Abstract
Mismatch repair (MMR) system alterations can trigger transient hypermutation, promoting adaptive mutations under stress, such as antibiotic exposure. While most organisms use MutS and MutL protein families for MMR, many archaea and actinobacteria, including the major human pathogen Mycobacterium tuberculosis, lack these components and instead rely on NucS, a structurally distinct enzyme driving a non-canonical MMR pathway. Given the role of MMR in mutation control, understanding how nucS expression is regulated could be essential for uncovering the molecular basis of antibiotic resistance development in mycobacteria. In this study, we characterized the nucS promoter and transcription start site in Mycobacterium smegmatis. We found that nucS expression declines during the stationary phase in both M. smegmatis and M. tuberculosis, paralleling replication activity and canonical MMR downregulation. Our data suggest that the alternative sigma factor σB may negatively regulate nucS expression during this phase. Additionally, we identified candidate compounds that may modulate nucS expression, underscoring its responsiveness to environmental cues. These findings enhance our understanding of mycobacterial stress responses and lay the groundwork for exploring antibiotic resistance mechanisms. Strikingly, our work reveals a case of double convergent evolution: both canonical (MutS/MutL) and non-canonical (NucS) pathways have independently evolved not only the same DNA repair function, but also similar regulatory frameworks for genome integrity preservation under stress conditions.
1. Introduction
Bacteria continuously face fluctuating environmental conditions that demand rapid and coordinated stress responses. These adaptive strategies frequently involve a transient elevation of mutation rates, thereby increasing genetic variability and enhancing survival under specific adverse conditions [1].
In Escherichia coli and related microorganisms, nutrient limitation during the stationary phase triggers a general stress response that can increase the mutation rate either by inducing the expression of the error-prone DNA polymerase (Pol IV) or by repressing the expression of key components of the mismatch repair (MMR) system [1]. Moreover, since MMR system constrains recombination between non-identical (homeologous) sequences, its downregulation in the stationary phase may further facilitate the exchange of divergent alleles, broadening the adaptive landscape available to the cell [1].
Detailed investigation in E. coli have shown that levels of MMR proteins MutS and MutH are reduced by approximately four-fold and two-fold, respectively, during the stationary phase compared to the exponential phase of growth [2], whereas MutL levels remain constant across both phases [2,3]. Even though MutL levels appear stable, evidence suggests that its active concentration becomes limited during the stationary phase, contrasting with the proportional decline in MutS and MutH, which likely mirrors the reduced replication demand [2,4,5].
Global regulators such as the alternative sigma factor RpoS (σS) and the RNA chaperone Hfq play essential roles in adjusting MutS and MutH protein levels during stress [2]. In the exponential phase, Hfq destabilizes mutS transcripts via an RpoS-independent mechanism. As cells transition to the stationary phase, Hfq further downregulate MutS via both RpoS-dependent and independent pathways, potentially through intermediaries like RNases, proteases, or other RpoS-controlled factors [2]. Hfq and RpoS also seem to regulate MutH levels through the same pathway during the stationary phase [2]. In addition, subinhibitory concentrations of β-lactam antibiotics induce the rpoS regulon. Under these conditions, the RpoS-controlled regulatory sRNA SdsR targets mutS mRNA, preventing its translation and consequently reducing MMR activity [6]. Recent evidence also implicates an RNA G-quadruplex structure, formed by guanine-rich sequences within the mutS coding region, as a potential regulatory element [7].
While the canonical MMR proteins MutS and MutL are conserved across all domains of life, from bacteria to humans, many archaea and nearly all members of the phylum Actinobacteria, including major pathogens such as Mycobacterium tuberculosis, lack these components [8,9,10,11]. Remarkably, these microorganisms maintain low mutation rates, suggesting the existence of an alternative MMR pathway. This observation led our research group to identify a non-canonical MMR system in Actinobacteria, based on a homolog of the archaeal endonuclease NucS [10]. The in vivo role of NucS as an MMR protein was first demonstrated in the nonpathogenic species Mycobacterium smegmatis, where its disruption leads to a hypermutator phenotype, a bias towards transition mutations, and increased homeologous recombination, hallmark features of MMR deficiency [10,12]. These findings underscored the essential role of NucS in maintaining genome integrity in Actinobacteria [10,11,12,13,14,15].
Despite these advances, the molecular mechanisms regulating nucS expression remain unknown. We hypothesized that nucS expression is subject to transcriptional and/or post-transcriptional regulation, influenced by the physiological state of the cells, environmental stressors (including certain antibiotics), and host factors such as oxidative and nitrosative stress. Indeed, recent evidence has suggested that nitrosative agents may influence nucS expression [16]. Understanding the regulatory mechanisms controlling nucS expression could open new avenues for modulating mutation rates in mycobacteria, for instance by enhancing nucS transcription to reduce mutagenesis and limit resistance development in clinical settings. Moreover, the transient suppression of nucS expression in vitro could increase mutation rates and facilitate the discovery of novel resistance pathways, ultimately contributing to more effective and targeted antibiotic therapies.
In this work, we investigated the expression profile of mycobacterial nucS across growth phases and examined the potential regulatory role of the sigma factor σB, a functional analog of RpoS [17,18,19,20], after identifying putative σB binding boxes in the nucS promoter region. Furthermore, to explore the influence of environmental factors, we evaluated the impact of a number of chemical compounds on nucS expression using a nucS::gfp transcriptional fusion. The dynamic regulation of nucS expression may induce transient hypermutation, potentially increasing genetic variability and providing an adaptive advantage under certain stressful conditions.
2. Results
2.1. Determination of the Transcription Start Site of nucS in M. smegmatis
To initiate the study of nucS expression, we determined its transcription start site (TSS) using the 5′-RACE technique. A TSS was identified that coincides with the gene’s translation start site, consistent with findings from the global analysis by Martini et al. [21] (Figure 1A). This overlap between the transcription and translation start sites, resulting in the absence of a 5′-UTR, is commonly observed in mycobacteria [22].
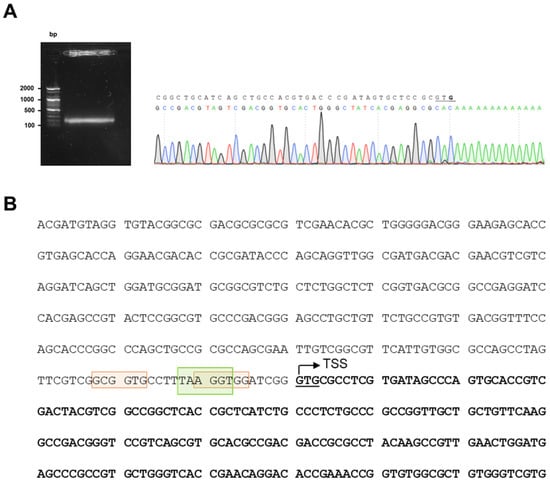
Figure 1.
Identification of nucS TSS. (A) Results of 5′-RACE experiment. A gel image showing the band obtained after performing the 5′-RACE technique, which was sequenced to identify the TSS of nucS in M. smegmatis. The TSS of nucS is highlighted in bold, corresponding to the complementary base to the first nucleotide following the poly-A tail. The TSS coincides with the translation start of nucS (the first G of the start codon GTG, which is underlined). (B) Upstream sequence of nucS in M. smegmatis. The start of the coding sequence of the nucS gene is shown in bold, with the GTG start codon underlined. The identified transcription start site (TSS), determined by 5′-RACE, is indicated with an arrow. The colored boxes represent potential −10 regions for binding of sigma factors in M. smegmatis: green for σA (TANNNT) [22] and orange for σB (NNGNNG) [23].
The determination of the TSS of nucS allows us to better locate the promoter region of the gene. Close to the TSS, we identified the consensus sequence of the −10 box for σA factor binding (TANNNT), homologous to σ70 of E. coli and commonly found in constitutively expressed genes [22] (Figure 1B). We did not identify the consensus sequence for the −35 box for σA binding, as the −35 box is rarely conserved in mycobacteria [23]. Based on the consensus sequences reported by Newton-Foot and Gey van Pittius [23], potential −10 boxes for other sigma factors were detected near the σA binding site. While these sequences are less conserved than those of σA, we identified two putative consensus sequences for the −10 box of σB (NNGNNG), a sigma factor that plays a crucial role in response to various stress conditions and adaptation to the stationary phase (Figure 1B).
2.2. Construction of GFP Reporter Strains for the Study of nucS Expression
In this study, reporter strains were constructed to analyze the expression levels and regulatory mechanisms of the nucS gene. Specifically, three transcriptional reporter fusions were constructed, each containing nucS upstream regions of varying lengths followed by the GFP gene. The first fusion, referred to as the “short fusion,” included a 73 bp region directly upstream of the nucS start codon (pSGV53-PnucS-73-gfp), which may correspond to the core promoter region according to the identified TSS and the predicted sigma factor binding sites. The second fusion, referred to as the “long fusion,” encompassed a 408 bp region upstream of the nucS start codon (pSGV53-PnucS-408-gfp), including the same region plus additional upstream sequences that may contain regulatory elements. The third fusion spanned the same 408 bp region but excluded the 46 bp just upstream the TSS (pSGV53-PnucS-408∆46-gfp), which corresponds to the intergenic sequence immediately upstream of nucS, and therefore likely lacked the core promoter region (Figure 2).
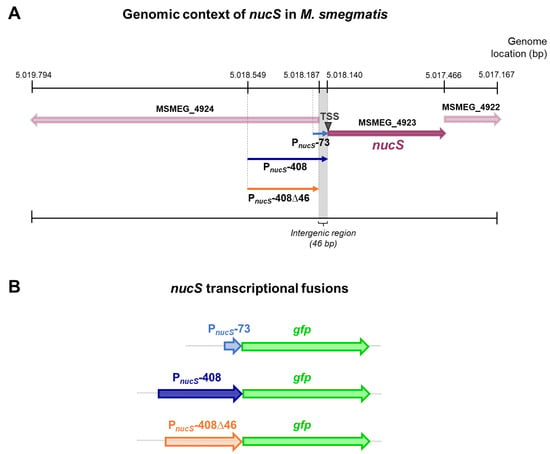
Figure 2.
Genomic context of nucS in M. smegmatis and schematic of nucS transcriptional fusions. (A) Genomic context of the nucS gene in M. smegmatis mc2 155. The position of nucS in the genome of M. smegmatis mc2 155 (NC_008596) is shown, along with the upstream regions of the gene (PnucS-73, PnucS-408 and PnucS-408∆46) that were included in the transcriptional fusions with gfp. The position of the nucS TSS is also indicated. (B) Schematic representation of the nucS::gfp transcriptional fusions. The three nucS upstream regions of different lengths cloned into the reporter vectors are shown, each followed by the gfp gene.
The plasmid pSGV53 served as the backbone for constructing the reporter fusions (Table S1). This plasmid is a replicative vector with a replication origin derived from pAL5000, maintaining approximately 5 copies per cell [24,25], and originally carries the gene for the eGFP protein under the control of the constitutive promoter Pmpt64 [26]. To generate the reporter plasmids, the constitutive promoter was replaced with the upstream regions of nucS, enabling the fluorescence signal to reflect the activity of these regulatory regions.
2.3. nucS Expression Is Growth Phase-Dependent
To investigate nucS expression, the reporter plasmids were transformed into the wild—type M. smegmatis strain, and fluorescence levels during growth were monitored using a spectrofluorometer. Fluorescence intensity (FI) was normalized to OD595 values at each time point (Figure 3A). No differences in growth were observed between the strains carrying the reporter vectors and the non-transformed control strain (Figure S1).
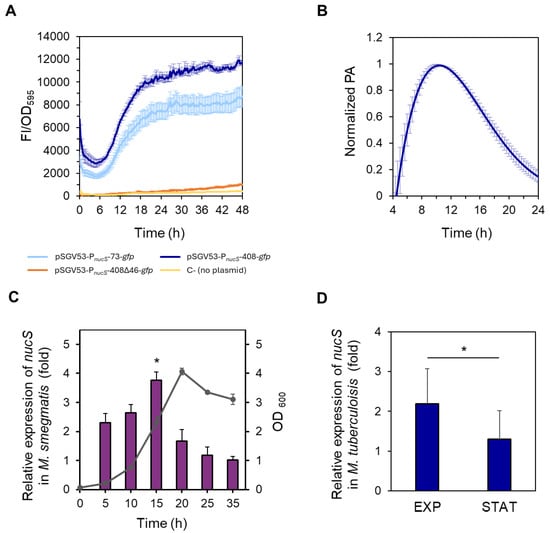
Figure 3.
Study of nucS expression during growth. (A) Spectrofluorometric analysis of reporter strains during growth. Normalized fluorescence (FI/OD595, arbitrary units) of M. smegmatis strains carrying different nucS::gfp transcriptional fusions: pSGV53-PnucS-73-gfp (light blue), pSGV53-PnucS-408-gfp (dark blue) and pSGV53-PnucS-408∆46-gfp (orange). Autofluorescence of non-transformed cells (no plasmid) is shown in yellow (C-, negative control). Data represent the mean ± standard error (SE) of eight biological replicates (n = 8). (B) Promoter activity of M. smegmatis nucS. The promoter activity (PA) values of the M. smegmatis reporter strain harboring the plasmid pSGV53-PnucS-408-gfp normalized to their respective maximum are shown (see Materials and Methods). Error bars: SE (n = 8). (C) Measurement of nucS expression in M. smegmatis during growth by RT-qPCR. Bars show the relative expression levels of nucS normalized to the endogenous control sigA at different growth times. Relative expression was calculated using the 2−ΔΔCt method, with the latest time point (35 h) serving as the control with baseline levels, assigned a value of 1. The growth curve showing OD600 values at the different time points when samples were collected is represented in gray. Error bars: SE (n = 3). *: p < 0.05 (15 h vs. 35 h), Student’s t-test for paired samples. (D) Measurement of nucS expression in M. tuberculosis H37Rv at different growth phases by RT-qPCR. Bars show the expression levels (2−ΔΔCt method) of nucS normalized to the endogenous control lpqM at late exponential phase (EXP) (OD600 = 1) relative to the value at stationary phase (STAT) (OD600 = 5). Error bars: SE (n = 3). *: p < 0.05, Student’s t-test for paired samples. A growth curve of M. tuberculosis H37Rv is shown in Figure S2.
When comparing the fluorescence levels of the short (73 bp) and long (408 bp) fusions, we observed that the fluorescence was moderately higher (approximately 30–40%) in the strains carrying the long fusion, suggesting the presence of additional regulatory elements between 74 and 408 bp upstream of the nucS TSS. However, the presence of the short region alone was sufficient to drive high fluorescence levels (Figure 3A). In contrast, the strain carrying the vector pSGV53-PnucS-408∆46-gfp exhibited fluorescence levels comparable to the non-transformed negative control (Figure 3A), indicating that the intergenic region between nucS and the upstream gene is essential for nucS expression. These results further support the location of the promoter within this region.
Focusing on the fluorescence pattern during bacterial growth of the reporter strains carrying the short and long fusions, we found that fluorescence intensity steadily increased during the exponential phase in both strains. This suggests that the highest expression of nucS occurs during this phase, coinciding with active cell division. Fluorescence continued to increase into the early stationary phase, reaching maximum levels, and then remained constant throughout the rest of the stationary phase (Figure 3A). This stable signal is likely due to the long half-life of GFP [27,28], which allows fluorescence to accumulate even after transcriptional activity has decreased.
To quantify promoter activity (PA), the rate of GFP accumulation was calculated from the spectrofluorometric data (see Section 4. Materials and Methods). Maximum promoter activity for the reporter fusion with the longest upstream region of nucS (pSGV53-PnucS-408-gfp) was observed approximately at 10 h of growth under the conditions tested in the microplate reader, coinciding with the late exponential phase (see Figure S1). Following this peak, the PA gradually declined (Figure 3B).
The assays performed with the reporter fusions suggested that the highest expression of nucS occurred during the exponential phase. The transcriptional profile of nucS throughout the growth cycle was validated by RT-qPCR, confirming that nucS expression is growth phase-dependent (Figure 3C). In this assay, the highest levels of nucS transcript were observed towards the end of the exponential phase (15 h vs. 35 h expression ratio of 3.8-fold, p = 0.0105) (Figure 3C). However, when the cells entered the stationary phase, nucS transcription experienced a significant decrease, with the gene expression levels remaining low for the rest of the stationary phase (Figure 3C). A similar pattern was observed in M. tuberculosis, where nucS transcription levels were higher in the late exponential phase than in the stationary phase (p = 0.0172) (Figure 3D).
It was also analyzed whether the decrease in nucS expression observed at the transcript level during the stationary phase was noticeable at the protein level. To investigate this, we performed Western blot analyses using protein extracts from M. smegmatis collected at different growth times (Figure 4). To normalize NucS expression levels, the expression of the constitutive protein FtsZ, which remains at similar levels during both the exponential and stationary growth phases [29], was also measured. Similar to the transcript levels, NucS protein abundance was higher in the exponential phase than in the stationary phase, particularly when comparing the end of the exponential phase to the late stationary phase (18 h vs. 36 h expression ratio of 2.3-fold, p = 0.0207) (Figure 4B).
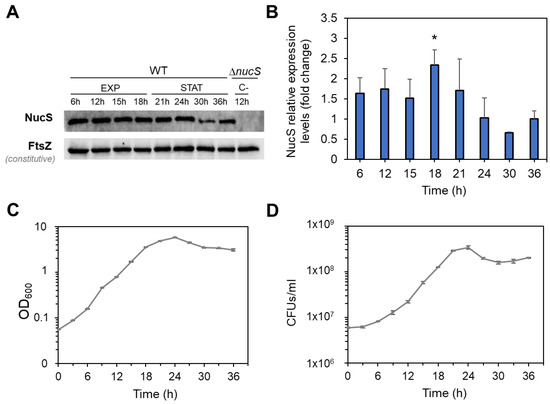
Figure 4.
NucS levels in M. smegmatis during bacterial growth. (A) Western blot analysis of NucS expression. Representative Western blot experiment showing NucS and FtsZ (loading control) bands in wild-type M. smegmatis (WT) at various time points during exponential (EXP) and stationary (STAT) phases. A ΔnucS extract collected at 12 h was included as a negative control. Images of the full membrane are provided in Figure S3. (B) Quantification of NucS levels. NucS band intensities were normalized to FtsZ and expressed relative to the 36h time point (baseline = 1). Error bars represent SE from three independent experiments using extracts from different biological replicates. *: p < 0.05 (18 h vs. 36 h), paired Student’s t-test. (C) Growth curve of M. smegmatis based on optical density. OD600 measurements of the cultures used for the Western blot analysis. Error bars represent SE (n = 3). (D) Viability curve based on colony-forming units (CFU). Average CFU/mL for each time point. Error bars represent SE (n = 3).
In summary, the analyses performed using reporter fusions, RT-qPCR and Western blot, indicate that NucS expression at both the transcript and protein levels is growth phase-dependent, decreasing during the stationary phase.
2.4. Analysis of nucS Expression in a M. smegmatis sigB-Deficient Strain
Reduced expression of canonical MMR system components during the stationary phase has been linked to indirect negative regulation by rpoS, which encodes the σS factor, in microorganisms like E. coli [2,4,5]. In mycobacteria, the gene sigB, encoding the sigma factor σB, is functionally analogous to rpoS and, like rpoS, is induced during the stationary phase and under stress conditions [17,18,19,20]. Moreover, two putative consensus sequences for σB binding (NNGNNG) were identified in the upstream region of nucS (see Figure 2B). This observation suggests that sigB may play a role in the regulation of nucS during the stationary phase. To investigate this, we compared nucS expression in a ΔsigB mutant with that of the wild-type strain.
Given our hypothesis that sigB negatively regulates nucS expression during the stationary phase, we anticipated higher nucS expression in the ΔsigB mutant compared to the wild-type strain during this phase. RT-qPCR analysis revealed no differences in nucS expression between the two strains during the exponential phase. However, during the stationary phase, nucS expression in the ΔsigB strain was approximately twofold higher than in the wild-type strain (p = 0.0259), suggesting negative regulation of nucS by sigB in the stationary phase (Figure 5).
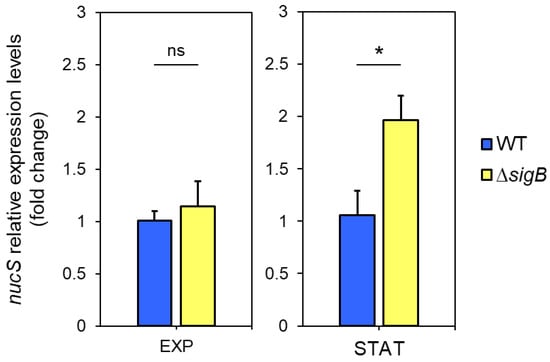
Figure 5.
Expression of nucS in M. smegmatis ΔsigB during exponential and stationary phases. Expression levels of nucS in the ΔsigB mutant relative to the wild-type strain during late exponential (EXP, 15 h) and stationary (STAT, 40 h) phases, as determined by RT-qPCR. Cultures were initiated at OD600 = 0.05, and both strains exhibited similar growth rates (see Figure S4). Bars represent nucS expression normalized to the endogenous control sigA. Relative expression was calculated using the 2−ΔΔCt method, with the wild-type strain set as the reference (value = 1). Error bars: SE (n = 3). Statistical significance was assessed using a one-tailed t-test for independent samples (alternative hypothesis: ΔsigB > wild type; specified as “greater” in R). ns: not significant (p > 0.05); *: p < 0.05.
Additionally, to assess whether the potential regulation of nucS by sigB during the stationary phase affects the phenotype, the mutant frequency in the presence of rifampicin was calculated for the WT and ΔsigB strains during both the exponential and stationary phases. Rifampicin is commonly used to calculate mutant frequency or mutation rate because resistance to this antibiotic typically arises from well-characterized point mutations in the rpoB gene, allowing reliable detection and quantification of spontaneous mutation events in M. smegmatis and other bacterial species. The results showed a higher mutant frequency during the exponential phase compared to the stationary phase for both strains. While the mutant frequency in the WT and ΔsigB strains was similar during the exponential phase, the ΔsigB strain exhibited a slight (nearly twofold) reduction in mutant frequency relative to the WT strain during the stationary phase, although this difference was not statistically significant (Figure 6). Further studies are required to confirm the relevance of potential nucS regulation by the σB factor in the mutator phenotype.
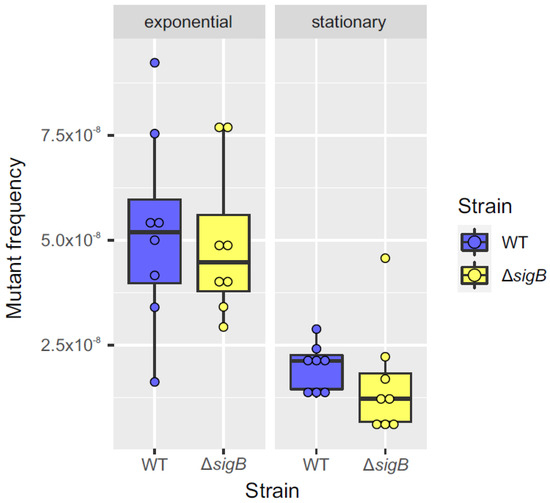
Figure 6.
Comparison of mutant frequency between WT and ΔsigB strains during exponential and stationary phases. Box plots comparing mutant frequencies between the WT (blue) and ΔsigB (yellow) strains during the exponential and stationary phases. The thick black line inside each box represents the median, and the dots show mutant frequency values for each replicate (n = 8). No significant differences in mutant frequencies were observed between the two strains in either the exponential or stationary phase (p > 0.05; Mann–Whitney U test). The plots were generated using the ggplot2 package (v3.3.3) in R (v4.0.5).
2.5. Screening for Compounds Regulating nucS Expression
Fluorescent reporter fusions are powerful tools for investigating gene expression under diverse conditions. To identify potential compounds regulating nucS expression in M. smegmatis—either inducers or repressors—a screening was performed using Phenotype MicroArrays (PMs) (Biolog™) in combination with a reporter strain carrying the vector pSGV-PnucS-408-gfp (Figure 2, Table S1). Biolog™ PMs, designed to evaluate bacterial responses to a wide array of chemical agents, have been successfully applied with fluorescent reporter fusions in prior studies [30]. For this screening, chemical sensitivity assay plates (PM11-PM20) were selected. Each plate contained 24 compounds, tested at four increasing concentrations, resulting in the analysis of 240 compounds (Table S2).
To establish baseline fluorescence, normalized fluorescence levels (FI/OD595) of the reporter strain were measured under screening conditions in the absence of compounds. Upper and lower thresholds were then defined to identify compounds influencing nucS expression (Figure S5). The upper threshold was calculated as , where x represents the normalized fluorescence value of the reporter strain at each point, and SD is the standard deviation. The lower threshold was defined as . Compounds producing fluorescence values above the upper threshold were considered potential inducers, while those yielding values below the lower threshold were classified as potential repressors. This screening identified 22 candidate compounds: 13 putative inducers and 9 putative repressors (Table S3).
A follow-up screening was conducted using a disk diffusion assay with the reporter strain and the candidate compounds. This method allows testing a wide range of compound concentrations. Induction of nucS expression was observed as an increase in fluorescence intensity around the inhibition halo, while repression was indicated by a decrease in fluorescence in the same region.
Through this assay, only two compounds showed a specific effect on nucS expression, producing an increase or decrease in fluorescence relative to the negative control (disk with solvent only) that is not observed in the constitutive expression control (carrying the pSGV53 vector). Specifically, 8-hydroxyquinoline was identified as a potential inducer (Figure 7A), and D,L-thioctic acid as a potential repressor (Figure 7B). 8-hydroxyquinoline is a quinoline-family antibiotic with metal-chelating properties [31] and has been reported to exhibit potential mutagenic effects [32]. D,L-thioctic acid, also known as α-lipoic acid, also exerts metal-chelating capacity and is primarily recognized for its antioxidant properties, acting as a free radical scavenger and contributing to the repair of oxidative damage [33,34].
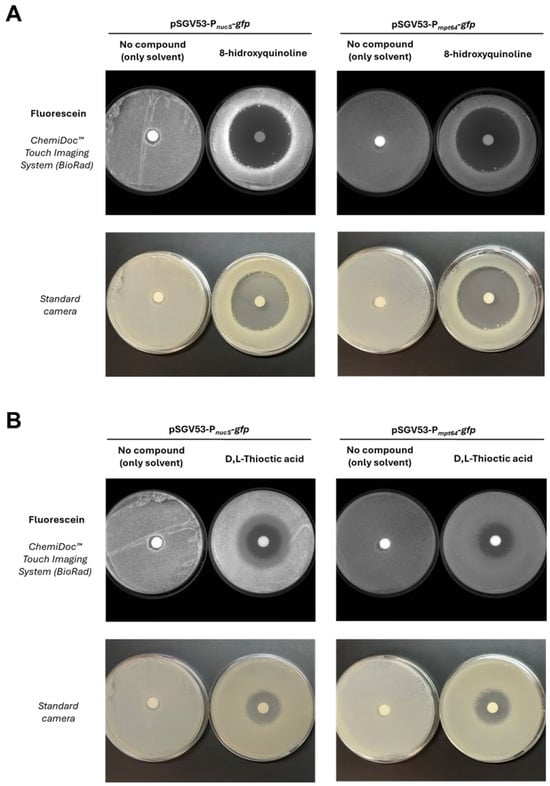
Figure 7.
Effect of candidate compounds on nucS expression assessed by disk diffusion assay. (A). Results with 8-hydroxyquinoline, identified as a potential inducer of nucS expression. (B). Results with D,L-thioctic acid, identified as a potential repressor. The upper panels (fluorescence images) show the effect of each compound on the expression of the nucS::gfp transcriptional fusion in M. smegmatis. Induction is observed as increased fluorescence around the inhibition halo, while repression appears as reduced fluorescence in the same region. Disks containing no compound were used as negative controls. A control strain expressing gfp under a constitutive promoter (Pmpt64) was included (right side of each panel) to rule out nonspecific effects. The lower panels display the corresponding photographs of the plates, showing cell mass distribution.
In contrast, most of the compounds tested did not cause fluorescence changes in the reporter strain (Figure S6A), whereas some produced fluorescence changes around the inhibition halo in both the reporter and control strains, indicating nonspecific effects on protein expression unrelated to nucS regulation (Figure S6B).
3. Discussion
Variations in the expression of the nucS gene may influence the bacterial mutation rate, thereby impacting the acquisition of antibiotic resistance in mycobacterial pathogens, including M. tuberculosis. Despite its importance, the expression and regulation of this gene had not been thoroughly studied.
In this study, we identified the transcription start site (TSS) for the nucS gene in M. smegmatis, which enabled us to identify several candidate −10 regions for sigma factor binding. These included sequences recognized by the primary sigma factor σA and the alternative stationary phase sigma factor σB [23]. To monitor nucS expression, we constructed three reporter strains carrying nucS::gfp transcriptional fusions with different lengths of upstream regions. Our findings revealed that the minimal 73 bp upstream fragment was sufficient to drive basal nucS expression, suggesting that the core promoter is situated near the TSS. However, the higher fluorescence observed with the 408 bp fusion implies that additional regulatory elements between 74 and 408 bp may enhance expression. In contrast, the absence of detectable fluorescence from the fusion lacking the immediate upstream intergenic region (46 bp) underscores the critical role of this segment as the key promoter element. The use of these reporter fusions is an important first step in decoding the regulatory architecture of the nucS promoter and exploring regulatory mechanisms governing nucS expression. Further studies will be essential to address their specific roles in controlling nucS expression.
Our analyses further show that nucS expression in M. smegmatis is strongly dependent on the growth phase. We observed the highest promoter activity during exponential growth, with a marked reduction in the stationary phase. This finding was corroborated by RT-qPCR and Western blot analyses, all of which suggested that nucS transcript and protein level correlate with the physiological state of the cell. During exponential growth, when the rates of cell division and DNA replication are high [35], elevated NucS levels may be required to correct replication errors. Conversely, the reduced replication rate in the stationary phase aligns with lower nucS expression. These observations are consistent with previous global transcriptomic studies in both M. smegmatis and M. tuberculosis [36,37,38], as well as with the known downregulation of canonical MMR components during periods of reduced cellular replication [2,3]. Altogether, the correlation between NucS levels, growth phase, and replication rates aligns with the behavior expected for an MMR protein that is functionally coupled to DNA replication.
Drawing a parallel with E. coli, where the canonical MMR genes mutS and mutH system are downregulated during the stationary phase via rpoS and hfq [2,4,5], we explored whether the mycobacterial functional analog σB factor (encoded by sigB) [17,18,19,20], might similarly influence nucS expression. In mycobacteria, no hfq homolog has been identified, although the existence of a functional homolog or a direct role by regulatory sRNAs cannot be excluded [39]. In a ΔsigB mutant of M. smegmatis, we observed an approximately twofold increase in nucS transcription during stationary phase. Additionally, the ΔsigB strain exhibited a roughly two-fold lower mutation frequency than the wild type during the same phase, suggesting that an increase of two-fold in nucS transcription is enough to decrease mutation rate at the same level. These results support the idea that σB may modulate mutation rates, at least in part, by influencing nucS transcription. Whether σB exerts its effect directly—via binding competition with σA—or indirectly through σB-dependent effectors remains to be determined. It is possible that the downregulation of nucS in the stationary phase involves an intermediate regulator whose transcription is promoted by σB, similar to the negative regulation of mutS by rpoS in E. coli during the stationary phase [2]. Moreover, the low conservation of mycobacterial promoter sequences, especially in the −35 region, could facilitate the exchange of sigma factors in response to different stress conditions [22,23,40]. Given the complexity of the mycobacterial transcriptional machinery, with multiple sigma factors (28 in M. smegmatis and 13 in M. tuberculosis) [18,23,41], further experiments are warranted to elucidate these mechanisms at genetic and biochemical level.
Beyond growth phase regulation, our data, along with literature and database analysis [36,37,42,43,44], indicate that nucS expression is likely modulated by additional endogenous and exogenous factors. For instance, an antisense ncRNA targeting nucS has been reported in M. tuberculosis under nitric oxide exposure [16], suggesting a layer of regulation. Similarly, canonical MMR components such as mutS have been shown to respond to DNA-damaging agents like mitomycin C [45] and to subinhibitory concentrations of β-lactam antibiotics, which downregulate mutS expression in E. coli via the induction of the rpoS regulon under diverse stress conditions [6]. These conditions include nutrient limitation, high osmolarity, extreme temperatures, and low pH [17,46].
To further identify potential regulators of nucS expression, we screened 240 compounds from the Biolog™ bacterial chemical sensitivity plates, using a reporter strain harboring the vector pSGV-PnucS-408-gfp. From this screen, 22 candidate compounds were identified. A subsequent disk diffusion assay highlighted only two compounds with significant effect on nucS expression: the quinoline-family antimicrobial 8-hydroxyquinoline, identified as a potential inducer, and the potent antioxidant D,L-thioctic acid (α-lipoic acid), which may act as a repressor. 8-hydroxyquinoline is of both natural (plant-derived) and synthetic origin and has been used as a fungicide in agriculture. D,L-thioctic acid, in contrast, is endogenously produced by plants, animals and humans. Both compounds have multiple potential medical applications [31,33,34]. The precise mechanisms by which these compounds influence nucS regulation require further clarification. However, these findings support the hypothesis that nucS expression may be modulated by both environmental and/or host-derived factors. Notably, the co-administration of different molecules, as well as patient-specific physiological conditions, may influence bacterial mutation rate and should be considered when designing effective antimicrobial therapies. Beyond the Biolog™ compounds tested here, future studies could explore the effects of additional molecules on nucS expression, including DNA-damaging agents, mutagens, antimicrobials, and oxidative stress inducers, using the reporter fusions developed in this study.
In conclusion, our work provides foundational insights into the regulation of nucS expression in Mycobacteria, demonstrating its growth-phase dependency and initial responsiveness to potential chemical modulators. These findings underscore the role of NucS as an integral component of the mismatch repair system and suggest that its dynamic regulation may be critical for modulating mutation rates, with possible implications for antibiotic resistance. Additionally, the existence of two distinct pathways at the molecular and evolutionary levels—the nucS-mediated non-canonical and the MutS/MutL-mediated canonical MMR systems—which perform almost identical functions, is the result of convergent evolution. Moreover, the fact that these two systems are also regulated in a similar manner provides an example of double homoplasy, both in activity and regulation, across two genetic traits. Future studies should focus on delineating the detailed transcriptional and post-transcriptional mechanisms controlling nucS expression, as well as exploring the impact of these regulatory pathways in pathogenic mycobacteria such as M. tuberculosis.
4. Materials and Methods
4.1. Bacterial Strains
In this study, we used the M. smegmatis mc2 155 (American Type Culture Collection, Manassas, VI, USA, 700084) wild-type (WT) strain, its noncanonical MMR-deficient (ΔnucS) derivative [10] and the ΔsigB mutant (this study). M. tuberculosis H37Rv was also used in some experiments. E. coli DH5α strain was used to obtain recombinant plasmids.
4.2. Culture Media and Growth Conditions
For liquid cultures of M. smegmatis, Middlebrook 7H9 broth (Difco, Franklin Lakes, NJ, USA) supplemented with 0.5% glycerol and 0.5% Tween 80 was used. All cultures were grown in Erlenmeyer flasks with a 1:5 medium-to-flask volume ratio and incubated at 37 °C with orbital shaking (250 rpm). For solid cultures of M. smegmatis, Middlebrook 7H10 agar (Difco) supplemented with 0.5% glycerol and 0.05% Tween 80 was used.
Liquid cultures of M. tuberculosis were grown in 7H9 broth supplemented with 0.5% glycerol, 0.05% Tween 80, 10% OADC (Oleic Albumin Dextrose Catalase), and incubated in roller bottles at 37 °C. The 7H10 agar plates were further supplemented with 5 µg/mL amphotericin.
E. coli was cultured in LB broth or LB agar at 37 °C.
4.3. Extraction of Total RNA from M. smegmatis
A volume of 1–2 mL was collected from various M. smegmatis cultures under the appropriate conditions for each experiment. After centrifugation (5 min, 12,000× g), the supernatant was discarded, and the pellets were stored at −80 °C for further processing. Initially, cells were washed with 300 μL of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8) and then resuspended in 300–400 μL of lysis buffer. The suspensions were transferred to 2 mL BeadBug™ (Benchmark Scientific, Inc., Sayreville, NJ, USA) tubes containing 0.1 mm zirconium beads. Cell lysis was achieved using two cycles of 1 min at maximum speed, with 2 min on ice between cycles, in a BeadBug™ microtube homogenizer. After cell lysis, total RNA was extracted using the RNeasy Mini Kit, Part 1 (QIAGEN, Hilden, Germany), following the manufacturer’s instructions. Residual genomic DNA was removed from the RNA samples using DNase treatment with the Turbo DNA-Free Kit (Invitrogen, Carlsbad, CA, USA). RNA concentration was measured with a NanoDrop® (Thermo Fisher Scientific, Waltham, MA, USA), and sample integrity was confirmed by electrophoresis on a 1.2% agarose gel (100 V, 20–30 min).
4.4. Extraction of Total RNA from M. tuberculosis
40-mL cultures were collected at late exponential (OD600 = 1) and stationary (OD600 = 5) phases. Cell pellets were resuspended in 6M guanidine chloride for inactivation and stored at −80 °C for a minimum of 7 days. Subsequently, cells were harvested and resuspended in 1 mL of TRIzol™ reagent (Invitrogen), then transferred to 2 mL BeadBug™ tubes containing 0.1 mm zirconium beads. Mechanical cell disruption was performed using a BeadBug™ microtube homogenizer with three cycles of 1 min at 400 rpm, with 2 min intervals on ice between cycles. RNA extraction was carried out following the protocol of Rustad et al. [47], with minor modifications. Genomic DNA was removed using the DNase I recombinant, RNase-free (Merck, Darmstadt, Germany), followed by phenol/chloroform extraction to eliminate DNase, and RNA was subsequently precipitated with ethanol. RNA concentration and sample integrity were assessed as described for M. smegmatis.
4.5. 5′-RACE
The transcription start site (TSS) was determined using the 5′-RACE technique, following the protocol by Scotto-Lavino and colleagues [48]. Total RNA from M. smegmatis RNA was extracted as previously described using the RNeasy Mini Kit, Part 1 (QIAGEN). Reverse transcription was performed using a reverse primer that hybridizes to the internal region of nucS (GSP-RT) (Table S4). RNAse H (New England Biolabs, NEB, Ipswich, MA, USA) was then added to destroy the RNA template, and the resulting cDNA was purified and polyadenylated at the 3′ end using terminal deoxynucleotidyl transferase (Tdt, NEB) and dATP.
Next, the first round of cDNA amplification was performed by PCR using QT, QO, and GSP1 primers (Table S4). The PCR product was diluted 1:20 and subjected to a second round of amplification to increase the yield of the specific product using the nested primers QI and GSP2 (Table S4). PCR product was visualized by electrophoresis on a 1% agarose gel. The resulting band was purified using the AccuPrep® Gel Purification Kit (BIONEER, Daejeon, Republic of Korea) and sequenced to determine the TSS.
4.6. Transformation of M. smegmatis
Transformation of M. smegmatis was performed by electroporation, following the protocol of Goude and Parish [49]. A total of 200 ng (for replicative or integrative plasmid) to 5 µg (for homologous recombination) of DNA (in a volume not exceeding 5 µL), previously dialyzed using MF-Millipore® MCE Membrane Filter (0.025 µm) (Merck Millipore, Darmstadt, Germany), was added to a 200 µL aliquot of electrocompetent cells. The cell-DNA mixture was transferred to pre-chilled 0.2 cm electroporation cuvettes (BioRad, Hercules, CA, USA) and given a single electric pulse (2.5 kV, 25 µF, 1000 Ω) using a Gene Pulser Xcell™ electroporator (BioRad). After the pulse, the cells were incubated on ice for 10 min, then transferred to a flask containing 5 mL of antibiotic-free 7H9 medium and incubated at 37 °C for 3 h. Finally, appropriate volumes (0.1–5 mL) were plated on 7H10 agar plates supplemented with the selection antibiotic and incubated at 37 °C for 3 to 5 days until colonies appeared.
4.7. Construction of GFP Reporter Plasmids
Upstream regions of different lengths from the nucS gene were amplified by PCR from M. smegmatis genomic DNA using the following primer pairs: 73up_nucS_NotI_F/nucS_up_NdeI_R, 408up_nucS_NotI_F/nucS_up_NdeI_R and 408up_nucS_NotI_F/msmeg4924startR (Table S4). The amplified fragments corresponded to (i) 73 bp upstream of nucS (“short region”), (ii) 408 bp upstream of nucS (“long region”), and (iii) a 408 bp region upstream of nucS excluding the first 46 bp (which correspond to the nucS upstream intergenic region). These fragments were cloned into the replicative plasmid pSGV53 using the restriction enzymes NotI-HF (NEB) and NdeI (NEB) to generate the vectors pSGV53-PnucS-73-gfp, pSGV53-PnucS-408-gfp and pSGV53-PnucS-408∆46-gfp (Table S1). The correct sequences were verified by sequencing using the primers ble_fw_1 and gfp_rev_seq_2 (Table S4).
4.8. Spectrofluorometric Assays for Monitoring Fluorescence During Growth
Fluorescence and OD595 measurements of M. smegmatis strains carrying GFP reporter plasmids were measured during growth using an Infinite® 200 spectrofluorometer (TECAN, Männedorf, Switzerland). Black, clear-bottom 96-well plates (Corning™, Corning, New York, USA) were used, inoculating 8 wells (replicates) with each strain, with an initial OD595 of 0.05–0.1 (measured in the spectrofluorometer) in a final volume of 200 μL of 7H9 medium per well. The i-control™ software version 1.6 (TECAN) was used to take measurements every 20 min over a 48-h growth period (145 cycles) at 37 °C. The program for each cycle was as follows: (i) orbital shaking for 10 s (5 mm amplitude), (ii) a waiting time of 15 s, (iii) absorbance measurement at 595 nm (25 flashes), (iv) fluorescence intensity (FI) measurement: excitation wavelength 485 nm, emission wavelength 530 nm, 25 flashes, Bottom mode, integration time 20 μs, manual gain of 100. Relative fluorescence (FI/OD595) was plotted by dividing the fluorescence intensity by the OD595 at each point.
Promoter activity (PA) was calculated following the method described by Camas et al. [50]. Briefly, relative fluorescence (FI/OD595) data were fitted to a sixth-order polynomial, and the derivative of the resulting curves was obtained. PA values were then normalized to their respective maximum.
4.9. Measurement of nucS Expression by RT-qPCR
For M. smegmatis, samples of 1–2 mL were taken from three independent cultures (biological replicates) of each strain under the specified conditions and time points for each case. Total RNA was extracted as previously described. The reverse transcription (RT) reaction was performed using 250 ng of RNA per sample in a final volume of 20 μL with the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR (qPCR) was carried out using Power SYBR® Green PCR Master Mix (Applied Biosystems) on a 7500 Real-Time PCR System (Applied Biosystems). Reactions were prepared in MicroAmp Optical 96-Well Reaction Plates (Applied Biosystems), with each containing 20 μL of Master Mix (10 μL of Power SYBR® Green, 9.2 μL of water, and 0.4 μL of each primer at 25 μM) and 5 μL of cDNA (5 ng/μL). Three biological and three technical replicates were used. The thermocycler conditions were: (i) 2 min at 50 °C, (ii) 10 min at 95 °C, (iii) 40 cycles of 15 s at 95 °C, followed by 1 min at 66 °C. Results were analyzed using the 2−ΔΔCT method (121), with the sigA housekeeping gene used for normalization.
For M. tuberculosis, total RNA was extracted from three independent cultures at exponential (OD600 = 1) or stationary phase (OD600 = 5), as previously described. Reverse transcription was performed using 5 μg of RNA in a final volume of 80 μL with the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems). qPCR reactions included 5 μL of a 1:25 cDNA dilution (2.5 ng/μL) and 20 μL of Master Mix (12.5 μL of Power SYBR® Green, 7.1 μL of nuclease-free water, and 0.2 μL of each primer at 10 μM). Three biological and three technical replicates were used per condition. Reactions were run on a 7500 Real-Time PCR System (Applied Biosystems) under the following conditions: (i) 2 min at 50 °C, (ii) 10 min at 95 °C, (iii) 40 cycles of 15 s at 95 °C, followed by 1 min at 60 °C. Relative expression was calculated using the 2−ΔΔCT method (121), with lpqM gene used as the reference gene, as it has been shown to maintain constant expression levels across many different conditions in M. tuberculosis [51].
The sequences of the primers used in the qPCR reactions are listed in Table S4.
4.10. Generation of a Polyclonal Mouse Anti-NucS Antibody
For antigen production, the first 294 bp of the nucS gene from M. smegmatis were cloned into the pET-24a(+) vector (Novagen, Merck, Darmstadt, Germany) using the restriction enzymes NdeI and XhoI. The resulting recombinant 12 kDa N-terminal fragment of NucS was expressed in E. coli BL21(DE3) and purified by affinity chromatography using TALON® Metal Affinity Resin (TaKaRa, Kusatsu, Japan). The purified protein fragment was subsequently coupled to KLH following standard procedures. A polyclonal mouse antibody against NucS from M. smegmatis was generated by immunizing female BALB/c mice (n = 5; 6–8 weeks-old at the beginning of the study) with three subcutaneous doses (25 µg/dose) of the KLH-conjugated antigen. On day 14 after the third antibody boost, blood was collected from each mouse by submandibular bleeding to obtain serum samples that were stored at −20 °C until analysis of humoral immune responses. A pool of anti-NucS antibody was generated by mixing equal amounts of serum from mice selected for their high antibody titer.
This study involved only one cage with five mice. The mice were purchased from Harlan Laboratories and housed under specific pathogen-free conditions in the CNB-CSIC animal facility. They had not undergone any prior experimental procedures. The mice were acclimated to the facility for one week prior to immunization, and their health and general well-being were monitored at least twice a week. Only one polyclonal serum production experiment was conducted. Normal mouse serum and sera from mice previously immunized with unrelated antigens were used as negative controls.
No criteria were established to include or exclude animals during the experiment. The sample size, number of mice in each experimental group used for the production of polyclonal antibodies, was calculated based on the specific objectives of the research, the nature of the antigen, the volume of serum required, and the laboratory’s previous experience. Randomization was not used to assign experimental units to control and treatment groups. To minimize potential confounding, each animal was ear-marked. These data, along with the cage location, were recorded in the facility’s computer system. Research group staff and facility personnel had access to the experimental data.
4.11. Generation of a Polyclonal Anti-FtsZ Antibody
For antigen production, the ftsZ gene from M. tuberculosis was cloned into plasmid pET-28a(+) (Novagen) using the restriction enzymes NcoI and XhoI. Polyclonal antibodies against mycobacterial FtsZ, capable of detecting FtsZ from both M. tuberculosis and M. smegmatis, were generated in rabbits by Charles River Laboratories (Wilmington, MA, USA) using their standard immunization protocol.
4.12. Analysis of NucS Protein Levels in M. smegmatis by Western Blot
Samples of 1.5–12 mL were taken from three independent cultures of M. smegmatis at the indicated time points and centrifugated to pellet the cells, which were stored at −80 °C until further processing. For cell lysis, pellets were resuspended in ~300 μL of PBS supplemented with cOmplete™ protease inhibitor cocktail (Roche) (25X) and sonicated (minimum of 4 cycles of 20 pulses at 0.7 s). Protein concentration was measured using the Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific).
Proteins were separated by SDS-PAGE using the Mini-PROTEAN III vertical electrophoresis system (BioRad) on 12% resolving gels and 4% stacking gels. A total of 20 μg of protein per sample was mixed with loading buffer (4X) and 0.35 M DTT, heated at 100 °C for 10 min, and separated by electrophoresis in Tris-Glycine-SDS running buffer (25 mM Tris, 190 mM glycine, 0.1% SDS) at 200 V for 5 min, then at 130 V for about 1 h and 20 min. Transfer to an Immobilon-P PVDF membrane (0.45 μm) (Merck Millipore) was performed using the Trans-Blot® SD Semi-Dry Transfer Cell (BioRad) for 70 min at 15 V.
The membrane was blocked by incubation with gentle agitation in PBS—0.05% Tween 20 for 30 min, followed by PBS—0.05% Tween 20—1% BSA for 1 h, and finally in PBS—0.05% Tween 20—1% BSA—5% milk powder for 30 min. The membrane was then incubated overnight at 4 °C with the polyclonal mouse anti-NucS antibody (1/8000). After washing in PBS—0.05% Tween 20, the membrane was incubated for 1 h with goat anti-mouse (IgG-h + I, HRP conjugated) secondary antibody (1:2500, Bethyl Laboratories, Montgomery, TX, USA) at room temperature. After washing with PBS—0.05% Tween 20 and water, signal detection was performed using ECL Western detection reagents (GE Healthcare, Chicago, IL, USA) and imaged using a ChemiDoc™ Touch Imaging System (BioRad).
For FtsZ detection (loading control), the membrane was re-incubated with PBS—0.02% sodium azide to inhibit the HRP peroxidase of the previous secondary antibody and blocked again with PBS—0.05% Tween 20—1% BSA—5% milk powder. Rabbit anti-FtsZ antibody (1:1500) was incubated overnight at 4 °C, followed by detection with HRP-Protein A (1:10,000, Invitrogen) for 40 min at room temperature. Band intensity was quantified using ImageJ software (version 1.53k, Bio-Formats plugin), and NucS signal was normalized to FtsZ.
4.13. Growth Curve (OD600 and CFUs/mL) of M. smegmatis
Three independent M. smegmatis cultures were inoculated at an initial OD600 of 0.05 in 500 mL flasks containing 100 mL of medium and incubated at 37 °C with shaking at 250 rpm. OD600 measurements were taken every 3 h (approximately the generation time of M. smegmatis) over a period of 36 h using a spectrophotometer, and samples were collected for CFU/mL counts. Appropriate dilutions from each culture were plated in duplicate on 7H10 solid medium to obtain colony counts between 30 and 200 (from 200 × 10−4 μL at t = 0 to 500 × 10−6 μL at t = 36 h). Plates were incubated at 37 °C for 3–4 days before counting the colonies.
4.14. Construction of the M. smegmatis ΔsigB Mutant
The mutant was constructed following the method of Parish and Stoker [52]. Two ~ 1 kb fragments flanking the sigB gene, both upstream (sigB5′) and downstream (sigB3′) of the gene, were amplified by PCR from M. smegmatis genomic DNA using the primers sigB5_PstIF/sigB5_HindIIIR and sigB3_HindIIIF/sigB3_BamHIR (Table S4). Both fragments were sequentially cloned into the p2NIL plasmid [52] (Table S1) using the appropriate restriction enzymes. The final suicide plasmid was generated by inserting the pGOAL19 marker cassette into the unique PacI site of the p2NIL plasmid containing the sigB flanking fragments.
M. smegmatis was transformed by electroporation with 5 μL of the suicide plasmid (500–1000 ng/μL). Merodiploid selection (single recombination event) was performed on 7H10 medium with kanamycin (25 μg/mL) and X-gal (100 μg/mL). Blue colonies resistant to hygromycin (50 μg/mL) were cultured on 7H10 without antibiotics to promote the second recombination event. After one day of growth, the cultures were transferred to liquid 7H9 medium without antibiotics for another 24 h. Serial dilutions (10−1, 10−2, and 10−3) were plated on 7H10 with X-gal (100 μg/mL) and 10% sucrose. White colonies (with both WT and ΔsigB genotypes) were tested for kanamycin (25 μg/mL) and hygromycin (50 μg/mL) sensitivity. PCR verification of the sigB deletion was performed using the primers sigB5_end_F/sigB3_start_R and sigB.intF/sigB.intR (Table S4, Figure S6).
4.15. Estimation of Mutant Frequency in Different Growth Phases of M. smegmatis Wild-Type and ΔsigB Strains
Eight independent cultures per strain were inoculated and incubated overnight. A 1:10,000 dilution was performed to avoid the selection of pre-existing rifampicin-resistant mutants. Viable and mutant counts were conducted during the exponential phase (29–32 h of incubation, OD600 = 0.6–0.8) and the stationary phase (52–54 h, OD600 = 4–6).
In the exponential phase, 300 μL of a 10−5 dilution of all cultures were plated on solid 7H10 medium without antibiotics for viable counts. For mutant counts, 30 mL of culture were plated on 7H10 medium with rifampicin (100 μg/mL).
In the stationary phase, 300 μL of a 10−6 dilution were plated on 7H10 without antibiotics for viable counts. For mutant counts, 10 mL of culture were plated on 7H10 medium with rifampicin (100 μg/mL). Plates without antibiotics were incubated for 4 days, and rifampicin plates for 7 days, after which colonies were counted. Mutant frequency per ml for each strain was determined by dividing the number of mutants/mL by the number of viable cells/mL for each culture, and the median was calculated across all cultures.
4.16. Screening for Compounds Regulating nucS Expression Using Biolog™ Phenotype MicroArrays
Biolog™ PM11-PM20 plates were used for the compound screening (Table S2). To prepare the plates, the lyophilized compounds at the bottom of each well were dissolved in 80 μL of liquid 7H9 medium, followed by gentle shaking for 2–3 h. The plates were then inoculated with the wild-type M. smegmatis strain harboring the plasmid pSGV53-PnucS-408-gfp at an initial OD595 of approximately 0.1 (measured using an Infinite® 200 spectrofluorometer [TECAN]) in a final volume of 100 μL of medium per well.
The plates were incubated at 37 °C with periodic shaking for 72 h in an Infinite® 200 spectrofluorometer (TECAN), with measurements of OD595 and fluorescence intensity (FI, excitation/emission: 485/530 nm) taken every 10 min (433 cycles). Relative fluorescence was calculated as the ratio of fluorescence intensity to OD595 over the entire growth period.
To establish fluorescence thresholds for identifying compounds that could induce or repress nucS expression, a control assay was performed using the same M. smegmatis reporter strain grown under identical conditions in a plate without compounds. The thresholds served as benchmarks to identify wells with fluorescence values indicative of potential overexpression or repression of nucS.
4.17. Effect of Candidate Compounds in nucS Expression Using Disk Diffusion Assays
The effect of candidate compounds from the Biolog™ PM screening on nucS expression was qualitatively evaluated using the M. smegmatis strain carrying the reporter plasmid pSGV53-PnucS-408-gfp. To rule out nonspecific effects on protein expression, a control strain harboring the pSGV53 vector (with the gfp gene under the constitutive promoter Pmpt64, referred to as pSGV53-Pmpt64-gfp in Figure 7 and Figure S6) was included.
Overnight cultures were adjusted to the same OD600 and diluted 1:10 before being plated on 7H10 agar using the flood inoculation method. Filter disks (Whatman® Antibiotic Assay Discs diam. 9 mm, Merck) were placed on the agar surface and spotted with 30 μL of compounds stock solutions, prepared with the appropriate solvent and concentration (Table S3). Disks containing only the corresponding solvent were used as negative controls. After incubation at 37 °C for 72 h, fluorescence was visualized using a ChemiDoc™ Touch Imaging System (BioRad) under fluorescein settings (Blot > Fluorescein). Photographs of the plates were also taken to provide a visual reference for cell mass distribution. For compounds showing a specific effect on nucS expression through this assay, the experiment was performed twice to confirm reproducibility.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics14111065/s1, Table S1: Vectors used in this study; Table S2: Compounds tested from the Biolog™ Phenotype MicroArrays™; Table S3: Compounds tested in the disk diffusion assay; Table S4: Primers used in this study; Figure S1: Growth curves of the reporter strains in the microplate reader; Figure S2: Growth curve of M. tuberculosis H37Rv; Figure S3: Full membrane images from a representative Western blot experiment; Figure S4: Growth curves of WT and ∆sigB strains of M. smegmatis mc2 155; Figure S5: Selection of thresholds for filtering candidate compounds regulating nucS expression; Figure S6: Example of results from disk diffusion assays for discarded compounds.
Author Contributions
Conceptualization, E.C.-S., A.C.-G. and J.B.; methodology, E.C.-S., Á.R.-E., A.C.-G., S.G., L.K. and J.B.; validation, E.C.-S., A.C.-G., L.K. and J.B.; formal analysis, E.C.-S.; investigation, E.C.-S., Á.R.-E., A.C.-G., P.G.-B. and J.B.; resources, S.G., L.K. and J.B.; data curation, E.C.-S.; writing—original draft preparation, E.C.-S. and J.B.; writing—review and editing, E.C.-S., Á.R.-E., A.C.-G., S.G., L.K. and J.B.; visualization, E.C.-S. and J.B.; supervision, A.C.-G. and J.B.; project administration, J.B.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants PCI2019-103488/AEI/10.13039/501100011033 and PID2020-112865RBI00/AEI/10.13039/501100011033 from the State Research Agency (AEI), and FIS PI17/00159 from the Carlos III Institute of Health (ISCIII) and the European Regional Development Fund (FEDER, EU). E.C.-S. is the recipient of a PFIS predoctoral fellowship (FI18/00036), co-funded by the ISCIII and the European Social Fund. Á.R.-E. is the recipient of an FPU fellowship from the Ministry of Science, Innovation and Universities (FPU2020/01676).
Institutional Review Board Statement
The mouse studies were approved by the Ethical Committee of Animal Experimentation (CEEA) of the CNB-CSIC, the CSIC Ethics Committee and the Division of Animal Protection of the Comunidad de Madrid (PROEX 184.0_21, 1 May 2021). Animal procedures conformed with international guidelines and with Spanish law under the Royal Decree (RD) 53/2013. The mouse studies were conducted in accordance with the local legislation and institutional requirements.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank José R. Valverde and A. Couce for statistical and evolutionary advice, respectively, Renata Moreno for her helpful input on some of the experiments and the staff of the CNB Protein Tools Unit for their help with mouse immunizations and immune response analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Foster, P.L. Stress-Induced Mutagenesis in Bacteria. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 373–397. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.C.; Feng, G.; Winkler, M.E. Negative Regulation of mutS and mutH Repair Gene Expression by the Hfq and RpoS Global Regulators of Escherichia coli K-12. J. Bacteriol. 1997, 179, 7476–7487. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Tsui, H.C.; Winkler, M.E. Depletion of the Cellular Amounts of the MutS and MutH Methyl-Directed Mismatch Repair Proteins in Stationary-Phase Escherichia coli K-12 Cells. J. Bacteriol. 1996, 178, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.S.; Feng, G.; Ross, K.J.; Sidhu, R.; Thulin, C.; Longerich, S.; Szigety, S.K.; Winkler, M.E.; Rosenberg, S.M. Mismatch Repair Protein MutL Becomes Limiting during Stationary-Phase Mutation. Genes. Dev. 1997, 11, 2426–2437. [Google Scholar] [CrossRef]
- Harris, R.S.; Feng, G.; Ross, K.J.; Sidhu, R.; Thulin, C.; Longerich, S.; Szigety, S.K.; Hastings, P.J.; Winkler, M.E.; Rosenberg, S.M. Mismatch Repair Is Diminished during Stationary-Phase Mutation. Mutat. Res. 1999, 437, 51–60. [Google Scholar]
- Gutierrez, A.; Laureti, L.; Crussard, S.; Abida, H.; Rodríguez-Rojas, A.; Blázquez, J.; Baharoglu, Z.; Mazel, D.; Darfeuille, F.; Vogel, J.; et al. β-Lactam Antibiotics Promote Bacterial Mutagenesis via an RpoS-Mediated Reduction in Replication Fidelity. Nat. Commun. 2013, 4, 1610. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, W.; Umar, M.I.; Wong, H.Y.; Seng, Z.; Xie, Y.; Zhang, Y.; Yang, L.; Kwok, C.K.; Deng, X. RNA G-Quadruplex Structures Mediate Gene Regulation in Bacteria. mBio 2020, 11. [Google Scholar] [CrossRef]
- Mizrahi, V.; Andersen, S.J. DNA Repair in Mycobacterium tuberculosis. What Have We Learnt from the Genome Sequence? Mol. Microbiol. 1998, 29, 1331–1339. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the Biology of Mycobacterium tuberculosis from the Complete Genome Sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Castañeda-García, A.; Prieto, A.I.; Rodríguez-Beltrán, J.; Alonso, N.; Cantillon, D.; Costas, C.; Pérez-Lago, L.; Zegeye, E.D.; Herranz, M.; Plociński, P.; et al. A Non-Canonical Mismatch Repair Pathway in Prokaryotes. Nat. Commun. 2017, 8, 14246. [Google Scholar] [CrossRef]
- Cebrián-Sastre, E.; Martín-Blecua, I.; Gullón, S.; Blázquez, J.; Castañeda-García, A. Control of Genome Stability by EndoMS/NucS-Mediated Non-Canonical Mismatch Repair. Cells 2021, 10, 1314. [Google Scholar] [CrossRef]
- Castañeda-García, A.; Martín-Blecua, I.; Cebrián-Sastre, E.; Chiner-Oms, A.; Torres-Puente, M.; Comas, I.; Blázquez, J. Specificity and Mutagenesis Bias of the Mycobacterial Alternative Mismatch Repair Analyzed by Mutation Accumulation Studies. Sci. Adv. 2020, 6, eaay4453. [Google Scholar] [CrossRef]
- Takemoto, N.; Numata, I.; Su’etsugu, M.; Miyoshi-Akiyama, T. Bacterial EndoMS/NucS Acts as a Clamp-Mediated Mismatch Endonuclease to Prevent Asymmetric Accumulation of Replication Errors. Nucleic Acids Res. 2018, 46, 6152–6165. [Google Scholar] [CrossRef] [PubMed]
- Ishino, S.; Skouloubris, S.; Kudo, H.; l’Hermitte-Stead, C.; Es-Sadik, A.; Lambry, J.-C.; Ishino, Y.; Myllykallio, H. Activation of the Mismatch-Specific Endonuclease EndoMS/NucS by the Replication Clamp Is Required for High Fidelity DNA Replication. Nucleic Acids Res. 2018, 46, 6206–6217. [Google Scholar] [CrossRef] [PubMed]
- Fressatti Cardoso, R.; Martín-Blecua, I.; Pietrowski Baldin, V.; Meneguello, J.E.; Valverde, J.R.; Blázquez, J.; Castañeda-García, A. Noncanonical Mismatch Repair Protein NucS Modulates the Emergence of Antibiotic Resistance in Mycobacterium abscessus. Microbiol. Spectr. 2022, 10, e0222822. [Google Scholar] [CrossRef] [PubMed]
- Namouchi, A.; Gómez-Muñoz, M.; Frye, S.A.; Moen, L.V.; Rognes, T.; Tønjum, T.; Balasingham, S.V. The Mycobacterium tuberculosis Transcriptional Landscape under Genotoxic Stress. BMC Genom. 2016, 17, 791. [Google Scholar] [CrossRef]
- Battesti, A.; Majdalani, N.; Gottesman, S. The RpoS-Mediated General Stress Response in Escherichia coli. Annu. Rev. Microbiol. 2011, 65, 189–213. [Google Scholar] [CrossRef]
- Sachdeva, P.; Misra, R.; Tyagi, A.K.; Singh, Y. The Sigma Factors of Mycobacterium tuberculosis: Regulation of the Regulators. FEBS J. 2010, 277, 605–626. [Google Scholar] [CrossRef]
- Manganelli, R.; Dubnau, E.; Tyagi, S.; Kramer, F.R.; Smith, I. Differential Expression of 10 Sigma Factor Genes in Mycobacterium tuberculosis. Mol. Microbiol. 1999, 31, 715–724. [Google Scholar] [CrossRef]
- Hu, Y.; Coates, A.R.M. Transcription of Two Sigma 70 Homologue Genes, sigA and sigB, in Stationary-Phase Mycobacterium tuberculosis. J. Bacteriol. 1999, 181, 469–476. [Google Scholar] [CrossRef]
- Martini, M.C.; Zhou, Y.; Sun, H.; Shell, S.S. Defining the Transcriptional and Post-Transcriptional Landscapes of Mycobacterium smegmatis in Aerobic Growth and Hypoxia. Front. Microbiol. 2019, 10, 591. [Google Scholar] [CrossRef]
- Cortes, T.; Schubert, O.T.; Rose, G.; Arnvig, K.B.; Comas, I.; Aebersold, R.; Young, D.B. Genome-Wide Mapping of Transcriptional Start Sites Defines an Extensive Leaderless Transcriptome in Mycobacterium tuberculosis. Cell Rep. 2013, 5, 1121–1131. [Google Scholar] [CrossRef]
- Newton-Foot, M.; Gey van Pittius, N.C. The Complex Architecture of Mycobacterial Promoters. Tuberculosis 2013, 93, 60–74. [Google Scholar] [CrossRef]
- Ranes, M.G.; Rauzier, J.; Lagranderie, M.; Gheorghiu, M.; Gicquel, B. Functional Analysis of pAL5000, a Plasmid from Mycobacterium fortuitum: Construction of a “Mini” Mycobacterium-Escherichia coli Shuttle Vector. J. Bacteriol. 1990, 172, 2793–2797. [Google Scholar] [CrossRef] [PubMed]
- Bourn, W.R.; Jansen, Y.; Stutz, H.; Warren, R.M.; Williamson, A.-L.; van Helden, P.D. Creation and Characterisation of a High-Copy-Number Version of the pAL5000 Mycobacterial Replicon. Tuberculosis 2007, 87, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Gola, S.; Munder, T.; Casonato, S.; Manganelli, R.; Vicente, M. The Essential Role of SepF in Mycobacterial Division. Mol. Microbiol. 2015, 97, 560–576. [Google Scholar] [CrossRef] [PubMed]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green Fluorescent Protein as a Marker for Gene Expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef]
- Corish, P.; Tyler-Smith, C. Attenuation of Green Fluorescent Protein Half-Life in Mammalian Cells. Protein Eng. Des. Sel. 1999, 12, 1035–1040. [Google Scholar] [CrossRef]
- Liu, D.; Hao, K.; Wang, W.; Peng, C.; Dai, Y.; Jin, R.; Xu, W.; He, L.; Wang, H.; Wang, H.; et al. Rv2629 Overexpression Delays Mycobacterium smegmatis and Mycobacteria tuberculosis Entry into Log-Phase and Increases Pathogenicity of Mycobacterium smegmatis in Mice. Front. Microbiol. 2017, 8, 2231. [Google Scholar] [CrossRef]
- Blanco, P.; Corona, F.; Martínez, J.L. Biolog Phenotype Microarray Is a Tool for the Identification of Multidrug Resistance Efflux Pump Inducers. Antimicrob. Agents Chemother. 2018, 62, e01263-18. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A Review of Their Metal Chelating Properties and Medicinal Applications. DDDT 2013, 7, 1157–1178. [Google Scholar] [CrossRef]
- Talcott, R.; Hollstein, M.; Wei, E. Mutagenicity of 8-Hydroxyquinoline and Related Compounds in the Salmonella typhimurium Bioassay. Biochem. Pharmacol. 1976, 25, 1323–1328. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K.; Bishen, S.M.; Mishra, H.; Khurana, A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev. Bras. Farmacogn. 2023, 33, 272–287. [Google Scholar] [CrossRef]
- Shahid, A.; Nasir, K.; Bhatia, M. Therapeutic Potential of Alpha-Lipoic Acid: Unraveling Its Role in Oxidative Stress and Inflammatory Conditions. Curr. Issues Mol. Biol. 2025, 47, 322. [Google Scholar] [CrossRef]
- Cook, G.M.; Berney, M.; Gebhard, S.; Heinemann, M.; Cox, R.A.; Danilchanka, O.; Niederweis, M. Physiology of Mycobacteria. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2009; Volume 55, pp. 81–319. [Google Scholar]
- Voskuil, M.I.; Visconti, K.C.; Schoolnik, G.K. Mycobacterium tuberculosis Gene Expression during Adaptation to Stationary Phase and Low-Oxygen Dormancy. Tuberculosis 2004, 84, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Uplekar, S.; Rougemont, J.; Cole, S.T.; Sala, C. High-Resolution Transcriptome and Genome-Wide Dynamics of RNA Polymerase and NusA in Mycobacterium tuberculosis. Nucleic Acids Res. 2013, 41, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mei, H.; Chen, F.; Tang, Q.; Yu, Z.; Cao, X.; Andongma, B.T.; Chou, S.-H.; He, J. Transcriptome Landscape of Mycobacterium smegmatis. Front. Microbiol. 2017, 8, 2505. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhulin, I.; Wartell, R.M. Predicted Structure and Phyletic Distribution of the RNA-Binding Protein Hfq. Nucleic Acids Res. 2002, 30, 3662–3671. [Google Scholar] [CrossRef]
- Chiner-Oms, Á.; Berney, M.; Boinett, C.; González-Candelas, F.; Young, D.B.; Gagneux, S.; Jacobs, W.R.; Parkhill, J.; Cortes, T.; Comas, I. Genome-Wide Mutational Biases Fuel Transcriptional Diversity in the Mycobacterium tuberculosis Complex. Nat. Commun. 2019, 10, 3994. [Google Scholar] [CrossRef]
- Rodrigue, S.; Provvedi, R.; Jacques, P.-É.; Gaudreau, L.; Manganelli, R. The σ Factors of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 2006, 30, 926–941. [Google Scholar] [CrossRef]
- Kapopoulou, A.; Lew, J.M.; Cole, S.T. The MycoBrowser Portal: A Comprehensive and Manually Annotated Resource for Mycobacterial Genomes. Tuberculosis 2011, 91, 8–13. [Google Scholar] [CrossRef]
- Galagan, J.E.; Sisk, P.; Stolte, C.; Weiner, B.; Koehrsen, M.; Wymore, F.; Reddy, T.B.K.; Zucker, J.D.; Engels, R.; Gellesch, M.; et al. TB Database 2010: Overview and Update. Tuberculosis 2010, 90, 225–235. [Google Scholar] [CrossRef]
- Boshoff, H.I.M.; Myers, T.G.; Copp, B.R.; McNeil, M.R.; Wilson, M.A.; Barry, C.E. The Transcriptional Responses of Mycobacterium tuberculosis to Inhibitors of Metabolism: Novel Insights into Drug Mechanisms of Action. J. Biol. Chem. 2004, 279, 40174–40184. [Google Scholar] [CrossRef]
- Khil, P.P.; Camerini-Otero, R.D. Over 1000 Genes Are Involved in the DNA Damage Response of Escherichia coli. Mol. Microbiol. 2002, 44, 89–105. [Google Scholar] [CrossRef]
- Tenaillon, O.; Denamur, E.; Matic, I. Evolutionary Significance of Stress-Induced Mutagenesis in Bacteria. Trends Microbiol. 2004, 12, 264–270. [Google Scholar] [CrossRef]
- Rustad, T.R.; Roberts, D.M.; Liao, R.P.; Sherman, D.R. Isolation of Mycobacterial RNA. In Mycobacteria Protocols, 2nd ed.; Parish, T., Brown, A.C., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 13–22. ISBN 978-1-59745-207-6. [Google Scholar]
- Scotto–Lavino, E.; Du, G.; Frohman, M.A. 5′ End cDNA Amplification Using Classic RACE. Nat. Protoc. 2006, 1, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Goude, R.; Parish, T. Electroporation of Mycobacteria. In Mycobacteria Protocols, 2nd ed.; Parish, T., Brown, A.C., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; pp. 203–215. ISBN 978-1-59745-207-6. [Google Scholar]
- Camas, F.M.; Blázquez, J.; Poyatos, J.F. Autogenous and Nonautogenous Control of Response in a Genetic Network. Proc. Natl. Acad. Sci. USA 2006, 103, 12718–12723. [Google Scholar] [CrossRef] [PubMed]
- Bei, C.; Zhu, J.; Culviner, P.H.; Gan, M.; Rubin, E.J.; Fortune, S.M.; Gao, Q.; Liu, Q. Genetically Encoded Transcriptional Plasticity Underlies Stress Adaptation in Mycobacterium tuberculosis. Nat. Commun. 2024, 15, 3088. [Google Scholar] [CrossRef] [PubMed]
- Parish, T.; Stoker, N.G. Use of a Flexible Cassette Method to Generate a Double Unmarked Mycobacterium tuberculosis tlyA plcABC Mutant by Gene Replacement. Microbiology 2000, 146, 1969–1975. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).