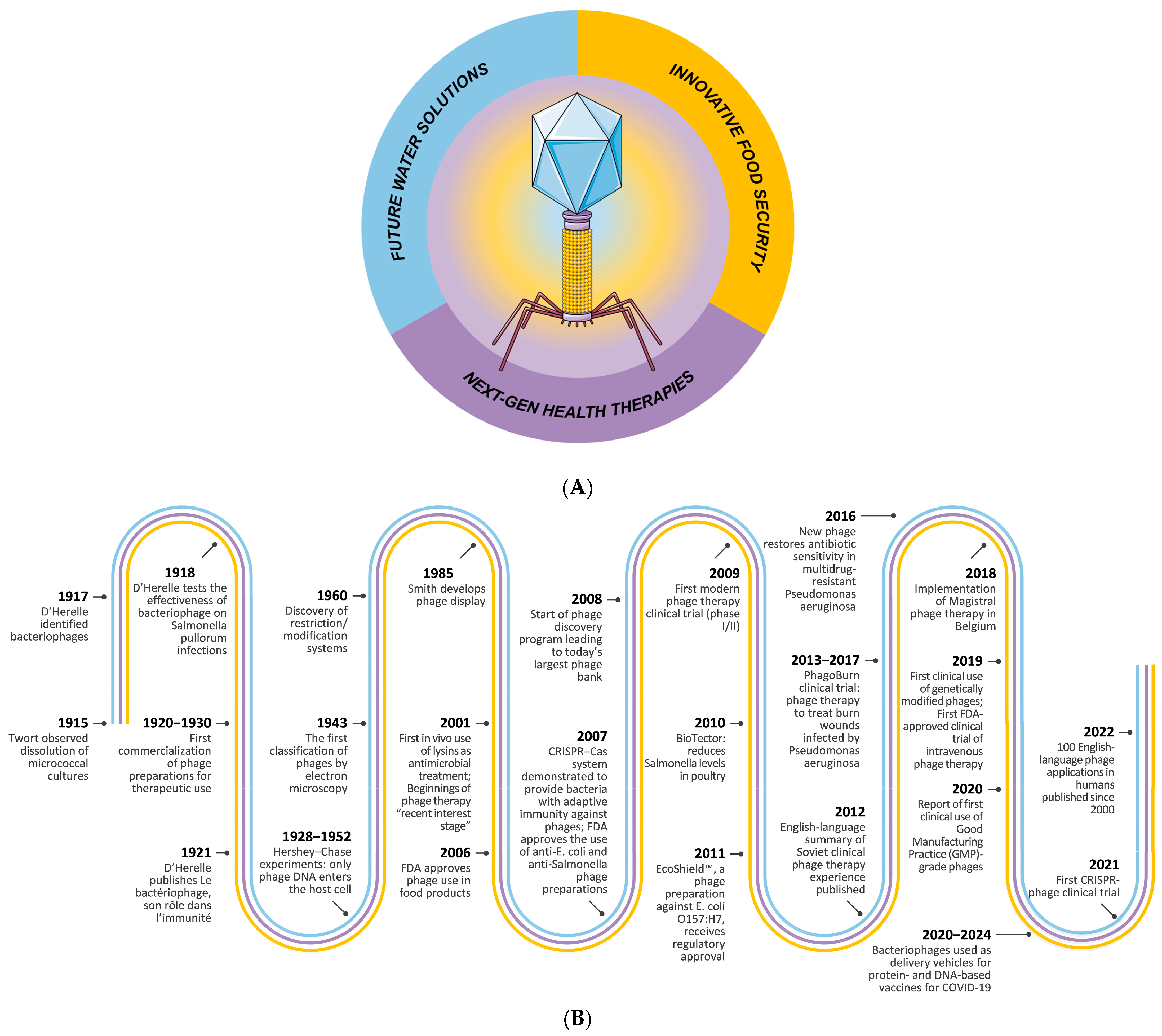
1. Introduction
2. Bacteriophages
2.1. Suitability of Phages for Different Applications
2.2. Legislation for Bacteriophage Preparations
3. Bacteriophage Applications
3.1. Bacteriophages and Molecular Biology
3.2. Phage Therapy
| Clinical Trial/Study | Outcomes | Country | Reference |
|---|---|---|---|
| A Human Experimental Model to Evaluate the Safety and Efficacy of the ShigActive™ Bacteriophage Preparation for Shigellosis | ShigActive™, a bacteriophage preparation, is being evaluated in a clinical trial to assess its safety and efficacy for treating shigellosis. | USA | NCT05182749 |
| Assessing the Safety, Tolerability, and Pharmacokinetics of LBP-EC01 in Patients with E. coli Colonization of the Lower Urinary Tract | No drug-related treatment-emergent adverse events (TEAEs) were observed in this Phase 1b study, confirming that LBP-EC01 is safe and well tolerated. The clear pharmacodynamic difference between LBP-EC01 and placebo, regardless of MDR status, supports its potential for treating antibiotic-resistant E. coli urinary tract infections. | USA | NCT04191148 |
| Bacteriophage PhiX174-based assay of in vivo immune competence in patients with HIV | Immunization with bacteriophage PhiX174 revealed persistent abnormalities in CD4(+) T-cell function in most patients with HIV, even after adequate antiretroviral therapy. | USA | NCT00001540 |
| Bacteriophage-Based Treatment for Urinary Tract Infections in Patients Undergoing Transurethral Resection of the Prostate | Although efficacy was similar to placebo, this trial provides valuable insights for designing future large-scale studies on phage therapy for urinary tract infections. | Georgia | NCT03140085 |
| Beta Testing of S. aureus/MSSA/MRSA Blood Culture with Microphage Agent | Microphage is developing innovative phage-based immunoassay solutions for rapid bacterial identification and antibiotic susceptibility testing. | USA | NCT00814151 |
| Efficacy of T4N5 Liposomal Lotion in Preventing Nonmelanoma Skin Cancer Recurrence in Kidney Transplant Patients | T4N5 liposomal lotion has shown promise in reducing nonmelanoma skin cancer recurrence among kidney transplant recipients. | USA | NCT00089180 |
| Evaluating the Safety, Tolerability, and Fecal Pharmacokinetics of BX002-A in Healthy Adults: A Phase 1 Study | BX002-A was safe and well tolerated in healthy adults. Pharmacokinetic analysis revealed high levels of viable phages in stool, confirming interaction with target bacteria after gastrointestinal passage. | USA | NCT04737876 |
| Evaluation of Changes in Inflammatory Markers Following Treatment with Bacterial Viruses | This case study showed that a single bacteriophage can effectively resolve a bacterial infection. | Poland | [83] |
| Evaluation of the bacteriophage cocktail TP-102 for treating diabetic foot ulcers | A preliminary safety assessment revealed no serious adverse events related to the preparation. Final efficacy results are pending. | Israel | NCT04803708 |
| In subjects with non-cystic fibrosis bronchiectasis and chronic pulmonary P. aeruginosa infection, the safety, phage kinetics, and efficacy of inhaled AP-PA02 were evaluated (Tailwind). | A Phase II clinical trial, named Tailwind, is currently recruiting to evaluate the safety, efficacy, and kinetics of AP-PA02 in patients with non-CF bronchiectasis and chronic P. aeruginosa infections. | USA | NCT05616221 |
| Intravenous and Nebulized Bacteriophage Therapy for Multidrug-Resistant Acinetobacter baumannii Respiratory Infection | Successful treatment led to favorable clinical response and bacterial eradication. | USA | [84] |
| Phage Therapy for Multidrug-Resistant Burkholderia multivorans Infection in a Cystic Fibrosis and Lung Transplant Patient | Phage therapy was followed by transient clinical improvement and decreased bacterial DNA in respiratory samples without seroneutralization. | USA | [85] |
| PhagoDAIR I: Pilot Study of Phage Therapy for Osteoarticular Infections on Prostheses (Targeting S. aureus). | The PhagoDAIR I study demonstrated excellent safety for PHAXIAM’s phages, with a 74% infection control rate after a single intra-articular injection. These results support the Phase II GLORIA study. | France | NCT05369104 |
| Pregnane X Receptor (PXR) as a Regulator of Intestinal Permeability and Indole Signaling in Inflammatory Bowel Disease | The study emphasizes the need for both in vitro and in vivo analyses to fully understand the therapeutic utility of PXR-directed analogs. | USA | NCT04089501 |
| Prospective Evaluation of Direct-from-Positive Blood Culture Performance of the Microphage S. aureus/MSSA Test | The KeyPath MRSA/MSSA test achieved 91.8% sensitivity and 98.3% specificity for MRSA/MSSA detection, providing faster and more effective results than traditional methods. | Not specified | NCT01184339 |
| Prospective, Randomized, Double-Blind, Controlled Study of WPP-201 for Venous Leg Ulcers | The product was deemed safe; a Phase II study is planned to evaluate its efficacy. | USA | NCT00663091 |
| Safety and Immunogenicity of a Recombinant Human CD40 Ligand in Patients with X-Linked Hyper-IgM Syndrome | The findings suggest potential improvements in dendritic cell activation and tumor immunity, with possible applications for cancer vaccines. | USA | NCT00001145 |
| Safety, Recovery, and Pharmacodynamics of Multiple Oral Administrations of SNIPR001 in Healthy Subjects | SNIPR001 demonstrated safety and efficacy in Phase 1, supporting further development of an intravenous formulation for preventing bloodstream infections in cancer patients. | USA | NCT05277350 |
| Therapeutic Efficacy and Safety Profile of the Bacteriophage Preparation Biophage-PA in Antibiotic-Resistant P. aeruginosa Chronic Otitis. | A double-blind, placebo-controlled Phase I/II trial demonstrated that phage treatment was safe and improved symptoms. | United Kingdom | [86] |
| Treatment of Multidrug-Resistant P. aeruginosa in Cystic Fibrosis with Personalized Inhaled Bacteriophage Therapy | Phage therapy reduced bacterial density in sputum and improved lung function, with no serious adverse effects. | USA | [87] |
Phage Therapy in the Context of Antimicrobial Stewardship
3.3. Detection of Bacteria
3.4. Phage Remediation
3.4.1. Control and Prevention of Foodstuff Contamination
3.4.2. Water Treatment
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMS | Antimicrobial Stewardship |
| AMR | Antimicrobial resistance |
| ICTV | International Committee on Taxonomy of Viruses |
| dsDNA | Double-Stranded Deoxyribonucleic Acid |
| ssRNA | Single-Stranded Ribonucleic Acid |
| ssDNA | Single-Stranded DNA |
| dsRNA | Double-Stranded RNA |
| R-M | Restriction–Modification |
| R&D | European Research & Development |
| E. coli | Escherichia coli |
| P. aeruginosa | Pseudomonas aeruginosa |
| US | United States |
| FDA | Food and Drug Administration |
| EFSA | European Food Safety Authority |
| CRISPR-Cas | Clustered Regularly Interspaced Short Palindromic Repeats Associated Protein Cas system |
| ESBL | Extended-Spectrum Beta-Lactamase |
| K. pneumoniae | Klebsiella pneumoniae |
| L. monocytogenes | Listeria monocytogenes |
| VLPs | Virus-Like Particles |
| COVID-19 | Coronavirus Disease of 2019 |
| HPV | Human Papillomavirus |
| S. aureus | Staphylococcus aureus |
| M. abscessus | Mycobacterium abscessus |
| PFGE | Pulsed Field Gel Electrophoresis |
| S. typhimurium | Salmonella typhimurium |
| S. Enteritidis | Salmonella enteritidis |
| Y. ruckeri | Yersinia ruckeri |
| C. perfringens | Clostridium perfringens |
References
- Blasdel, B.G.; Chevallereau, A.; Monot, M.; Lavigne, R.; Debarbieux, L. Comparative transcriptomics analyses reveal the conservation of an ancestral infectious strategy in two bacteriophage genera. ISME J. 2017, 11, 1988–1996. [Google Scholar] [CrossRef]
- Mathieu, J.; Yu, P.; Zuo, P.; Da Silva, M.L.B.; Alvarez, P.J.J. Going Viral: Emerging Opportunities for Phage-Based Bacterial Control in Water Treatment and Reuse. Acc. Chem. Res. 2019, 52, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Atterbury, R.J.; Van Bergen, M.A.; Ortiz, F.; Lovell, M.A.; Harris, J.A.; De Boer, A.; Wagenaar, J.A.; Allen, V.M.; Barrow, P.A. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007, 73, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- D’Herelle, F. Sur un microbe invisible antagoniste des bacilles dysentériques. Comptes Rendus Acad. Sci. Paris 1917, 165, 373–375. [Google Scholar]
- Ruffat, M. 175 ans D’industrie Pharmaceutique Française; La découverte: Paris, France, 1996. [Google Scholar]
- Ackermann, H.W. 5500 Phages examined in the electron microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef]
- Almeida, G.M.; Leppanen, M.; Maasilta, I.J.; Sundberg, L.R. Bacteriophage imaging: Past, present and future. Res. Microbiol. 2018, 169, 488–494. [Google Scholar] [CrossRef]
- Zhu, Y.; Shang, J.; Peng, C.; Sun, Y. Phage family classification under Caudoviricetes: A review of current tools using the latest ICTV classification framework. Front. Microbiol. 2022, 13, 1032186. [Google Scholar] [CrossRef]
- Chow, C.-E.T.; Suttle, C.A. Biogeography of Viruses in the Sea. Annu. Rev. Virol. 2015, 2, 41–66. [Google Scholar] [CrossRef]
- Kasman, L.M.; Porter, L.D. Bacteriophages; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Lim, E.S.; Zhou, Y.; Zhao, G.; Bauer, I.K.; Droit, L.; Ndao, I.M.; Warner, B.B.; Tarr, P.I.; Wang, D.; Holtz, L.R. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 2015, 21, 1228–1234. [Google Scholar] [CrossRef]
- Loeb, T.; Zinder, N.D. A bacteriophage containing RNA. Proc. Natl. Acad. Sci. USA 1961, 47, 282–289. [Google Scholar] [CrossRef]
- Mertens, P. The dsRNA viruses. Virus Res. 2004, 101, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Watanabe, S.; Sharmin, S.; Kawaguchi, T.; Tan, X.E.; Wannigama, D.L.; Cui, L. RNA and Single-Stranded DNA Phages: Unveiling the Promise from the Underexplored World of Viruses. Int. J. Mol. Sci. 2023, 24, 17029. [Google Scholar] [CrossRef]
- Ackermann, H.W.; Elzanowski, A.; Fobo, G.; Stewart, G. Relationships of tailed phages: A survey of protein sequence identity. Arch. Virol. 1995, 140, 1871–1884. [Google Scholar] [CrossRef]
- Vale, F.F.; Roberts, R.J.; Kobayashi, I.; Camargo, M.C.; Rabkin, C.S.; Hp, G.P.R.N. Gene content, phage cycle regulation model and prophage inactivation disclosed by prophage genomics in the Helicobacter pylori Genome Project. Gut Microbes 2024, 16, 2379440. [Google Scholar] [CrossRef]
- Hernandez, V. The Hershey-Chase Experiments (1952), by Alfred Hershey and Martha Chase; Embryo Project Encyclopedia: Tempe, AZ, USA, 2019. [Google Scholar]
- Enikeeva, F.N.; Severinov, K.V.; Gelfand, M.S. Restriction-modification systems and bacteriophage invasion: Who wins? J. Theor. Biol. 2010, 266, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Dublanchet, A.; Fruciano, E. Brève histoire de la phagothérapie. Med. Mal. Infect. 2008, 38, 415–420. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Abu Lila, A.S.; Moin, A.; Syed, S.; Khafagy, E.; Askoura, M.; Rajab, A.A.H.; Hegazy, W.A.H. Bacteriophage resurrection: Innovative impacts in medicine, biotechnology, and environmental solutions. Sci. Afr. 2025, 27, e02506. [Google Scholar] [CrossRef]
- Chanishvili, N. Phage therapy—History from Twort and d’Herelle through Soviet experience to current approaches. Adv. Virus Res. 2012, 83, 3–40. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef]
- Clark, J.R.; March, J.B. Bacteriophages and biotechnology: Vaccines, gene therapy and antibacterials. Trends Biotechnol. 2006, 24, 212–218. [Google Scholar] [CrossRef]
- Fruciano, D.E.; Bourne, S. Phage as an antimicrobial agent: D’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 19–26. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B. Phage Therapy—Challenges, Opportunities and Future Prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Kingwell, K. Bacteriophage therapies re-enter clinical trials. Nat. Rev. Drug Discov. 2015, 14, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, T.; Yu, M.; Chen, Y.L.; Jin, M. The Life Cycle Transitions of Temperate Phages: Regulating Factors and Potential Ecological Implications. Viruses 2022, 14, 1904. [Google Scholar] [CrossRef]
- Canchaya, C.; Fournous, G.; Chibani-Chennoufi, S.; Dillmann, M.L.; Brussow, H. Phage as agents of lateral gene transfer. Curr. Opin. Microbiol. 2003, 6, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Doub, J.B. Risk of Bacteriophage Therapeutics to Transfer Genetic Material and Contain Contaminants Beyond Endotoxins with Clinically Relevant Mitigation Strategies. Infect. Drug Resist. 2021, 14, 5629–5637. [Google Scholar] [CrossRef] [PubMed]
- Merril, C.R.; Scholl, D.; Adhya, S.L. The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discov. 2003, 2, 489–497. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Mackiewicz, P.; Kesik-Szeloch, A.; Maciaszczyk-Dziubinska, E.; Weber-Dabrowska, B.; Dorotkiewicz-Jach, A.; Augustyniak, D.; Majkowska-Skrobek, G.; Bocer, T.; Empel, J.; et al. Isolation and characterisation of KP34—A novel phiKMV-like bacteriophage for Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2011, 90, 1333–1345. [Google Scholar] [CrossRef]
- Merabishvili, M.; Vervaet, C.; Pirnay, J.P.; De Vos, D.; Verbeken, G.; Mast, J.; Chanishvili, N.; Vaneechoutte, M. Stability of Staphylococcus aureus phage ISP after freeze-drying (lyophilization). PLoS ONE 2013, 8, e68797. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortes, P.; Maspoch, D.; Llagostera, M. Liposome-Encapsulated Bacteriophages for Enhanced Oral Phage Therapy against Salmonella spp. Appl. Environ. Microb. 2015, 81, 4841–4849. [Google Scholar] [CrossRef]
- Hamdi, S.; Rousseau, G.M.; Labrie, S.J.; Tremblay, D.M.; Kourda, R.S.; Ben Slama, K.; Moineau, S. Characterization of two polyvalent phages infecting Enterobacteriaceae. Sci. Rep. 2017, 7, 40349. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, M.; Strain, R.; Neve, H.; Franz, C.M.A.P.; Cousin, F.J.; Ross, R.P.; Hill, C. Three New Phages from the Human Gut Show Promising Potential for Phage Therapy. PLoS ONE 2016, 11, e0156773. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, S.; Rousseau, G.M.; Labrie, S.J.; Kourda, R.S.; Tremblay, D.M.; Moineau, S.; Slama, K.B. Characterization of Five Podoviridae Phages Infecting Citrobacter freundii. Front. Microbiol. 2016, 7, 1023. [Google Scholar] [CrossRef]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good Manufacturing Practice (GMP) Compliance for Phage Therapy Medicinal Products. Front. Microbiol. 2020, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2021, 21, 427–436. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Kutter, E. Bacteriophages: It’s a medicine, Jim, but not as we it. Lancet Infect. Dis. 2021, 21, 309–311. [Google Scholar] [CrossRef]
- Patey, O.; McCallin, S.; Mazure, H.; Liddle, M.; Smithyman, A.; Dublanchet, A. Clinical Indications and Compassionate Use of Phage Therapy: Personal Experience and Literature Review with a Focus on Osteoarticular Infections. Viruses 2018, 11, 18. [Google Scholar] [CrossRef]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current State of Compassionate Phage Therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Merabishvili, M.; Van Raemdonck, H.; De Vos, D.; Verbeken, G. Bacteriophage Production in Compliance with Regulatory Requirements. Methods Mol. Biol. 2018, 1693, 233–252. [Google Scholar] [CrossRef]
- Lin, R.C.Y.; Sacher, J.C.; Ceyssens, P.-J.; Zheng, J.; Khalid, A.; Iredell, J.R. Phage Biobank: Present Challenges and Future Perspectives. Curr. Opin. Biotechnol. 2021, 68, 221–230. [Google Scholar] [CrossRef]
- Hershey, A.D.; Chase, M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J. Gen. Physiol. 1952, 36, 39–56. [Google Scholar] [CrossRef]
- Volkin, E.; Astrachan, L.; Countryman, J.L. Metabolism of RNA phosphorus in Escherichia coli infected with bacteriophage T7. Virology 1958, 6, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Duin, J.V. Single-Stranded RNA Phages (Leviviridae). In Encyclopedia of Virology, 2nd ed.; Granoff, A., Webster, R.G., Eds.; Elsevier: Oxford, UK, 1999; pp. 1663–1668. [Google Scholar]
- Roberts, R.J. How restriction enzymes became the workhorses of molecular biology. Proc. Natl. Acad. Sci. USA 2005, 102, 5905–5908. [Google Scholar] [CrossRef] [PubMed]
- Salmond, G.P.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Wang, X.; Shi, L. The immune system of prokaryotes: Potential applications and implications for gene editing. Biotechnol. J. 2024, 19, e2300352. [Google Scholar] [CrossRef]
- Pacia, D.M.; Brown, B.L.; Minssen, T.; Darrow, J.J. CRISPR-phage antibacterials to address the antibiotic resistance crisis: Scientific, economic, and regulatory considerations. J. Law Biosci. 2024, 11, lsad030. [Google Scholar] [CrossRef] [PubMed]
- Mireille, A.; Pascale, B.; Charlotte, B.; Laurent, D.; Nicolas, D.; Rémy, F.; Sylvain, G.; Claire Le, H.; Marie-Agnès, P.; Eduardo, R.; et al. Les applications antibactériennes des bactériophages. Virologie 2020, 24, 23–36. [Google Scholar] [CrossRef]
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef]
- D’Herelle, F. Le Bacteriophage Son Role Dans L’Immunite (1921); Kessinger Publishing: Whitefish, MT, USA, 2010. [Google Scholar]
- Summers, W.C. Afterword: Phage, History and Historiography. Notes Rec. 2020, 74, 653–656. [Google Scholar] [CrossRef]
- Marongiu, L.; Burkard, M.; Lauer, U.M.; Hoelzle, L.E.; Venturelli, S. Reassessment of Historical Clinical Trials Supports the Effectiveness of Phage Therapy. Clin. Microbiol. Rev. 2022, 35, e0006222. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Panagiotopoulos, A.P.; Ferous, S.; Tsakris, A. Phages connect the biological dots of antimicrobial resistance: From genesis and spread to alternative treatment modules. J Antimicrob Chemother 2025, dkaf293. [Google Scholar] [CrossRef] [PubMed]
- Trotereau, A.; Kern, J.; Viardot, A.; Thiriet, A.; Schouler, C. Explorer le potentiel thérapeutique des coliphages: Approche curative des infections causées par Escherichia coli chez les oiseaux. Innov. Agron. 2018, 66, 9–17. [Google Scholar] [CrossRef]
- Sheng, H.; Knecht, H.J.; Kudva, I.T.; Hovde, C.J. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 2006, 72, 5359–5366. [Google Scholar] [CrossRef]
- Smith, H.W.; Huggins, M.B. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 1983, 129, 2659–2675. [Google Scholar] [CrossRef]
- Penziner, S.; Schooley, R.T.; Pride, D.T. Animal Models of Phage Therapy. Front. Microbiol. 2021, 12, 631794. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, B.; Xu, M.; Yan, Q.; Liu, S.; Zhu, X.; Sun, Z.; Tao, D.; Ding, L.; Reed, E.; et al. Therapeutic effectiveness of bacteriophages in the rescue of mice with extended spectrum β-lactamase-producing Escherichia coli bacteremia. Int. J. Mol. Med. 2006, 17, 347–355. [Google Scholar] [CrossRef]
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages Reduce Pathogenic Escherichia coli Counts in Mice Without Distorting Gut Microbiota. Front. Microbiol. 2019, 10, 1984. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P.; Tamhankar, A.J.; Lundborg, C.S.; Ramesh, N. Isolation, characterization and in vivo efficacy of Escherichia phage myPSH1131. PLoS ONE 2018, 13, e0206278. [Google Scholar] [CrossRef]
- Chhibber, S.; Kaur, S.; Kumari, S. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J. Med. Microbiol. 2008, 57, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Bruttin, A.; Brussow, H. Human volunteers receiving Escherichia coli phage T4 orally: A safety test of phage therapy. Antimicrob. Agents Chemother. 2005, 49, 2874–2878. [Google Scholar] [CrossRef]
- McCallin, S.; Alam Sarker, S.; Barretto, C.; Sultana, S.; Berger, B.; Huq, S.; Krause, L.; Bibiloni, R.; Schmitt, B.; Reuteler, G.; et al. Safety analysis of a Russian phage cocktail: From metagenomic analysis to oral application in healthy human subjects. Virology 2013, 443, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Mateu, M.G. Virus engineering: Functionalization and stabilization. Protein Eng. Des. Sel. 2011, 24, 53–63. [Google Scholar] [CrossRef]
- Kaur, T.; Nafissi, N.; Wasfi, O.; Sheldon, K.; Wettig, S.; Slavcev, R. Immunocompatibility of Bacteriophages as Nanomedicines. J. Nanotechnol. 2012, 2012, 247427. [Google Scholar] [CrossRef][Green Version]
- Ul Haq, I.; Krukiewicz, K.; Yahya, G.; Haq, M.U.; Maryam, S.; Mosbah, R.A.; Saber, S.; Alrouji, M. The Breadth of Bacteriophages Contributing to the Development of the Phage-Based Vaccines for COVID-19: An Ideal Platform to Design the Multiplex Vaccine. Int. J. Mol. Sci. 2023, 24, 1536. [Google Scholar] [CrossRef]
- Venkataraman, S.; Shahgolzari, M.; Yavari, A.; Hefferon, K. Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities. Bioengineering 2025, 12, 469. [Google Scholar] [CrossRef]
- Wenger, S.L.; Turner, J.H.; Petricciani, J.C. The cytogenetic, proliferative and viability effects of four bacteriophages on human lymphocytes. In Vitro 1978, 14, 543–549. [Google Scholar] [CrossRef]
- Majewska, J.; Beta, W.; Lecion, D.; Hodyra-Stefaniak, K.; Kłopot, A.; Kaźmierczak, Z.; Miernikiewicz, P.; Piotrowicz, A.; Ciekot, J.; Owczarek, B.; et al. Oral Application of T4 Phage Induces Weak Antibody Production in the Gut and in the Blood. Viruses 2015, 7, 4783–4799. [Google Scholar] [CrossRef]
- Clark, J.R.; March, J.B. Bacterial viruses as human vaccines? Expert. Rev. Vaccines 2004, 3, 463–476. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B. Bacteriophages and phage-derived proteins—Application approaches. Curr. Med. Chem. 2015, 22, 1757–1773. [Google Scholar] [CrossRef] [PubMed]
- Borysowski, J.; Weber-Dabrowska, B.; Gorski, A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 2006, 231, 366–377. [Google Scholar] [CrossRef]
- Golban, M.; Charostad, J.; Kazemian, H.; Heidari, H. Phage-Derived Endolysins Against Resistant Staphylococcus spp.: A Review of Features, Antibacterial Activities, and Recent Applications. Infect. Dis. Ther. 2025, 14, 13–57. [Google Scholar] [CrossRef]
- Comeau, A.M.; Tétart, F.; Trojet, S.N.; Prère, M.-F.; Krisch, H.M. Phage-Antibiotic Synergy (PAS): β-Lactam and Quinolone Antibiotics Stimulate Virulent Phage Growth. PLoS ONE 2007, 2, e799. [Google Scholar] [CrossRef]
- Comeau, A.M.; Tetart, F.; Trojet, S.N.; Prere, M.F.; Krisch, H.M. The discovery of a natural phenomenon, “Phage-Antibiotic Synergy”. Implications for phage therapy. Med. Sci. 2008, 24, 449–451. [Google Scholar] [CrossRef]
- Abedon, S.T. How Simple Maths Can Inform Our Basic Understanding of Phage Therapy. Clin. Infect. Dis. 2023, 77, S401–S406. [Google Scholar] [CrossRef]
- Kutateladze, M.; Adamia, R. Phage therapy experience at the Eliava Institute. Med. Mal. Infect. 2008, 38, 426–430. [Google Scholar] [CrossRef]
- Chan, B.K.; Turner, P.E.; Kim, S.; Mojibian, H.R.; Elefteriades, J.A.; Narayan, D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health 2018, 2018, 60–66. [Google Scholar] [CrossRef]
- Rao, S.; Betancourt-Garcia, M.; Kare-Opaneye Yetunde, O.; Swierczewski Brett, E.; Bennett Jason, W.; Horne, B.A.; Fackler, J.; Suazo Hernandez Lia, P.; Brownstein Michael, J. Critically Ill Patient with Multidrug-Resistant Acinetobacter baumannii Respiratory Infection Successfully Treated with Intravenous and Nebulized Bacteriophage Therapy. Antimicrob. Agents Chemother. 2022, 66, e00824-21. [Google Scholar] [CrossRef] [PubMed]
- Haidar, G.; Chan, B.K.; Cho, S.-T.; Hughes Kramer, K.; Nordstrom, H.R.; Wallace, N.R.; Stellfox, M.E.; Holland, M.; Kline, E.G.; Kozar, J.M.; et al. Phage therapy in a lung transplant recipient with cystic fibrosis infected with multidrug-resistant Burkholderia multivorans. Transpl. Infect. Dis. 2023, 25, e14041. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Hawkins, C.H.; Anggard, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Stanley, G.L.; Kortright, K.E.; Vill, A.C.; Modak, M.; Ott, I.M.; Sun, Y.; Würstle, S.; Grun, C.N.; Kazmierczak, B.I.; et al. Personalized inhaled bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis. Nat. Med. 2025, 31, 1494–1501. [Google Scholar] [CrossRef]
- Aswani, V.H.; Shukla, S.K. An Early History of Phage Therapy in the United States: Is it Time to Reconsider? Clin. Med. Res. 2021, 19, 82–89. [Google Scholar] [CrossRef]
- Abbasi, J. Patient Receives First Genetically Engineered Phage Treatment. J. Am. Med. Assoc. 2019, 322, 107. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Kim, M.K.; Suh, G.A.; Cullen, G.D.; Perez Rodriguez, S.; Dharmaraj, T.; Chang, T.H.W.; Li, Z.; Chen, Q.; Green, S.I.; Lavigne, R.; et al. Bacteriophage therapy for multidrug-resistant infections: Current technologies and therapeutic approaches. J. Clin. Investig. 2025, 135, e187996. [Google Scholar] [CrossRef]
- Rippon, M.G.; Rogers, A.A.; Ousey, K. Antimicrobial resistance and antimicrobial stewardship: An update. Wound Pract. Res. 2025, 33, 79–96. [Google Scholar] [CrossRef]
- James, R.; Hardefeldt, L.Y.; Ierano, C.; Charani, E.; Dowson, L.; Elkins, S.; Thursky, K. Antimicrobial stewardship from a One Health perspective. Nat. Rev. Microbiol. 2025. [Google Scholar] [CrossRef]
- Khakhria, R.; Duck, D.; Lior, H. Extended phage-typing scheme for Escherichia coli O157:H7. Epidemiol. Infect. 1990, 105, 511–520. [Google Scholar] [CrossRef]
- Frost, J.A.; Smith, H.R.; Willshaw, G.A.; Scotland, S.M.; Gross, R.J.; Rowe, B. Phage-typing of Vero-cytotoxin (VT) producing Escherichia coli O157 isolated in the United Kingdom. Epidemiol. Infect. 2009, 103, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cowley, L.A.; Beckett, S.J.; Chase-Topping, M.; Perry, N.; Dallman, T.J.; Gally, D.L.; Jenkins, C. Analysis of whole genome sequencing for the Escherichia coli O157:H7 typing phages. BMC Genom. 2015, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.; Ullah, M.W.; Farooq, U.; Aziz, A.; Wang, S. Bacteriophage-based advanced bacterial detection: Concept, mechanisms, and applications. Biosens. Bioelectron. 2021, 177, 112973. [Google Scholar] [CrossRef]
- Ahmed, R.; Bopp, C.; Borczyk, A.; Kasatiya, S. Phage-typing scheme for Escherichia coli O157:H7. J. Infect. Dis. 1987, 155, 806–809. [Google Scholar] [CrossRef]
- Barrett, T.J.; Lior, H.; Green, J.H.; Khakhria, R.; Wells, J.G.; Bell, B.P.; Greene, K.D.; Lewis, J.; Griffin, P.M. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J. Clin. Microbiol. 1994, 32, 3013–3017. [Google Scholar] [CrossRef]
- Preston, M.A.; Johnson, W.; Khakhria, R.; Borczyk, A. Epidemiologic subtyping of Escherichia coli serogroup O157 strains isolated in Ontario by phage typing and pulsed-field gel electrophoresis. J. Clin. Microbiol. 2000, 38, 2366–2368. [Google Scholar] [CrossRef]
- Nelson, D.; Loomis, L.; Fischetti, V.A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 4107–4112. [Google Scholar] [CrossRef] [PubMed]
- Al-Hindi, R.R.; Teklemariam, A.D.; Alharbi, M.G.; Alotibi, I.; Azhari, S.A.; Qadri, I.; Alamri, T.; Harakeh, S.; Applegate, B.M.; Bhunia, A.K. Bacteriophage-Based Biosensors: A Platform for Detection of Foodborne Bacterial Pathogens from Food and Environment. Biosensors 2022, 12, 905. [Google Scholar] [CrossRef]
- Lang, L.H. FDA Approves Use of Bacteriophages to be Added to Meat and Poultry Products. Gastroenterology 2006, 131, 1370. [Google Scholar] [CrossRef]
- Boehme, S.; Werner, G.; Klare, I.; Reissbrodt, R.; Witte, W. Occurrence of antibiotic-resistant enterobacteria in agricultural foodstuffs. Mol. Nutr. Food Res. 2004, 48, 522–531. [Google Scholar] [CrossRef]
- Liebana, E.; Carattoli, A.; Coque, T.M.; Hasman, H.; Magiorakos, A.P.; Mevius, D.; Peixe, L.; Poirel, L.; Schuepbach-Regula, G.; Torneke, K.; et al. Public health risks of enterobacterial isolates producing extended-spectrum beta-lactamases or AmpC beta-lactamases in food and food-producing animals: An EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin. Infect. Dis. 2013, 56, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Edrington, T.S.; Brabban, A.; Kutter, B.; Karriker, L.; Stahl, C.; Wagstrom, E.; Anderson, R.; Poole, T.L.; Genovese, K.; et al. Evaluation of Phage Treatment as a Strategy to Reduce Salmonella Populations in Growing Swine. Foodborne Pathog. Dis. 2011, 8, 261–266. [Google Scholar] [CrossRef]
- Hooton, S.P.; Atterbury, R.J.; Connerton, I.F. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food Microbiol. 2011, 151, 157–163. [Google Scholar] [CrossRef]
- Wall, S.K.; Zhang, J.; Rostagno, M.H.; Ebner, P.D. Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl. Environ. Microbiol. 2010, 76, 48–53. [Google Scholar] [CrossRef]
- Goodridge, L.D.; Bisha, B. Phage-based biocontrol strategies to reduce foodborne pathogens in foods. Bacteriophage 2011, 1, 130–137. [Google Scholar] [CrossRef]
- Rozema, E.A.; Stephens, T.P.; Bach, S.J.; Okine, E.K.; Johnson, R.P.; Stanford, K.; McAllister, T.A. Oral and rectal administration of bacteriophages for control of Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 2009, 72, 241–250. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, G.; Ross, R.P.; Fitzgerald, G.F.; Coffey, A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2004, 70, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Anany, H.; Chen, W.; Pelton, R.; Griffiths, M.W. Biocontrol of Listeria Monocytogenes and Escherichia coli O157:H7 in Meat by Using Phages Immobilized on Modified Cellulose Membranes. Appl. Environ. Microb. 2011, 77, 6379–6387. [Google Scholar] [CrossRef] [PubMed]
- Leverentz, B.; Conway, W.S.; Alavidze, Z.; Janisiewicz, W.J.; Fuchs, Y.; Camp, M.J.; Chighladze, E.; Sulakvelidze, A. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: A model study. J Food Prot. 2001, 64, 1116–1121. [Google Scholar] [CrossRef]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Diez-Gonzalez, F. Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food Microbiol. 2011, 28, 149–157. [Google Scholar] [CrossRef]
- Modi, R.; Hirvi, Y.; Hill, A.; Griffiths, M.W. Effect of phage on survival of Salmonella enteritidis during manufacture and storage of cheddar cheese made from raw and pasteurized milk. J. Food Prot. 2001, 64, 927–933. [Google Scholar] [CrossRef]
- Guenther, S.; Herzig, O.; Fieseler, L.; Klumpp, J.; Loessner, M.J. Biocontrol of Salmonella Typhimurium in RTE foods with the virulent bacteriophage FO1-E2. Int. J. Food Microbiol. 2012, 154, 66–72. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Ross, R.P.; Coffey, A. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 2009, 33, 801–819. [Google Scholar] [CrossRef]
- Coffey, B.; Mills, S.; Coffey, A.; McAuliffe, O.; Ross, R.P. Phage and their lysins as biocontrol agents for food safety applications. Annu. Rev. Food Sci. Technol. 2010, 1, 449–468. [Google Scholar] [CrossRef]
- Lopez, M.E.S.; Mendoza-Corvis, F.; Salgado-Behaine, J.J.; Hernandez-Arteaga, A.M.; González-Peña, V.; Burgos-Rivero, A.M.; Cortessi, D.; Vidigal, P.M.P.; Pérez-Sierra, O. Phage Endolysins as an Alternative Biocontrol Strategy for Pathogenic and Spoilage Microorganisms in the Food Industry. Viruses 2025, 17, 564. [Google Scholar] [CrossRef]
- Young, R. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef]
- Waseh, S.; Hanifi-Moghaddam, P.; Coleman, R.; Masotti, M.; Ryan, S.; Foss, M.; MacKenzie, R.; Henry, M.; Szymanski, C.M.; Tanha, J. Orally administered P22 phage tailspike protein reduces Salmonella colonization in chickens: Prospects of a novel therapy against bacterial infections. PLoS ONE 2010, 5, e13904. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Amjad, N.; Naseer, M.S.; Imran, A.; Menon, S.V.; Sharma, A.; Islam, F.; Tahir, S.; Shah, M.A. A mini-review on the role of bacteriophages in food safety. CyTa-J. Food 2024, 22. [Google Scholar] [CrossRef]
- Ranveer, S.A.; Dasriya, V.; Ahmad, M.F.; Dhillon, H.S.; Samtiya, M.; Shama, E.; Anand, T.; Dhewa, T.; Chaudhary, V.; Chaudhary, P.; et al. Positive and negative aspects of bacteriophages and their immense role in the food chain. NPJ Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Balogh, B.; Olson, S.M. Management of Tomato Bacterial Spot in the Field by Foliar Applications of Bacteriophages and SAR Inducers. Plant Dis. 2004, 88, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.; Simoes, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT-Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Omwenga, E.O.; Awuor, S.O. The Bacterial Biofilms: Formation, Impacts, and Possible Management Targets in the Healthcare System. Can. J. Infect. Dis. Med. Microbiol. 2024, 2024, 1542576. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, M.M.; Cooney, J.J.; Caldwell, D.E. Lytic infection of Escherichia coli biofilms by bacteriophage T4. Can. J. Microbiol. 1995, 41, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.A.; Nannapaneni, R.; Hagens, S. Reduction of Listeria monocytogenes on the surface of fresh channel catfish fillets by bacteriophage Listex P100. Foodborne Pathog. Dis. 2010, 7, 427–434. [Google Scholar] [CrossRef]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage LISTEX (TM) P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef]
- Iacumin, L.; Manzano, M.; Comi, G. Phage Inactivation of Listeria monocytogenes on San Daniele Dry-Cured Ham and Elimination of Biofilms from Equipment and Working Environments. Microorganisms 2016, 4, 4. [Google Scholar] [CrossRef]
- Oliveira, M.; Vinas, I.; Colas, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Xanthomonas campestris pv. Vesicatoria and Pseudomonas syringae pv. Tomato Specific Bacteriophages; Exemption from the Requirement of a Tolerance; 70 FR 76700; Environmental Protection Agency: Washington, DC, USA, 2005; pp. 76700–76704.
- García, P.; Rodríguez, L.; Rodríguez, A.; Martínez, B. Food biopreservation: Promising strategies using bacteriocins, bacteriophages and endolysins. Trends Food Sci. Technol. 2010, 21, 373–382. [Google Scholar] [CrossRef]
- Garcia, P.; Martinez, B.; Obeso, J.M.; Rodriguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef]
- Berchieri, A., Jr.; Lovell, M.A.; Barrow, P.A. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res. Microbiol. 1991, 142, 541–549. [Google Scholar] [CrossRef]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012, 2012, 863945. [Google Scholar] [CrossRef]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.D.; Nan, Y.C.; Stanford, K.; Holley, R.; McAllister, T.; Narvaez-Bravo, C. SalmoFresh (TM) effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int. J. Food Microbiol. 2019, 305, 108250. [Google Scholar] [CrossRef] [PubMed]
- Botsaris, G.; Liapi, M.; Kakogiannis, C.; Dodd, C.E.R.; Rees, C.E.D. Detection of Mycobacterium avium subsp paratuberculosis in bulk tank milk by combined phage-PCR assay: Evidence that plaque number is a good predictor of MAP. Int. J. Food Microbiol. 2013, 164, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Mujica, K.; Mora-Uribe, P.; Garcias-Papayani, H.; Paillavil, B.; Avendaño, C.; Flores-Crisosto, D.; Norambuena, R.; Rojas-Martínez, V.; Aguilera, M.; et al. Research Note: Reduction of Salmonella load in Brazilian commercial chicken farms using INSPEKTOR®: A bacteriophage-based product. Poult. Sci. 2025, 104, 104544. [Google Scholar] [CrossRef]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, B.; Zhang, T. Bacteria That Make a Meal of Sulfonamide Antibiotics: Blind Spots and Emerging Opportunities. Environ. Sci. Technol. 2018, 52, 3854–3868. [Google Scholar] [CrossRef]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; La Mastra, C.; Magnano San Lio, R.; Barchitta, M.; Agodi, A. The Impact of Wastewater on Antimicrobial Resistance: A Scoping Review of Transmission Pathways and Contributing Factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef]
- Jassim, S.A.; Limoges, R.G.; El-Cheikh, H. Bacteriophage biocontrol in wastewater treatment. World J. Microbiol. Biotechnol. 2016, 32, 70. [Google Scholar] [CrossRef]
- Withey, S.; Cartmell, E.; Avery, L.M.; Stephenson, T. Bacteriophages—Potential for application in wastewater treatment processes. Sci Total Environ. 2005, 339, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Liu, Z.; Sun, K.; Li, Z.; Fan, X.; Li, Q. Bacteriophages in water pollution control: Advantages and limitations. Front. Environ. Sci. Eng. 2021, 15, 84. [Google Scholar] [CrossRef]
- Samson, R.; Pawar, A.; Khairnar, K.; Dharne, M. Characterization of a novel Tequatrovirus phage from pristine stretch of the Ganges River, India, in reducing bacterial load from sewage water. J. Environ. Chem. Eng. 2025, 13, 116315. [Google Scholar] [CrossRef]
- Goldman, G.; Starosvetsky, J.; Armon, R. Inhibition of biofilm formation on UF membrane by use of specific bacteriophages. J. Membr. Sci. 2009, 342, 145–152. [Google Scholar] [CrossRef]
- Petrovski, S.; Seviour, R.J.; Tillett, D. Characterization of the genome of the polyvalent lytic bacteriophage GTE2, which has potential for biocontrol of Gordonia-, Rhodococcus-, and Nocardia-stabilized foams in activated sludge plants. Appl. Environ. Microbiol. 2011, 77, 3923–3929. [Google Scholar] [CrossRef]
- Khairnar, K.; Pal, P.; Chandekar, R.H.; Paunikar, W.N. Isolation and characterization of bacteriophages infecting nocardioforms in wastewater treatment plant. Biotechnol. Res. Int. 2014, 2014, 151952. [Google Scholar] [CrossRef]
- Gao, R.; Gao, S.H.; Li, J.; Su, Y.Y.; Huang, F.; Liang, B.; Fan, L.; Guo, J.H.; Wang, A.J. Emerging Technologies for the Control of Biological Contaminants in Water Treatment: A Critical Review. Engineering 2025, 48, 185–204. [Google Scholar] [CrossRef]
- Weinbauer, M.G. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef]
- Cristobal-Cueto, P.; García-Quintanilla, A.; Esteban, J.; García-Quintanilla, M. Phages in Food Industry Biocontrol and Bioremediation. Antibiotics 2021, 10, 786. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Jiang, X.; Yang, M.S.; Wang, Z. Biotechnology revival: Sludge minimization in wastewater. Front. Microbiol. 2025, 16, 1603215. [Google Scholar] [CrossRef] [PubMed]

| Product | Use | Company | Website |
|---|---|---|---|
| AgriPhageTM | Used on foods and crops targeting Xanthomonas pv. vesicatoria, Pseudomonas pv. tomato | OmniLytics (Sandy, UT, USA) | https://omnilytics.com/ |
| BioTector | Feed additive for controlling Salmonella in poultry | CheilJedang Corporation (Seoul, Republic of Korea) | https://cjbio.net/en/ |
| CUSTUS®YRS | Biocontrol against Yersinia ruckeri (aquaculture) | ACD Pharma (Leknes, Norway) | https://acdpharma.com/ |
| EcoShieldTM | Control of E. coli O157:H7 in foods and processing environments | Intralytix (Columbia, MD, USA) | https://www.intralytix.com/ |
| FASTPlaque-ResponseTM | Rapid detection of rifampicin resistance in Mycobacterium tuberculosis from sputum | Biotech Laboratories Ltd. (Ipswich, UK) | No longer available; acquired by Lab21 Ltd. (Cambridge, UK), then by Novacyt Group (Chandlers Ford, UK) |
| FASTPlaqueTBTM | Rapid detection of Mycobacterium tuberculosis in sputum | Biotech Laboratories Ltd. (Ipswich, UK) | No longer available; acquired by Lab21 Ltd. (Cambridge, UK), then by Novacyt Group (Chandlers Ford, UK) |
| INSPEKTOR® | Reducing Salmonella load in poultry farms | PHaGElab (Santiago, Chile) | https://phage-lab.com/ |
| LISTEXTM P100 | Food processing aid to eliminate Listeria monocytogenes strains on food products | Micreos Food Safety, rebranded PhageGuard (Wageningen, The Netherlands) | https://phageguard.com/ |
| ListShieldTM | Control of Listeria monocytogenes in foods and processing environments | Intralytix (Columbia, MD, USA) | https://www.intralytix.com/ |
| SalmoFree® | Feed additive for controlling Salmonella in poultry | SciPhage (Bogota, Colombia) | https://sciphage.com/ |
| SalmoFreshTM | Designed to control Salmonella enterica in ready-to-eat foods, surfaces and food contact environments | Intralytix (Columbia, MD, USA) | https://www.intralytix.com/ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dkhili, S.; Ribeiro, M.; Slama, K.B. A Century of Bacteriophages: Insights, Applications, and Current Utilization. Antibiotics 2025, 14, 1080. https://doi.org/10.3390/antibiotics14111080
Dkhili S, Ribeiro M, Slama KB. A Century of Bacteriophages: Insights, Applications, and Current Utilization. Antibiotics. 2025; 14(11):1080. https://doi.org/10.3390/antibiotics14111080
Chicago/Turabian StyleDkhili, Sadika, Miguel Ribeiro, and Karim Ben Slama. 2025. "A Century of Bacteriophages: Insights, Applications, and Current Utilization" Antibiotics 14, no. 11: 1080. https://doi.org/10.3390/antibiotics14111080
APA StyleDkhili, S., Ribeiro, M., & Slama, K. B. (2025). A Century of Bacteriophages: Insights, Applications, and Current Utilization. Antibiotics, 14(11), 1080. https://doi.org/10.3390/antibiotics14111080







