Occurrence and Antibiotic Resistance Risk Burden of Vibrio mimicus Isolates from Seafood and Aquatic Environments
Abstract
1. Introduction
2. Results
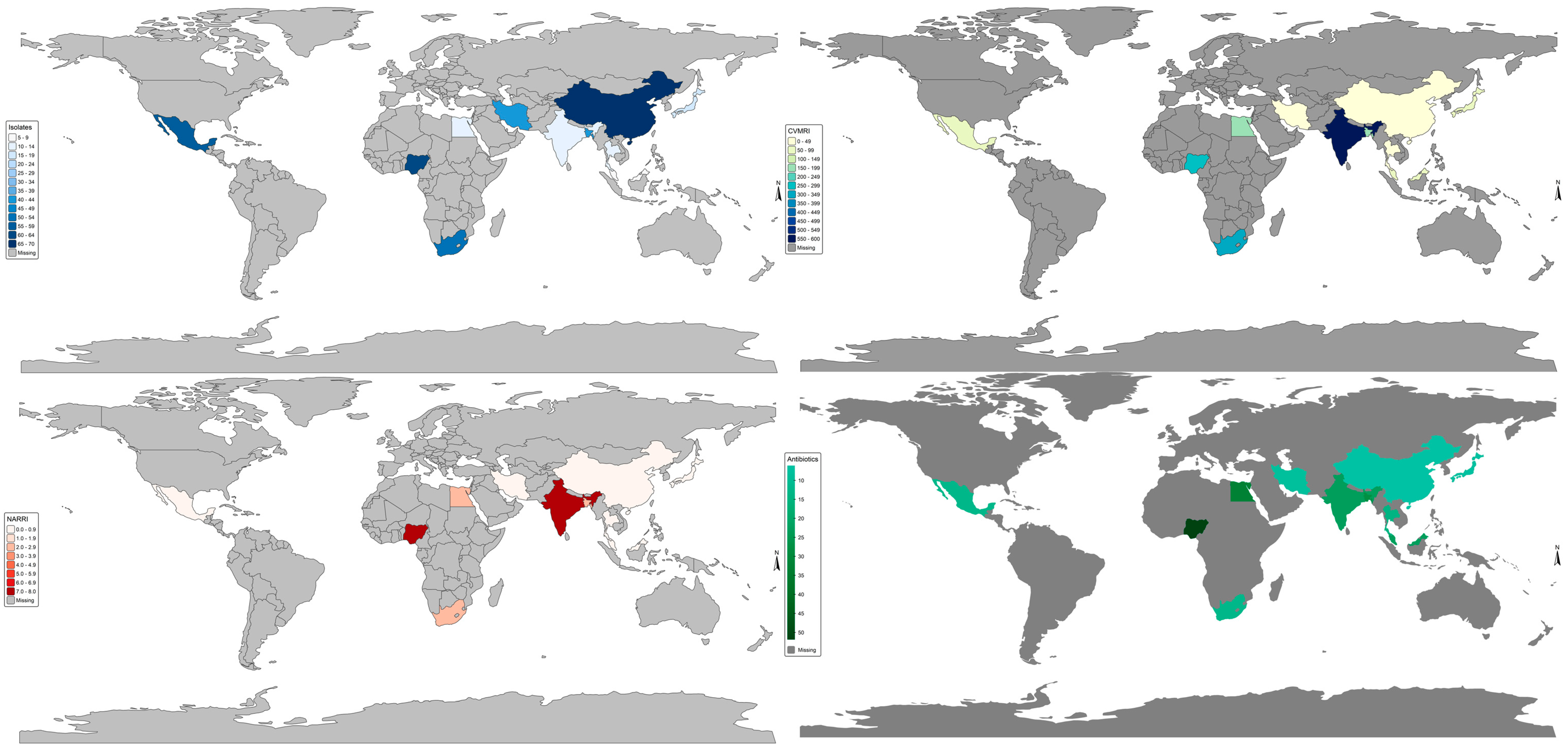
2.1. General Data Overview
Pooled Sample and Sample-Specific Prevalence of Different Antibiotic Resistance in V. mimicus
2.2. Aminoglycosides
2.3. Carbapenems
2.4. Cephalosporins
2.5. Chloramphenicol
2.6. Fluoroquinolones and Quinolones
2.7. Macrolides and Azalides
2.8. Penicillins
2.9. Polymyxins and Sulfonamides
2.10. Tetracyclines
2.11. Trimethoprim–Sulfamethoxazole
2.12. β-Lactam/β-Lactamase Inhibitor
2.13. Heterogeneity and Publication Bias
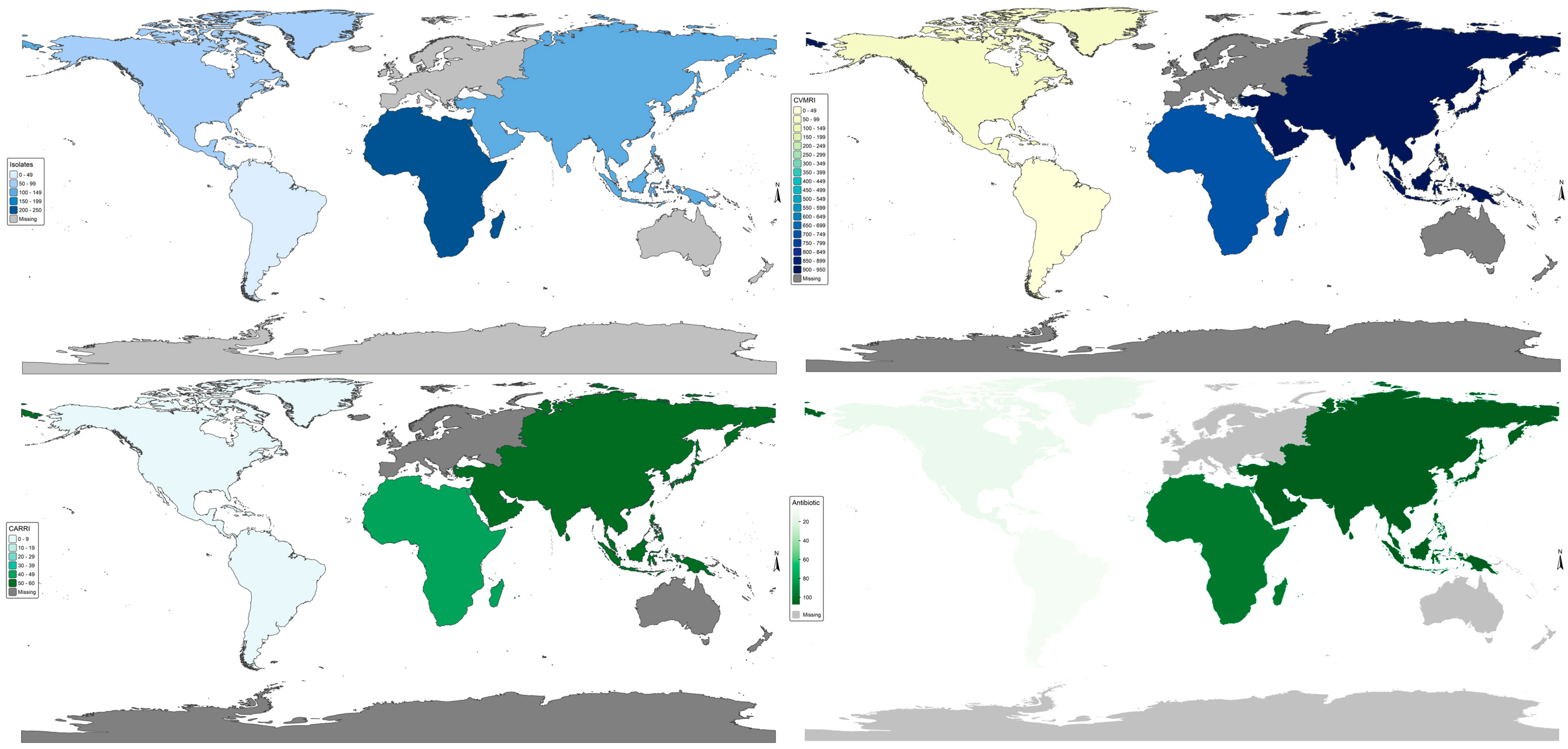
2.14. World Bank Income; GNIpc = Gross National Income per Capita. Vm Antibiotic Resistance Burden
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Strategy
4.2. Eligibility Criteria
4.3. Data Management
4.4. Data Items and Treatment
4.5. Data Synthesis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Public Health Aspects of Vibrio spp. Related to the Consumption of Seafood in the EU. EFSA J. 2024, 22, e8896. [Google Scholar] [CrossRef] [PubMed]
- Lajqi Berisha, N.; Poceva Panovska, A.; Hajrulai-Musliu, Z. Antibiotic Resistance and Aquatic Systems: Importance in Public Health. Water 2024, 16, 2362. [Google Scholar] [CrossRef]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne Pathogenic Vibrios: Antimicrobial Resistance. Front. Microbiol. 2021, 12, 638331. [Google Scholar] [CrossRef]
- Abioye, O.E.; Nontongana, N.; Osunla, C.A.; Okoh, A.I. Antibiotic Resistance and Virulence Genes Profiling of Vibrio cholerae and Vibrio mimicus Isolates from Some Seafood Collected at the Aquatic Environment and Wet Markets in Eastern Cape Province, South Africa. PLoS ONE 2023, 18, e0290356. [Google Scholar] [CrossRef]
- Alam, M.T.; Stern, S.R.; Frison, D.; Taylor, K.; Tagliamonte, M.S.; Nazmus, S.S.; Paisie, T.; Hilliard, N.B.; Jones, R.G.; Iovine, N.M.; et al. Seafood-Associated Outbreak of Ctx-Negative Vibrio mimicus Causing Cholera-Like Illness, Florida, USA. Emerg. Infect. Dis. 2023, 29, 2141. [Google Scholar] [CrossRef]
- Hirshfeld, B.; Lavelle, K.; Lee, K.Y.; Atwill, E.R.; Kiang, D.; Bolkenov, B.; Gaa, M.; Li, Z.; Yu, A.; Li, X.; et al. Prevalence and Antimicrobial Resistance Profiles of Vibrio spp. And Enterococcus spp. In Retail Shrimp in Northern California. Front. Microbiol. 2023, 14, 1192769. [Google Scholar] [CrossRef]
- Ljubojević Pelić, D.; Radosavljević, V.; Pelić, M.; Živkov, B.M.; Puvača, N.; Jug-Dujaković, J.; Gavrilović, A. Antibiotic Residues in Cultured Fish: Implications for Food Safety and Regulatory Concerns. Fishes 2024, 9, 484. [Google Scholar] [CrossRef]
- Albini, E.; Orso, M.; Cozzolino, F.; Sacchini, L.; Leoni, F.; Magistrali, C.F. A Systematic Review and Meta-Analysis on Antimicrobial Resistance in Marine Bivalves. Front. Microbiol. 2022, 13, 1040568. [Google Scholar] [CrossRef] [PubMed]
- Guedes, B.; Godinho, O.; Lage, O.M.; Quinteira, S. Microbiological Quality, Antibiotic Resistant Bacteria and Relevant Resistance Genes in Ready-to-Eat Pacific Oysters (Magallana Gigas). FEMS Microbiol. Lett. 2023, 370, fnad053. [Google Scholar] [CrossRef]
- Vu, T.T.T.; Alter, T.; Huehn, S. Prevalence of Vibrio spp. In Retail Seafood in Berlin, Germany. J. Food Prot. 2018, 81, 593–597. [Google Scholar] [CrossRef]
- Zeidler, C.; Szott, V.; Alter, T.; Huehn-Lindenbein, S.; Fleischmann, S. Prevalence of Vibrio spp. In Seafood from German Supermarkets and Fish Markets. Foods 2024, 13, 3987. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.A.; Mir, R.A.; Qadri, H.; Dhiman, R.; Almilaibary, A.; Alkhanani, M.; Mir, M.A. Integrons in the Development of Antimicrobial Resistance: Critical Review and Perspectives. Front. Microbiol. 2023, 14, 1231938. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic Resistance Genes in Bacteria: Occurrence, Spread, and Control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef]
- Adeleye, I.; Nwanze, R.; Daniels, F.; Eyinnia, V.; Smith, S.; Fowora, M.; Goodluck, H. Non-Plasmid Mediated Multi-Drug Resistance in Vibrio and Aeromonas Sp. Isolated from Seafoods in Lagos, Nigeria. Res. J. Microbiol. 2011, 6, 147. [Google Scholar] [CrossRef]
- Álvarez-Contreras, A.K.; Quiñones-Ramírez, E.I.; Vázquez-Salinas, C. Prevalence, Detection of Virulence Genes and Antimicrobial Susceptibility of Pathogen Vibrio Species Isolated from Different Types of Seafood Samples at “La Nueva Viga” Market in Mexico City. Antonie Van Leeuwenhoek 2021, 114, 1417–1429. [Google Scholar] [CrossRef]
- Banerjee, S.; Ooi, M.C.; Shariff, M.; Khatoon, H. Antibiotic Resistant Salmonella and Vibrio Associated with Farmed Litopenaeus Vannamei. Sci. World J. 2012, 2012, 130136. [Google Scholar] [CrossRef] [PubMed]
- Beshiru, A.; Okareh, O.; Okoh, A.; Igbinosa, E. Detection of Antibiotic Resistance and Virulence Genes of Vibrio Strains Isolated from Ready-to-Eat Shrimps in Delta and Edo States, Nigeria. J. Appl. Microbiol. 2020, 129, 17–36. [Google Scholar] [CrossRef]
- Chowdhury, M.; Aziz, K.; Rahim, Z.; Kay, B. Antibiotic Resistance Patterns of Vibrio mimicus Isolated from Human and Environmental Sources in Bangladesh. Antimicrob. Agents Chemother. 1986, 30, 622–623. [Google Scholar] [CrossRef]
- Elgendy, M.Y.; Abdelsalam, M.; Kenawy, A.M.; Ali, S.E. Vibriosis Outbreaks in Farmed Nile Tilapia (Oreochromis Niloticus) Caused by Vibrio mimicus and V. cholerae. Aquac. Int. 2022, 30, 2661–2677. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Feng, D.-Y.; Pan, X.-Y.; Yao, J.-Y.; Yin, W.-L.; Cao, Z.; Liu, Y.-H.; Xia, Y.-C.; Sehn, J.-Y. Identification, Virulence-Related Factors, and Antimicrobial Susceptibility of Vibrio Mimicus from Yellow Catfish, Pelteobagrus fulvidraco. J. Hydrobiol. 2020, 44, 799–810. [Google Scholar]
- Morshdy, A.E.M.; El-Ghandour, A.R.; Hussein, M.A.; El Bayomi, R.M. Prevalence of Antibiotic-Resistant Vibrio Isolated from Some Marketed Fish in Egypt with a Decontamination Trial by Lemon Juice. J. Adv. Vet. Res. 2022, 12, 353–357. [Google Scholar]
- Morshdy, A.E.M.; Abdelhameed, N.S.; Abdallah, K.; El-Tahlawy, A.S.; El Bayomi, R.M. Antibiotic Resistance Profile and Molecular Characterization of Vibrio parahaemolyticus and Vibrio cholerae Isolated from Fish. J. Adv. Vet. Res. 2023, 13, 443–448. [Google Scholar]
- Raissy, M.; Moumeni, M.; Ansari, M.; Rahimi, E. Antibiotic Resistance Pattern of Some Vibrio Strains Isolated. Iran. J. Fish. Sci. 2012, 11, 618–626. [Google Scholar]
- Raissy, M.; Moumeni, M.; Ansari, M.; Rahimi, E. Occurrence of Vibrio spp. In Lobster and Crab from the Persian Gulf. J. Food Saf. 2012, 32, 198–203. [Google Scholar] [CrossRef]
- Sheikh Aftabuddin, S.A.; Sikder, M.; Rahman, M.; Mohammad Zafar, M.Z. Antibiotic Resistance of Vibrio Bacteria Isolated from Mud Crab Scylla Serrata of Chakoria Coast, Bangladesh. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 325–334. [Google Scholar]
- Thongkao, K.; Sudjaroen, Y.; Payattikul, P. Bacterial Contamination and Antibiotic Resistance in Sushi from “Ready-to-Eat” Japanese Restaurants: Preliminary Study in Bangkok, Thailand. J. Pharm. Negat. Results 2022, 13. [Google Scholar]
- Adesiyan, I.M.; Bisi-Johnson, M.A.; Ogunfowokan, A.O.; Okoh, A.I. Occurrence and Antibiogram Signatures of Some Vibrio Species Recovered from Selected Rivers in South West Nigeria. Environ. Sci. Pollut. Res. 2021, 28, 42458–42476. [Google Scholar] [CrossRef]
- Chowdhury, M.; Aziz, K.; Rahim, Z.; Kay, B.A. Toxigenicity and Drug Sensitivity of Vibrio mimicus Isolated from Fresh Water Prawns in Bangladesh. J. Diarrhoeal Dis. Res. 1986, 4, 237–240. [Google Scholar] [PubMed]
- Chowdhury, M.; Yamanaka, H.; Miyoshi, S.; Aziz, K.; Shinoda, S. Ecology of Vibrio mimicus in Aquatic Environments. Appl. Environ. Microbiol. 1989, 55, 2073–2078. [Google Scholar] [CrossRef]
- Gxalo, O.; Digban, T.O.; Igere, B.E.; Olapade, O.A.; Okoh, A.I.; Nwodo, U.U. Virulence and Antibiotic Resistance Characteristics of Vibrio Isolates from Rustic Environmental Freshwaters. Front. Cell. Infect. Microbiol. 2021, 11, 732001. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Saha, S.; Mookerjee, S.; Palit, A. Exploring the Dynamics of Toxigenic Environmental Vibrio mimicus and Its Comparative Analysis with Vibrio cholerae of the Southern Gangetic Delta. Arch. Microbiol. 2022, 204, 420. [Google Scholar] [CrossRef]
- Rebouças, R.H.; Sousa, O.V.d.; Lima, A.S.; Vasconcelos, F.R.; Carvalho, P.B.d.; Fernandes Vieira, R.H.S.d. Antimicrobial Resistance Profile of Vibrio Species Isolated from Marine Shrimp Farming Environments (Litopenaeus vannamei) at Ceará, Brazil. Environ. Res. 2011, 111, 21–24. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, S.; Sinha, K.K. Evidence of Antibiotic Resistance and Virulence Factors in Environmental Isolates of Vibrio Species. Antibiotics 2023, 12, 1062. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.; Gräslund, S.; Wahlström, A.; Poungshompoo, S.; Bengtsson, B.-E.; Kautsky, N. Antibiotic Use in Shrimp Farming and Implications for Environmental Impacts and Human Health. Int. J. Food Sci. Technol. 2003, 38, 255–266. [Google Scholar] [CrossRef]
- Páll, E.; Niculae, M.; Brudașcă, G.F.; Ravilov, R.K.; Șandru, C.D.; Cerbu, C.; Olah, D.; Zăblău, S.; Potârniche, A.V.; Spinu, M.; et al. Assessment and Antibiotic Resistance Profiling in Vibrio Species Isolated from Wild Birds Captured in Danube Delta Biosphere Reserve, Romania. Antibiotics 2021, 10, 333. [Google Scholar] [CrossRef]
- Hankins, J.V.; Madsen, J.A.; Giles, D.K.; Brodbelt, J.S.; Trent, M.S. Amino Acid Addition to Vibrio Cholerae LPS Establishes a Link Between Surface Remodeling in Gram-Positive and Gram-Negative Bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 8722–8727. [Google Scholar] [CrossRef]
- Seethalakshmi, P.; Rajeev, R.; Prabhakaran, A.; Kiran, G.S.; Selvin, J. The Menace of Colistin Resistance Across Globe: Obstacles and Opportunities in Curbing Its Spread. Microbiol. Res. 2023, 270, 127316. [Google Scholar] [CrossRef]
- Heng, S.-P.; Letchumanan, V.; Deng, C.-Y.; Ab Mutalib, N.-S.; Khan, T.M.; Chuah, L.-H.; Chan, K.-G.; Goh, B.-H.; Pusparajah, P.; Lee, L.-H. Vibrio Vulnificus: An Environmental and Clinical Burden. Front. Microbiol. 2017, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.G.; You, L.; Ng, C.; Tong, X.; Mohapatra, S.; Khor, W.C.; Ong, H.M.G.; Aung, K.T.; Gin, K.Y.-H. A Multi-Pronged Approach to Assessing Antimicrobial Resistance Risks in Coastal Waters and Aquaculture Systems. Water Res. 2024, 266, 122353. [Google Scholar] [CrossRef]
- Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J.; Rücker, G. Seriously Misleading Results Using Inverse of Freeman-Tukey Double Arcsine Transformation in Meta-Analysis of Single Proportions. Res. Synth. Methods 2019, 10, 476–483. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and Influence Diagnostics for Meta-Analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. bmj 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A. A New Improved Graphical and Quantitative Method for Detecting Bias in Meta-Analysis. JBI Evid. Implement. 2018, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Higgins, J.P. Meta-Analysis and Subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Tabit, F.T. Global and Regional Trends of Antimicrobial Resistance in Vibrio alginolyticus from One-Health Perspective: Meta-Modelling and Antimicrobial Resistance Risk Index Assessment. One Health 2025. submitted. [Google Scholar]
| All Samples | Seafood | Environmental Water | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| s/n | Antibiotic | Class | Pooled study | Prevalence (95% CI) | I2 (95%CI) | ET/LFKi | k | Prevalence (95% CI) | I2 | k | Prevalence (95% CI) | I2 |
| 1 | Amikacin | Aminoglycosides | k = 6; e = 54 | 22.6 (4.6–64.1) | 87.7 (75.6; 93.8) | 0 | 4 | 19.2 (2.0–73.7) | 70.6 | 2 | 30.8 (0.0–100.0) | 89.1 |
| 2 | Gentamicin | Aminoglycosides | k = 12; e = 53 | 16.5 (2.8–57.4) | 78.6 (63.1–87.6) | 0.57 (−2.72–3.85, 0.74) | 9 | 26.7 (3.7–77.6) | 80.1 | 3 | 5.2 (0.1–80.4) | 0 |
| 3 | Kanamycin | Aminoglycosides | k = 9; e = 76 | 28.8 (3.5–81.9) | 75.4 (52.6–87.2) | 0 | 4 | 50.3 (5.8–94.4) | 0 | 4 | 25.1 (0.2–98.6) | 89.9 |
| 4 | Streptomycin | Aminoglycosides | k = 9; e = 71 | 54.6 (15.8–88.5) | 67.8 (35.2–84.0) | 0 | 5 | 51.8 (9.5–91.7) | 49.9 | 3 | 79.3 (0.1–100.0) | 81.4 |
| 5 | Imipenem | Carbapenems | k = 6; e = 24 | 10.7 (2.5–35.9) | 31.3 (0.0–72.1) | 0 | 3 | 2.1 (0.0–99.9) | 0 | 3 | 16.3 (1.9–65.6) | 71.7 |
| 6 | Meropenem | Carbapenems | k = 5; e = 27 | 12.3 (1.6–54.6) | 0.0 (0.0–79.2) | 0 | 2 | 6.6 (0.0–100.0) | 0 | 3 | 16.9 (1.7–70.6) | 0 |
| 7 | Cefotaxime | Cephalosporins | k = 7; e = 30 | 8.9 (2.3–29.3) | 70.0 (34.3–86.3) | 0 | 5 | 7.3 (2.3–21.13) | 11.3 | 2 | 8.6 (0.0–100.0) | 0 |
| 8 | Cefuroxime | Cephalosporins | k = 3; e = 77 | 60.1 (2.3–99.0) | 82.3 (45.7–94.3) | 0 | 1 | 10.0 (1.4–46.7) | – | 2 | 80.9 (13.1–99.2) | 0 |
| 9 | Cephalothin | Cephalosporins | k = 5; e = 30 | 28.7 (13.3–51.4) | 62.9 (2.0–86.0) | 0 | 4 | 27.6 (9.3–58.5) | 70.1 | 1 | 35.7 (15.7–62.4) | – |
| 10 | Chloramphenicol | Chloramphenicol | k = 10; e = 100 | 32.3 (2.7–89.0) | 68.8 (39.8–83.8) | −1.14 (−4.02–1.73, 0.5) | 6 | 20.8 (1.1–85.9) | 64.9 | 4 | 51.9 (0.0–100.0) | 0 |
| 11 | Ciprofloxacin | Fluoroquinolones and Quinolones | k = 10; e = 49 | 9.9 (1.7–41.6) | 71.0 (44.7–84.8) | −2.64 (−4.66–−0.62, 0.03) | 7 | 8.6 (0.7–56.4) | 34.5 | 3 | 12.9 (0.1–97.3) | 89.3 |
| 12 | Norfloxacin | Fluoroquinolones and Quinolones | k = 8; e = 56 | 8.9 (0.4–70.9) | 20.8 (0.0–63.1) | 0 | 5 | 18.0 (1.1–81.5) | 0 | 3 | 1.4 (0.0–100.0) | 0 |
| 13 | Nalidixic acid | Fluoroquinolones and Quinolones | k = 5; e = 50 | 47.2 (17.3–79.4) | 0.0 (0.0–79.2) | 0 | 4 | 39.7 (5.2–88.7) | 0 | 1 | 59.3 (45.8–71.5) | – |
| 14 | Ofloxacin | Fluoroquinolones and Quinolones | k = 4; e = 70 | 34.0 (1.7–94.0) | 69.1 (10.8–89.3) | 0 | 2 | 6.6 (0.0–100.0) | 0 | 2 | 71.0 (3.1–99.5) | 76.2 |
| 15 | Azithromycin | Macrolides and Azalides | k = 4; e = 50 | 65.8 (0.9–99.5) | 72.2 (21.3–90.2) | 0 | 2 | 46.2 (0.1–99.9) | 44 | 2 | 94.3 (0.0–100.0) | 0 |
| 16 | Erythromycin | Macrolides and Azalides | k = 7; e = 29 | 21.5 (3.0–70.8) | 51.7 (0.0–79.5) | 0 | 6 | 17.2 (4.3–49.2) | 0 | 1 | 92.9 (63.0–99.0) | – |
| 17 | Amoxicillin | Penicillins | k = 5; e = 46 | 83.7 (5.3–99.8) | 78.9 (49.7–91.1) | 0 | 3 | 98.0 (0.1–100.0) | 0 | 2 | 21.0 (0.0–100.0) | 91.3 |
| 18 | Ampicillin | Penicillins | k = 19; e = 203 | 61.11 (24.5–88.4) | 80.7 (70.7–87.2) | 1.14; −1.26–3.53; 0.4 | 12 | 76.1 (17.2–98.0) | 55.8 | 6 | 58.0 (19.4–88.8) | 91.6 |
| 19 | Penicillin | Penicillins | k = 4; e = 24 | 72.7 (43.5–90.3) | 16.5 (0.0–87.2) | 0 | 4 | 72.7 (43.5–90.3) | 16.5 | – | – | – |
| 20 | Colistin sulphate | Polymyxins | k = 3; e = 47 | 80.2 (0.0–100.0) | 54.9 (0.0–87.1) | 0 | – | – | – | – | – | – |
| 21 | Sulfamethoxazole | Sulfonamides | k = 7; e = 40 | 38.3 (3.6–91.2) | 79.5 (58.1–90.0) | 0 | 6 | 25.8 (1.8–86.9) | 71.6 | 1 | 92.9 (63.0–99.0) | – |
| 22 | Doxycycline | Tetracyclines | k = 5; e = 62 | 59.4 (3.6–98.3) | 49.9 (0.0–81.6) | 0 | 3 | 36.5 (0.0–100.0) | 0 | 2 | 76.5 (7.9–99.2) | 0 |
| 23 | Tetracycline | Tetracyclines | k = 16; e = 102 | 13.5 (2.9–45.2) | 69.7 (49.5–81.9) | −4.10 (−5.86–−2.34, <0.00) | 10 | 12.8 (2.6–45.0) | 52.5 | 5 | 35.1 (1.2–96.0) | 77.4 |
| 24 | Oxytetracycline | Tetracyclines | k = 4; e = 23 | 47.9 (26.1–70.5) | 30.8 (0.0–75.0) | 0 | 3 | 52.9 (20.3–83.3) | 36.9 | 1 | 35.7 (15.7–62.4) | 0 |
| 25 | Trimethoprim–Sulfamethoxazole | Trimethoprim and Combinations | k = 12; e = 69 | 5.8 (0.7–36.1) | 73.3 (52.5–85.0) | −3.75 (−5.48–−2.01, 0.002) | 6 | 4.9 (0.4–41.0) | 0 | 5 | 19.0 (0.4–92.6) | 84.4 |
| 26 | Amoxicillin–Clavulanic acid | β-Lactam/β-Lactamase Inhibitor Combos | k = 5; e = 46 | 31.8 (0.8–96.3) | 47.3 (0.0–80.7) | 0 | 4 | 27.2 (0.04–99.7) | 0 | 1 | 48.2 (35.3–61.3) | – |
| 27 | Ampicillin–Sulbactam | β-Lactam/β-Lactamase Inhibitor Combos | k = 3; e = 36 | 43.3 (5.1–91.5) | 84.4 (53.5–94.8) | 0 | 2 | 25.9 (0.1–98.9) | 0 | 1 | 72.5 (56.8–84.1) | – |
| Sample | Cvmrni | Total Antibiotic Tested | ARRI |
|---|---|---|---|
| Seafoods | 618 | 148 | 50.84 |
| Environmental water | 1179 | 71 | 46.53 |
| Human | 2 | 5 | 0.01 |
| Shellfish | 282 | 70 | 10.97 |
| Fish | 163 | 23 | 2.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekundayo, T.C.; Tabit, F.T. Occurrence and Antibiotic Resistance Risk Burden of Vibrio mimicus Isolates from Seafood and Aquatic Environments. Antibiotics 2025, 14, 1075. https://doi.org/10.3390/antibiotics14111075
Ekundayo TC, Tabit FT. Occurrence and Antibiotic Resistance Risk Burden of Vibrio mimicus Isolates from Seafood and Aquatic Environments. Antibiotics. 2025; 14(11):1075. https://doi.org/10.3390/antibiotics14111075
Chicago/Turabian StyleEkundayo, Temitope C., and Frederick T. Tabit. 2025. "Occurrence and Antibiotic Resistance Risk Burden of Vibrio mimicus Isolates from Seafood and Aquatic Environments" Antibiotics 14, no. 11: 1075. https://doi.org/10.3390/antibiotics14111075
APA StyleEkundayo, T. C., & Tabit, F. T. (2025). Occurrence and Antibiotic Resistance Risk Burden of Vibrio mimicus Isolates from Seafood and Aquatic Environments. Antibiotics, 14(11), 1075. https://doi.org/10.3390/antibiotics14111075







