Trends in Antimicrobial Resistance at a Greek Tertiary Hospital over a 7-Year Period, Including the COVID-19 Pandemic
Abstract
1. Introduction
2. Results
2.1. Description of the Clinical Isolates
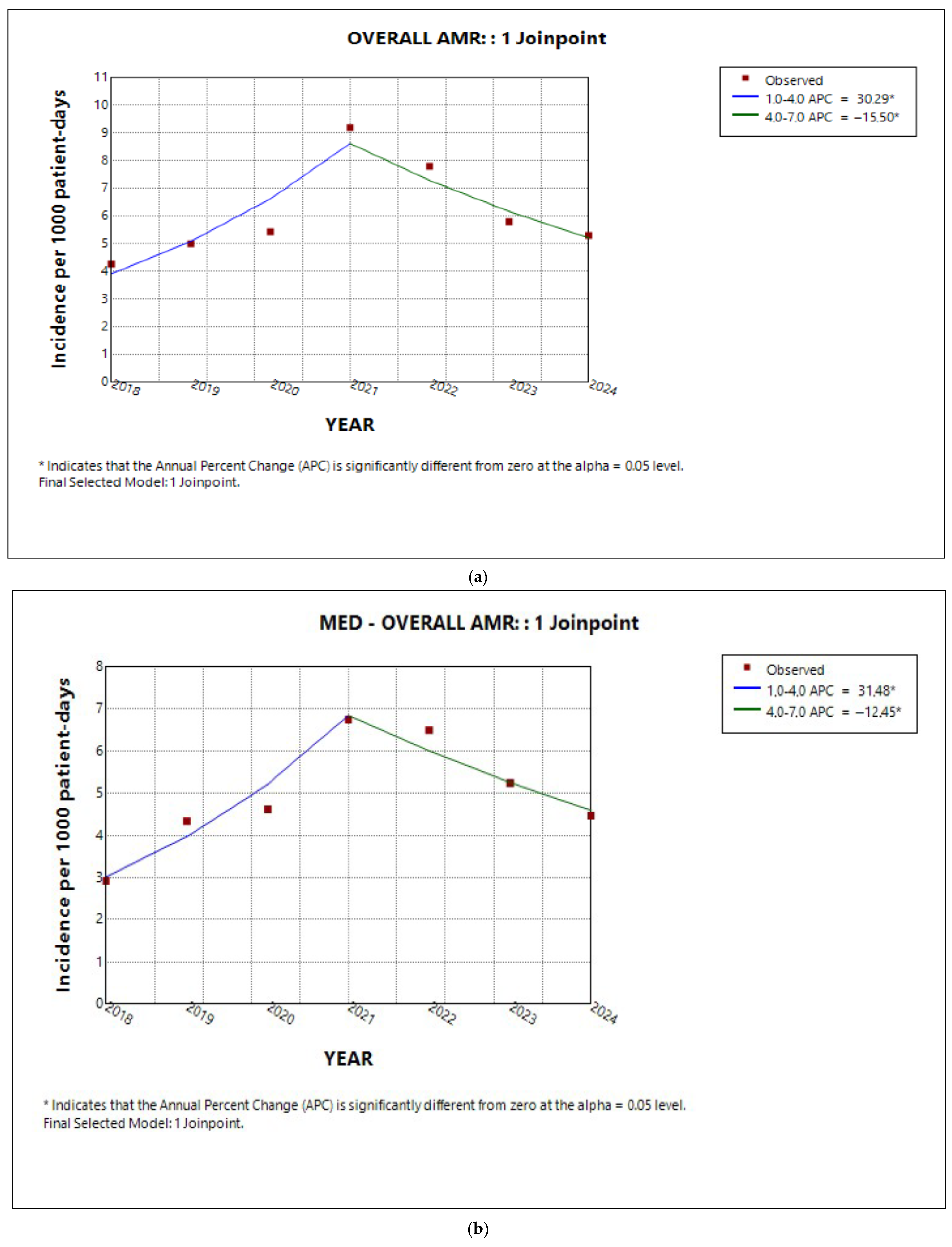
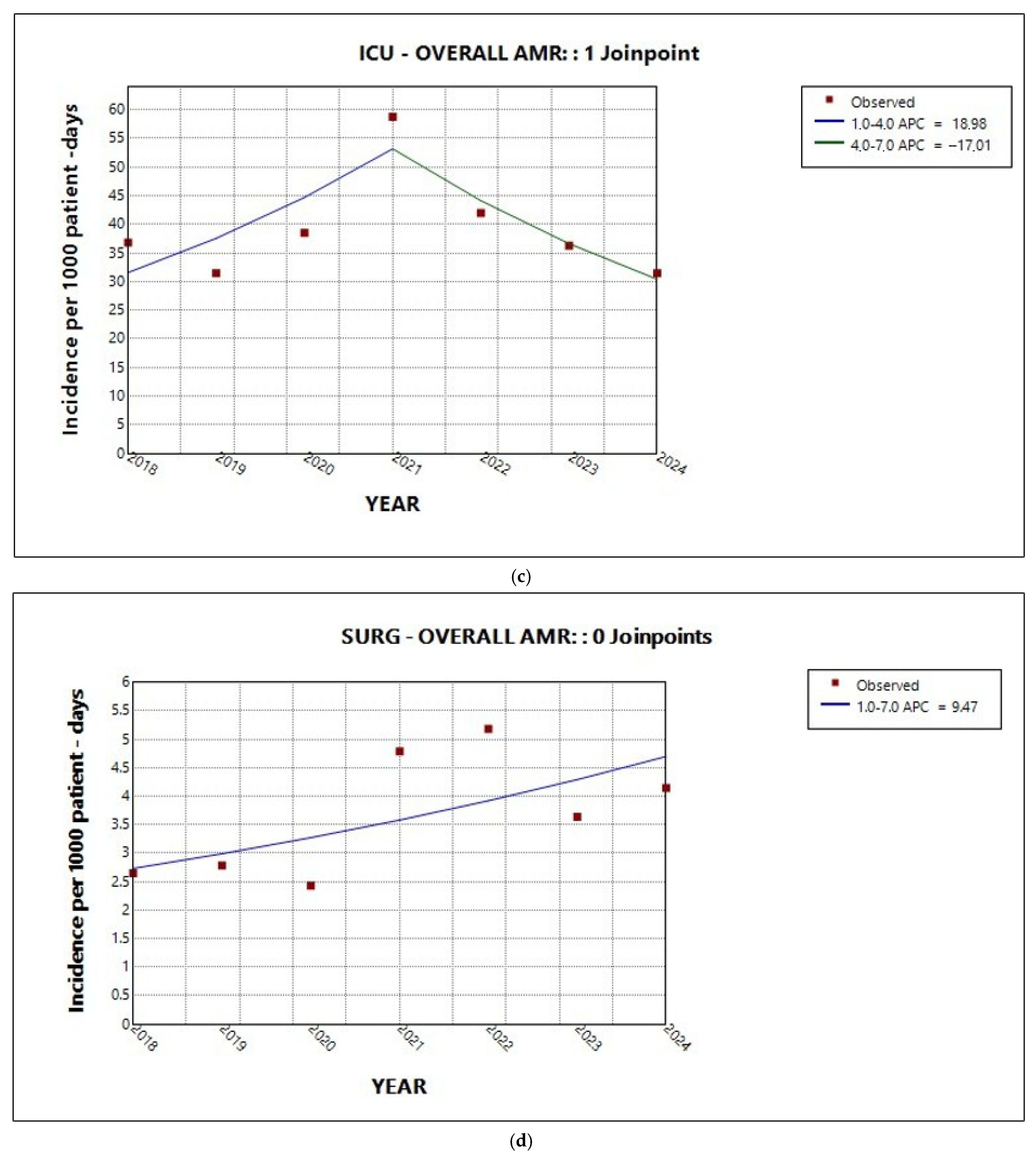
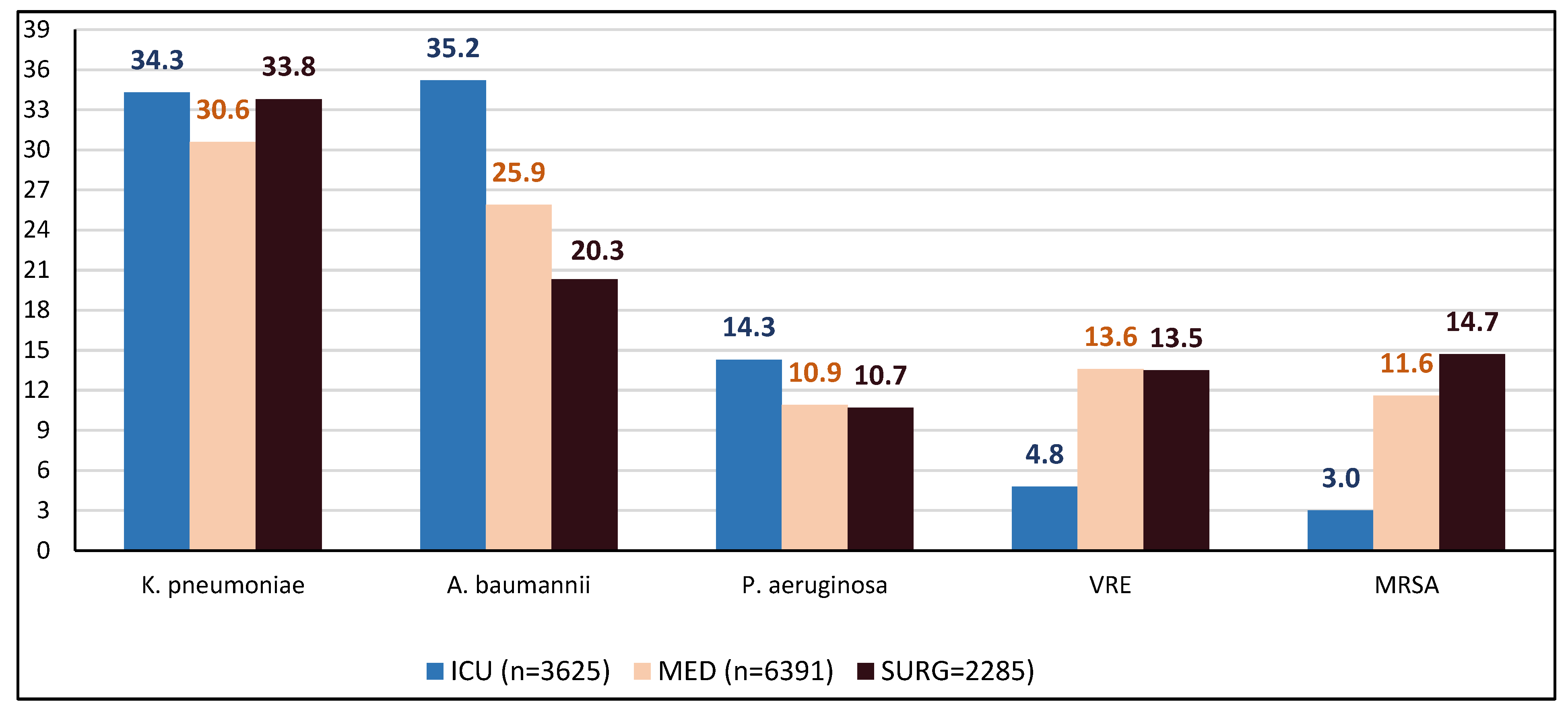
2.2. Trends in Total AMR in the Entire Hospital and per Sector
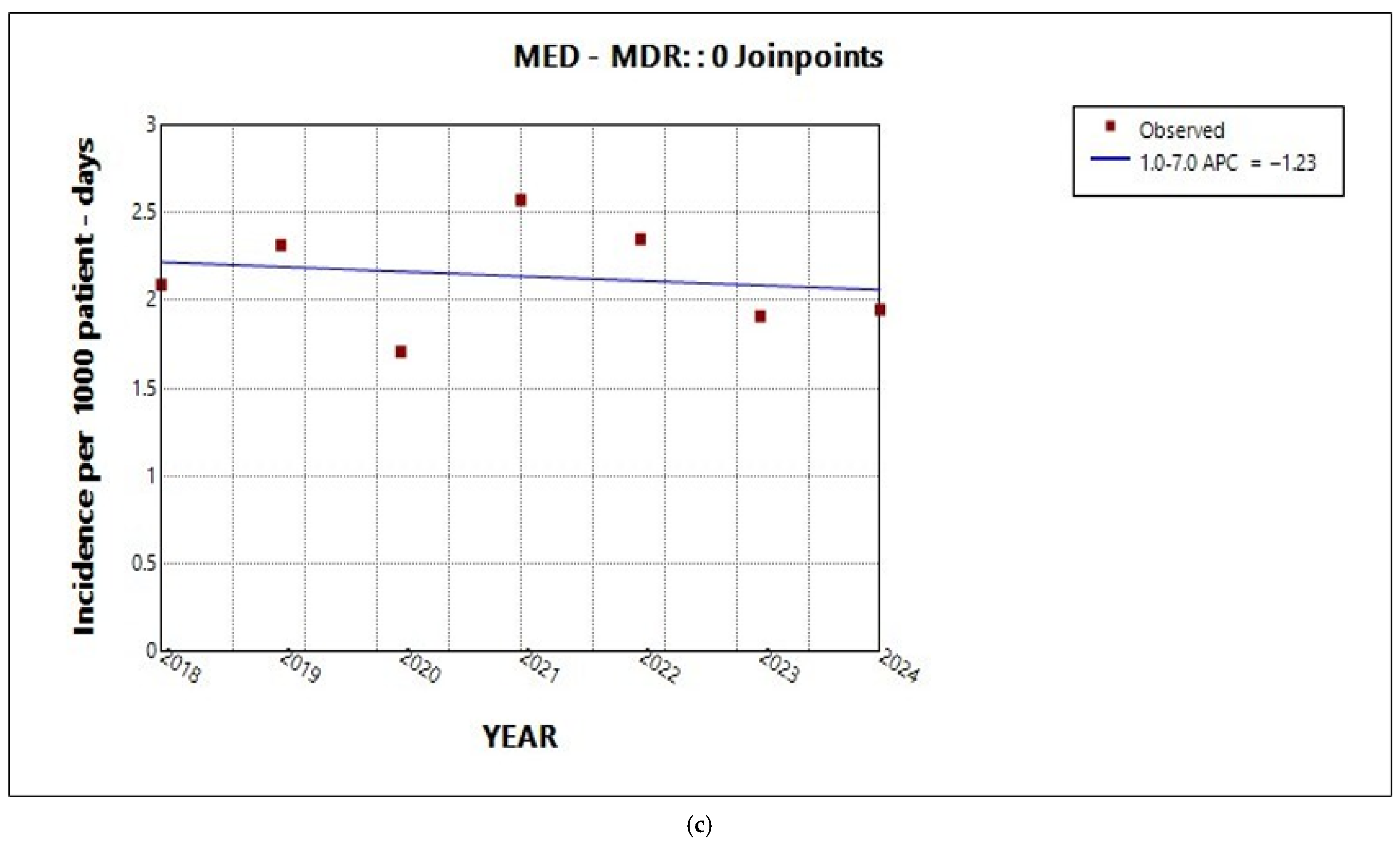
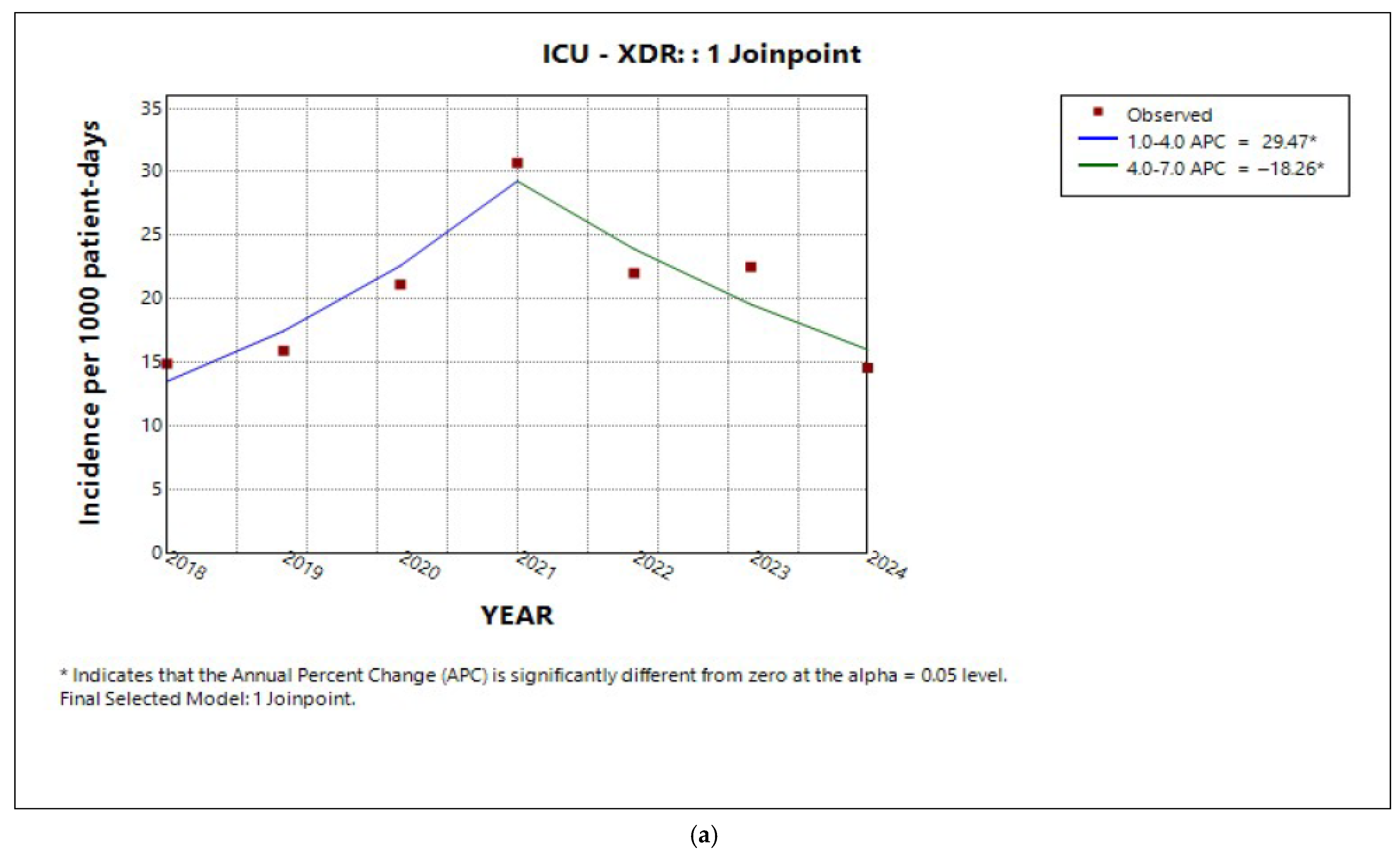
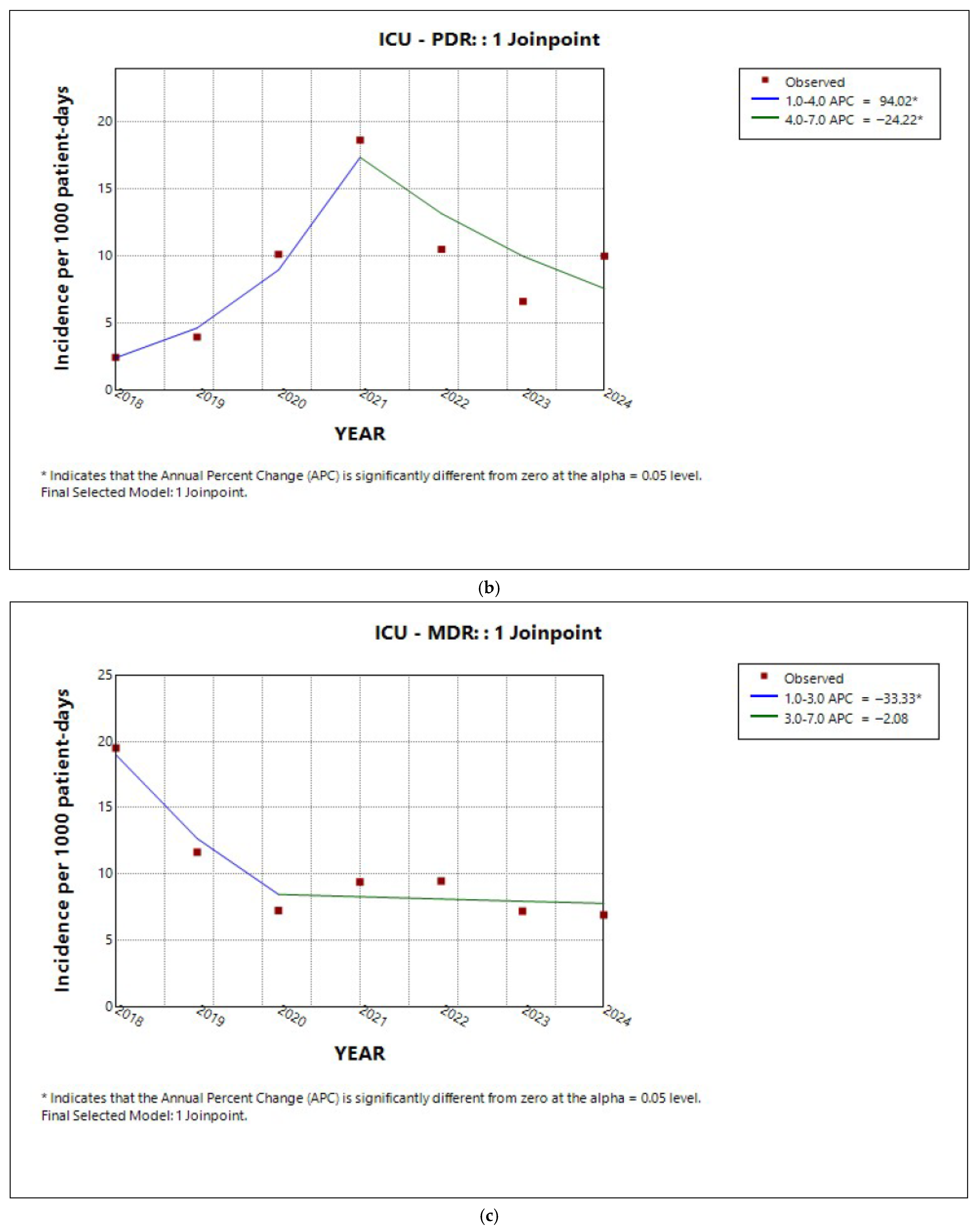
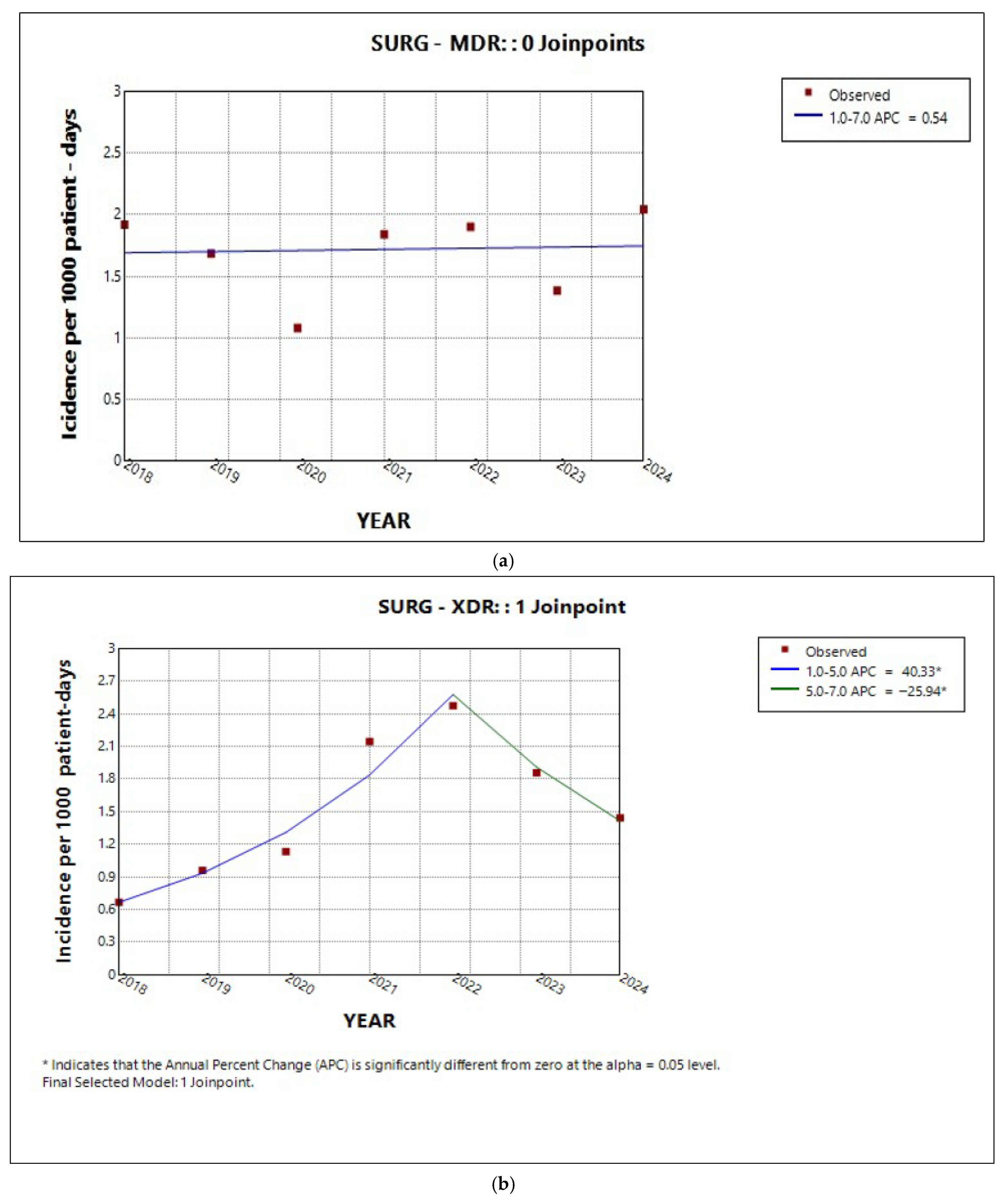
2.3. Trends in Incidence by Resistance Category
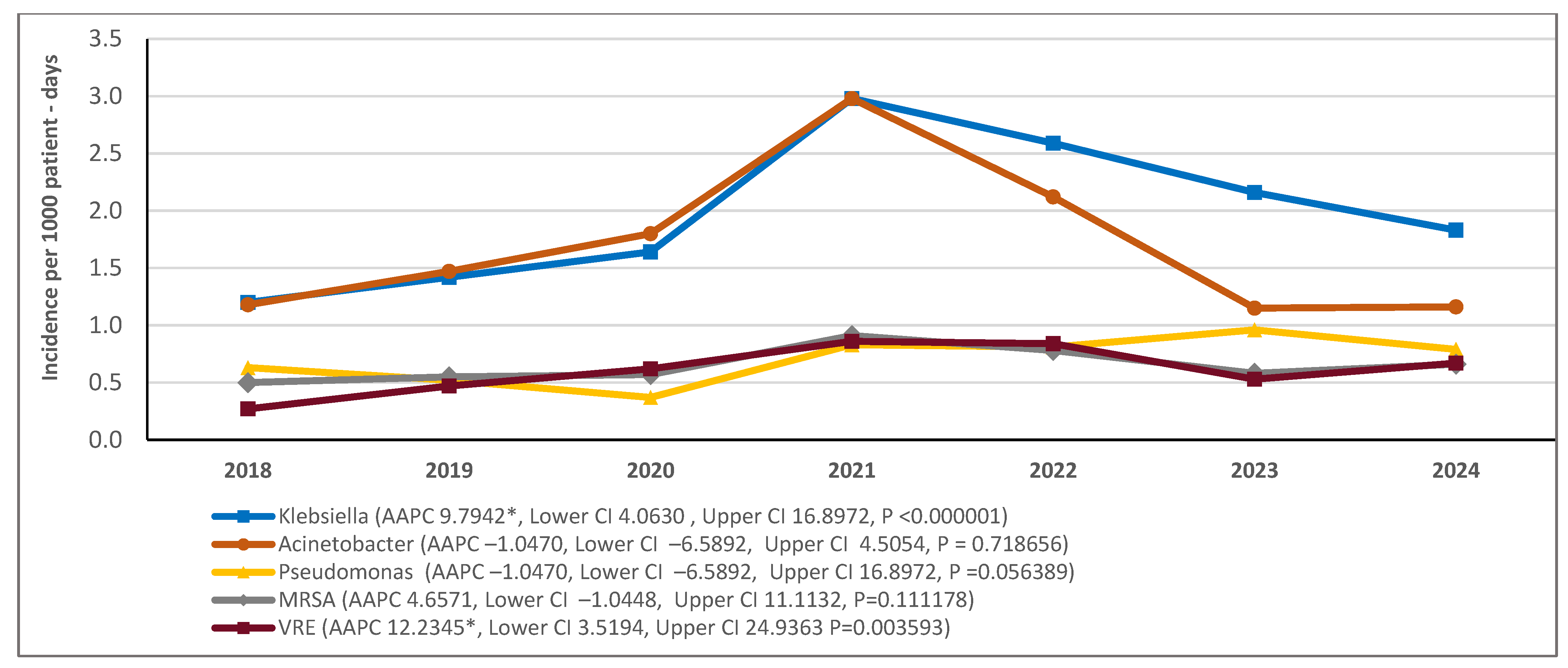
2.4. Incidence Trends by Bacterial Species
2.5. Trend in Total AMR in Isolates from Blood Cultures
3. Discussion
4. Materials and Methods
5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; WHO: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1 (accessed on 12 September 2025).
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Draft Global Action Plan for Antimicrobial Resistance. Available online: http://apps.who.int/gb/ebwha/pdf_files/EB136/B136_20-en.pdf (accessed on 12 September 2025).
- Simonsen, G.S. Antimicrobial resistance surveillance in Europe and beyond. Euro Surveill. 2018, 23, 1800560. [Google Scholar] [CrossRef] [PubMed]
- Iacchini, S.; Sabbatucci, M.; Gagliotti, C.; Rossolini, G.M.; Moro, M.L.; Iannazzo, S.; D’Ancona, F.; Pezzotti, P.; Pantosti, A. Bloodstream Infections Due to Carbapenemase-Producing Enterobacteriaceae in Italy: Results from Nationwide Surveillance, 2014–2017. Euro Surveill. 2019, 24, 1800159. [Google Scholar] [CrossRef]
- Tsioutis, C.; Kritsotakis, E.I.; Karageorgos, S.A.; Stratakou, S.; Gikas, A. Trends in antimicrobial resistance and antibiotic consumption in a tertiary care hospital in Crete, Greece. Infect. Drug Resist. 2021, 14, 1501–1511. [Google Scholar]
- Lim, C.; Takahashi, E.; Hongsuwan, M.; Wuthiekanun, V.; Day, N.P.J.; Peacock, S.J.; Sirijatuphat, R.; Pongsuttiyakorn, S.; Supapueng, O.; Kiratisin, P.; et al. The Global Antimicrobial Resistance Surveillance System (GLASS) pilot: Lessons from implementation in Thailand. Bull. World Health Organ. 2021, 99, 312–321. [Google Scholar]
- World Health Organization (WHO). GLASS Report: Early Implementation 2020–2021; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Ray, S.M.; Thompson, D.L.; Wilson, L.E.; Fridkin, S.K.; et al. Multistate point-prevalence surveys of healthcare-associated infections and antimicrobial use—United States, 2011–2015. Clin. Infect. Dis. 2021, 72, e1–e10. [Google Scholar]
- Yek, C.; Mongkolrattanothai, T.; Thielke, S.; Johannsson, B.; Evans, M.E.; Mancera, A.G.; Diao, G.; Walker, M.; Neupane, M.; Chishti, E.A.; et al. Impact of the COVID-19 pandemic on antibiotic-resistant infection burden in U.S. hospitals: A retrospective cohort study of trends and risk factors. Ann. Intern. Med. 2025, 178, 796–807. [Google Scholar] [CrossRef]
- Langford, B.J.; Soucy, J.-P.R.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic resistance associated with the COVID-19 pandemic: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 835–844. [Google Scholar] [CrossRef]
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodrígez-Baño, J. Impact of COVID-19 pandemic on multidrug resistant gram positive and gram-negative pathogens: A systematic review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). CDC Report Finds Sharp Rise in Dangerous Drug-Resistant Bacteria. U.S. Department of Health and Human Services, 12 March 2025. Available online: https://www.cdc.gov/media/releases/2025/2025-cdc-report-finds-sharp-rise-in-dangerous-drug-resistant-bacteria.html (accessed on 9 October 2025).
- Reffat, N.; Schwei, R.J.; Griffin, M.; Pop-Vicas, A.; Schulz, L.T.; Pulia, M.S. A scoping review of bacterial resistance among inpatients amidst the COVID-19 pandemic. J. Glob. Antimicrob. Res. 2024, 38, 49–65. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Simeonova, M.; Leung, V.; Lo, J.; Kan, T.; Raybardhan, S.; Sapin, M.E.; Mponponsuo, K.; Farrell, A.; et al. Antimicrobial resistance in patients with COVID-19: A systematic review and meta-analysis. Lancet Microbe. 2023, 4, e179–e191. [Google Scholar] [CrossRef] [PubMed]
- Rozman, U.; Kranjec, K.; Šeruga, A.; Kramar, U.; Vrbnjak, D.; Lavrič, M.; Turk, S. Impact of the COVID-19 pandemic on the consumption of antibiotics and the emergence of AMR: Case study in a general hospital. Front. Public Health 2025, 13, 1584574. [Google Scholar] [CrossRef] [PubMed]
- National Center for Emerging and Zoonotic Infectious Diseases. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; National Center for Emerging and Zoonotic Infectious Diseases: Atlanta, GA, USA, 2022.
- World Health Organization (WHO); European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance Surveillance in Europe 2023–2021 Data. Available online: https://who.int/europe/publications/i/item/9789289058537 (accessed on 12 September 2025).
- Spiliopoulou, A.; Giannopoulou, I.; Assimakopoulos, S.F.; Jelasopulu, E.; Bartzavali, C.; Marangos, M.; Paliogianni, F.; Kolonitsiou, F. Laboratory surveillance of Acinetobacter spp. bloodstream infections in a tertiary University hospital during a 9-year period. Trop. Med. Infect. Dis. 2023, 8, 503. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). ECDC Country Visit to Greece to Discuss Antimicrobial Resistance Issues, 15–19 April 2024; ECDC: Stockholm, Sweden, 2024; ISBN 978-92-9498-752-5. [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Rossolini, G.M.; Schultsz, C.; Tacconelli, E.; Murthy, S.; Ohmagari, N.; Holmes, A.; Bachmann, T.; Goossens, H.; Canton, R.; et al. Antimicrobial resistance research in a post-pandemic world: Insights on antimicrobial resistance research in the COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2021, 25, 5–7. [Google Scholar] [CrossRef]
- Ansari, S.; Hays, J.P.; Kemp, A.; Okechukwu, R.; Murugaiyan, J.; Ekwanzala, M.D.; Alvarez, M.J.R.; Paul-Satyaseela, M.; Iwu, C.D.; Balleste-Delpierre, C.; et al. The potential impact of the COVID-19 pandemic on global antimicrobial and biocide resistance: An AMR Insights global perspective. JAC Antimicrob. Resist. 2021, 3, dlab038. [Google Scholar] [CrossRef]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ. 2020, 98, 442. [Google Scholar] [CrossRef]
- Stevens, M.P.; Doll, M.; Pryor, R.; Godbout, E.; Cooper, K.; Bearman, G. Impact of COVID-19 on traditional healthcare-associated infection prevention efforts. Infect. Control. Hosp. Epidemiol. 2020, 41, 946–947. [Google Scholar] [CrossRef]
- Polly, M.; de Almeida, B.L.; Lennon, R.P.; Cortês, M.F.; Costa, S.F.; Guimarães, T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am. J. Infect. Control 2022, 50, 32–38. [Google Scholar] [CrossRef]
- Pascale, R.; Bussini, L.; Gaibani, P.; Bovo, F.; Fornaro, G.; Lombardo, D.; Ambretti, S.; Pensalfine, G.; Appolloni, L.; Bartoletti, M.; et al. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: A multicenter before-and-after cross-sectional study. Infect. Control. Hosp. Epidemiol. 2022, 43, 461–466. [Google Scholar] [CrossRef]
- Shbaklo, N.; Corcione, S.; Vicentini, C.; Giordano, S.; Fiorentino, D.; Bianco, G.; Francesco, C.; Rossana, C.; Maria, Z.C.; Rosa, D.; et al. An observational study of MDR hospital-acquired infections and antibiotic use during COVID-19 pandemic: A call for antimicrobial stewardship programs. Antibiotics 2022, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.J.; Song, W.; Kim, H.S.; Kim, J.-S. Impact of COVID-19 on antimicrobial consumption and spread of multidrug-resistance in bacterial infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Barnard, E. The impact of the COVID-19 pandemic on healthcare acquired infections with multidrug resistant organisms. Am. J. Infect. Control. 2021, 49, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial stewardship program, COVID-19, and infection control: Spread of carbapenem- resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Kariyawasan, R.M.; Julien, D.A.; Jelinski, D.C.; Larose, S.L.; Rennert-May, E.; Conly, J.M.; Dingle, T.C.; Chen, J.Z.; Tyrrell, G.J.; Ronkslely, P.E.; et al. Antimicrobial resistance (AMR) in COVID-19 patients: A systematic review and meta-analysis (November 2019–June 2021). Antimicrob. Res. Infect. Control. 2022, 11, 45. [Google Scholar] [CrossRef]
- Micheli, G.; Sangiorgi, F.; Catania, F.; Chiuchiarelli, M.; Frondizi, F.; Taddei, E.; Murri, R. The hidden cost of COVID-19: Focus on antimicrobial resistance in bloodstream infections. Microorganisms 2023, 11, 1299. [Google Scholar] [CrossRef]
- Bauer, K.A.; Puzniak, L.S.; Yu, K.C.; Klinker, K.P.; Watts, J.A.; Moise, P.A.; Fineli, L.; Ai, C.; Gupta, V. A Multicenter comparison of prevalence and predictors of antimicrobial resistance in hospitalized patients before and during the severe acute respiratory syndrome Coronavirus 2 Pandemic. Open Forum Infect. Dis. 2022, 9, ofac537. [Google Scholar] [CrossRef]
- Meschiari, M.; Onorato, L.; Bacca, E.; Orlando, G.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Santoro, A.; Sarti, M.; et al. Long-term impact of the COVID-19 pandemic on in-hospital antibiotic consumption and antibiotic resistance: A time series analysis (2015–2021). Antibiotics 2022, 11, 826. [Google Scholar] [CrossRef]
- Lagadinou, A.; Amerali, M.; Michailides, C.; Chondroleou, A.; Skintzi, K.; Spiliopoulou, A.; Kolonitsiou, F.; Leonidou, L.; Assimakopoulos, S.F.; Marangos, M. Antibiotic resistance trends in carbapenem-resistant gram-negative pathogens and eight-year surveillance of XDR bloodstream infection in a Western Greece tertiary hospital. Pathogens 2024, 13, 1136. [Google Scholar] [CrossRef]
- Abdelmoneim, S.A.; Ghazy, R.M.; Sultan, E.A.; Hassaan, M.A.; Mahgoub, M.A. Antimicrobial resistance presence burden pre and post-COVID-19 pandemic with mapping in the multidrug resistance in Egypt: A comparative cross-sectional study. Sci. Rep. 2024, 14, 7176. [Google Scholar] [CrossRef]
- Tsalidou, M.; Stergiopoulou, T.; Bostanitis, I.; Nikaki, C.; Skoumpa, K.; Koutsoukou, T.; Papaioannidou, P. Surveillance of antimicrobial resistance and multidrug resistance prevalence of clinical isolates in a general hospital in northern Greece. Antibiotics 2023, 12, 1595. [Google Scholar]
- Santoro, A.; Franceschini, E.; Meschiari, M.; Menozzi, M.; Zona, A.; Venturelli, C.; Digaetano, M.; Rogati, C.; Guaraldi, G.; Paul, M.; et al. Epidemiology and risk factors associated with mortality in consecutive patients with bacterial bloodstream infection: Impact of MDR and XDR Bacteria. Open Forum Infect. Dis. 2020, 7, ofaa461. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- John, T.J.; Kompithra, R.Z. Eco-epidemiology triad to explain infectious diseases. Indian. J. Med. Res. 2023, 158, 107–112. [Google Scholar] [CrossRef]
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One Health approach. Vet. World 2022, 15, 743–749. [Google Scholar] [CrossRef]
- European Commission. A European One Health Action Plan Against Antimicrobial Resistance (AMR); European Commission: Brussels, Belgium, 2017; Available online: https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf (accessed on 9 October 2025).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions or acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Clinical Breakpoints—Bacteria, Version 14.0; European Committee on Antimicrobial Susceptibility Testing (EUCAST): Växjö, Sweden, 2024. [Google Scholar]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for join-point regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
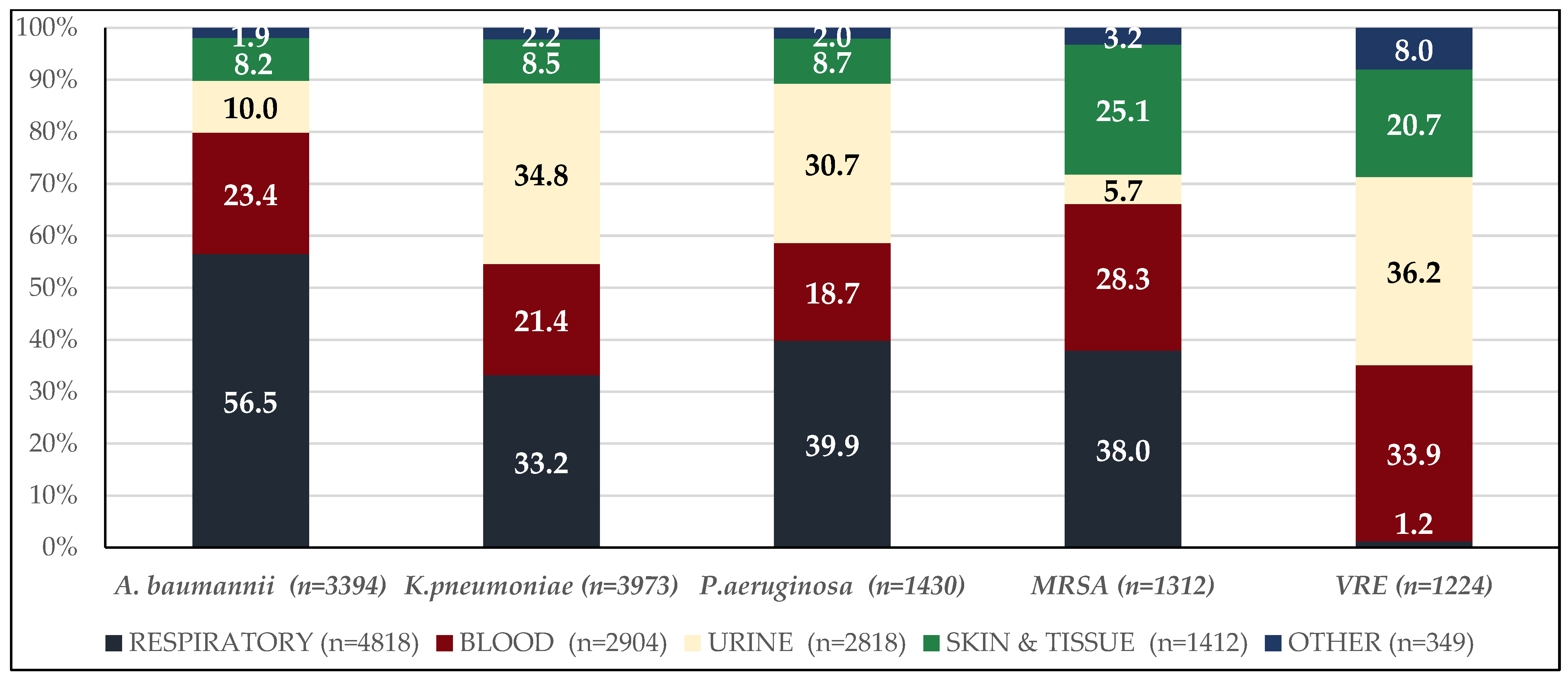
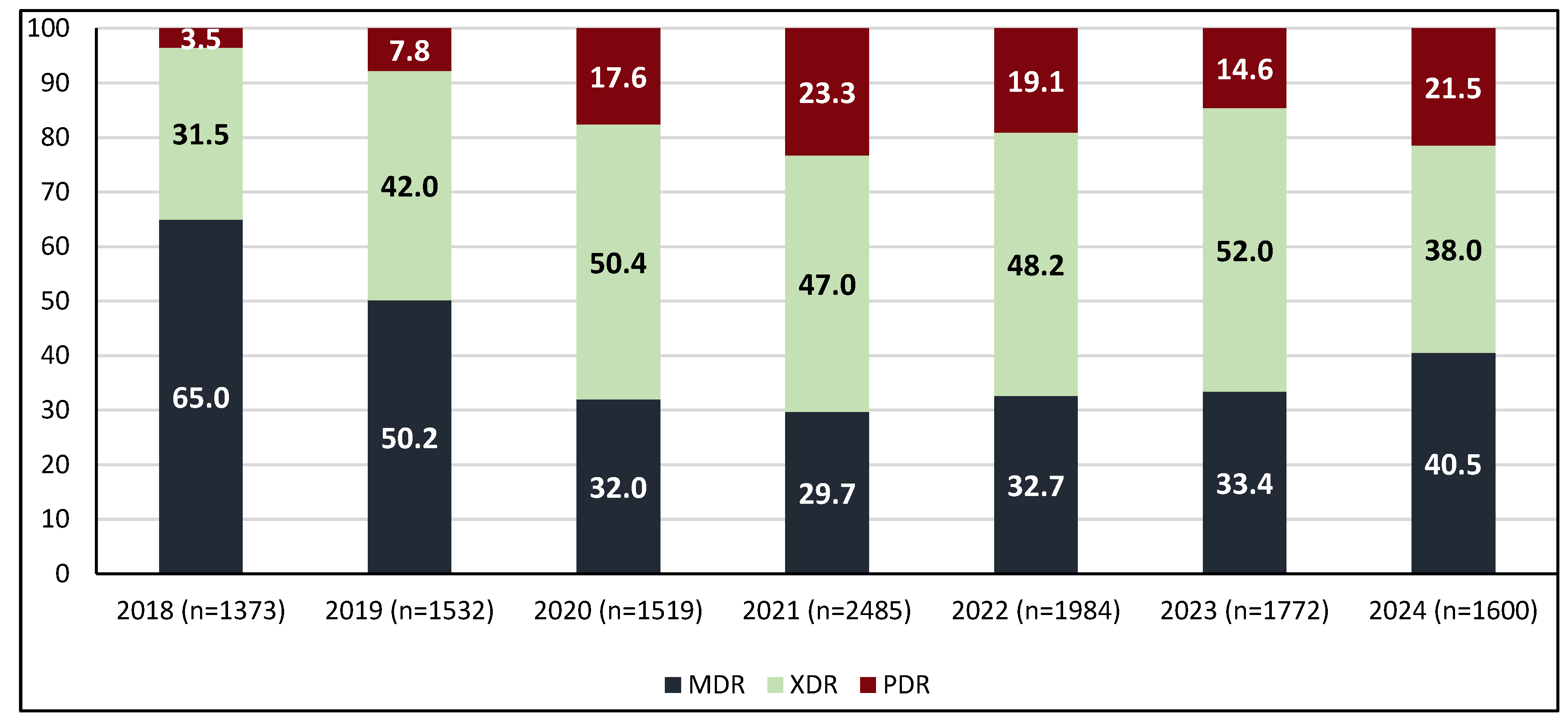








| Year | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
|---|---|---|---|---|---|---|---|
| No of Isolates | 1373 | 1568 | 1520 | 2485 | 1984 | 1772 | 1600 |
| Klebsiella pneumoniae | 388 (28.3) | 446 (28.4) | 461 (30.3) | 807 (32.5) | 658 (33.2) | 662 (37.4) | 552 (34.5) |
| Acinetobacter baumannii | 380 (27.7) | 461 (29.4) | 504 (33.2) | 807 (32.5) | 539 (27.2) | 352 (19.9) | 351 (21.9) |
| MRSA | 160 (11.7) | 174 (11.1) | 103 (6.8) | 246 (9.9) | 198 (10.0) | 177 (10.0) | 198 (12.4) |
| VRE | 88 (6.4) | 149 (9.5) | 161 (10.6) | 232 (9.3) | 214 (10.8) | 164 (9.3) | 204 (12.8) |
| Pseudomonas aeruginosa | 202 (14.7) | 161 (10.3) | 174 (11.4) | 225 (9.1) | 207 (10.4) | 293 (16.5) | 239 (14.9) |
| Stenotrophomonas maltophilia | 89 (6.5) | 89 (5.7) | 83 (5.5) | 132 (5.3) | 104 (5.2) | 77 (4.3) | 26 (1.6) |
| Providencia stuartii | 47 (3.4) | 48 (3.1) | 11 (0.7) | 7 (0.3) | 44 (2.2) | 22 (1.2) | 8 (0.5) |
| Proteus. mirabilis | 9 (0.7) | 15 (1.0) | 6 (0.4) | 7 (0.3) | 8 (0.4) | 7 (0.4) | 9 (0.6) |
| Escherichia coli | 0 | 6(0.4) | 5 (0.3) | 9 (0.4) | 1 (0.1) | 7 (0.4) | 7 (0.4) |
| other Gram- | 10 (0.7) | 19 (1.2) | 12 (0.8) | 13 (0.5) | 11 (0.6) | 11 (0.6) | 6 (0.4) |
| Sector | AAPC | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| Medical | 7.3 | 3.7 | 11.3 | <0.001 * |
| ICU | 0.6 | −18.3 | 17.5 | 0.08 |
| Surgical | 9.8 | −1.8 | 23.3 | 0.1 |
| Species | Segments of Study Period | APC | Lower CI | Upper CI | p Value |
|---|---|---|---|---|---|
| Klebsiella pneumoniae | 2018–2021 | 37.9 | 22 | 70.2 | <0.001 * |
| 2021–2024 | −12.6 | −26 | −2.5 | 0.003 * | |
| Acinetobacter baumannii | 2018–2021 | 36.6 | 22.8 | 55.1 | <0.001 * |
| 2021–2024 | −28.3 | −36.9 | −20.4 | <0.001 * | |
| Pseudomonas aeruginosa | 2018–2021 | 9.1 | −0.2 | 21.3 | 0.06 |
| 2021–2024 | 9.1 | −0.2 | 21.3 | 0.06 | |
| MRSA | 2018–2021 | 21.5 | 10.1 | 50.4 | <0.001 * |
| 2021–2024 | −9.8 | −25.1 | −0.9 | 0.02 * | |
| VRE | 2018–2021 | 41.9 | −21.7 | 106.3 | <0.001 * |
| 2021–2024 | −11.2 | −31.3 | 1.8 | 0.09 |
| Sector | AAPC | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| Entire hospital | 7.2 | −0.9 | 17.2 | 0.06 |
| Medical | 9.9 | 3.7 | 17.6 | <0.001 * |
| ICU | −3.5 | −10.7 | 2.8 | 0.2 |
| Surgical | 17.3 * | 4.9 | 35.0 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylona, E.; Kostourou, S.; Giankoula, D.; Spyrakou, E.; Michopanou, N.; Kolokotroni, C.; Papagianni, M.; Kounatidis, D.; Perivolioti, E.; Papastamopoulos, V. Trends in Antimicrobial Resistance at a Greek Tertiary Hospital over a 7-Year Period, Including the COVID-19 Pandemic. Antibiotics 2025, 14, 1067. https://doi.org/10.3390/antibiotics14111067
Mylona E, Kostourou S, Giankoula D, Spyrakou E, Michopanou N, Kolokotroni C, Papagianni M, Kounatidis D, Perivolioti E, Papastamopoulos V. Trends in Antimicrobial Resistance at a Greek Tertiary Hospital over a 7-Year Period, Including the COVID-19 Pandemic. Antibiotics. 2025; 14(11):1067. https://doi.org/10.3390/antibiotics14111067
Chicago/Turabian StyleMylona, Eleni, Sofia Kostourou, Dimitroula Giankoula, Efthimia Spyrakou, Nektaria Michopanou, Chrysoula Kolokotroni, Maria Papagianni, Dimitris Kounatidis, Efstathia Perivolioti, and Vasileios Papastamopoulos. 2025. "Trends in Antimicrobial Resistance at a Greek Tertiary Hospital over a 7-Year Period, Including the COVID-19 Pandemic" Antibiotics 14, no. 11: 1067. https://doi.org/10.3390/antibiotics14111067
APA StyleMylona, E., Kostourou, S., Giankoula, D., Spyrakou, E., Michopanou, N., Kolokotroni, C., Papagianni, M., Kounatidis, D., Perivolioti, E., & Papastamopoulos, V. (2025). Trends in Antimicrobial Resistance at a Greek Tertiary Hospital over a 7-Year Period, Including the COVID-19 Pandemic. Antibiotics, 14(11), 1067. https://doi.org/10.3390/antibiotics14111067







