1. Introduction
Healthcare-associated infections caused by multidrug-resistant (MDR) bacteria pose a significant and growing public health challenge, particularly in high-risk settings such as Intensive Care Units (ICUs), and especially in ICUs from infectious disease hospitals where patients frequently receive broad-spectrum antibiotic therapy [
1,
2]. These infections are notoriously difficult to treat due to limited antimicrobial options and are associated with increased morbidity, mortality, and healthcare costs [
3,
4,
5]. MDR bacteria are typically defined as bacteria resistant to at least three classes of antimicrobial agents, with key pathogens including
Staphylococcus aureus,
Enterococcus spp.,
Enterobacterales,
Pseudomonas aeruginosa, and
Acinetobacter spp. [
6]. ICU patients are especially vulnerable due to prolonged hospital stays, frequent invasive procedures, and immunosuppression, all of which facilitate colonization and infection by MDR bacteria [
7,
8,
9]. Colonization is often asymptomatic but can precede infection and contribute to hospital outbreaks, making early detection critical for infection control and containment [
10,
11].
Antimicrobial resistance (AMR) is currently recognized as one of the most significant threats to global health, necessitating immediate and coordinated action to curb its progression. The World Health Organization (WHO) has identified antimicrobial resistance as a critical public health concern and has developed a priority list of resistant pathogens based on factors such as infection-associated mortality, transmissibility, availability of effective treatments, and the feasibility of prevention in both healthcare and community settings. This prioritization aims to guide research and stimulate the development of new antimicrobial agents targeting the most urgent threats. The WHO list categorizes MDR bacteria into three priority tiers: critical, high, and medium. The critical-priority group includes carbapenem-resistant
Enterobacterales, third-generation cephalosporin-resistant
Enterobacterales, and carbapenem-resistant
Acinetobacter baumannii. The high-priority category encompasses vancomycin-resistant
Enterococcus faecium, carbapenem-resistant
Pseudomonas aeruginosa, methicillin-resistant
Staphylococcus aureus (MRSA), fluoroquinolone-resistant
Salmonella Typhi, non-typhoidal
Salmonella, and
Shigella species. The medium-priority group includes macrolide-resistant
Streptococcus pneumoniae and ampicillin-resistant
Haemophilus influenzae [
12].
AMR continues to pose a critical challenge to public health across Europe, with MDR bacteria contributing significantly to the burden of healthcare-associated infections (HAIs). An estimated 80,000 hospitalized patients suffer from at least one HAI on any given day, contributing to approximately 16 million additional hospital days annually. The prevalence of HAIs in high-income countries is around 7.5%, while in low- and middle-income countries it ranges between 5.7% and 19.2%. In Romania, HAIs remain a significantly underrecognized problem, with official reports indicating prevalence rates of only 0.2–0.25%, largely due to substantial underreporting. Across the EU, it is estimated that more than 4 million patients acquire a nosocomial infection each year, resulting in approximately 37,000 deaths [
13,
14].
Surveillance data from the European Centre for Disease Prevention and Control (ECDC) highlight alarming trends in the prevalence of resistance among key bacterial pathogens. Between 2016 and 2020, there was a marked increase in carbapenem-resistant
E. coli and
Klebsiella pneumoniae, as well as vancomycin-resistant
E. faecium across the region [
15]. By 2021, over half of
E. coli strains and approximately one-third of
K. pneumoniae strains exhibited resistance to at least one class of antibiotics, with combined resistance to multiple classes being increasingly common [
16]. The prevalence of carbapenem resistance in
K. pneumoniae has been particularly concerning. In 2021, over 25% of European countries reported carbapenem resistance rates exceeding 10%, and by 2022, these rates had increased by nearly 50% compared to the previous year.
E. coli and
K. pneumoniae remained the most frequently reported bacterial species causing bloodstream infections (BSIs) in Europe, with
E. coli accounting for 39.2% and
K. pneumoniae for 12.3% of cases in 2022 [
16,
17]. Although some declines in
E. coli resistance to third-generation cephalosporins and in methicillin-resistant
S. aureus (MRSA) BSIs were noted from 2019 to 2022, the incidence of carbapenem-resistant
K. pneumoniae continued to rise, signaling an urgent need for targeted interventions [
15,
16,
17]. In 2023,
E. coli and
K. pneumoniae remained the top contributors to bloodstream infections involving antimicrobial resistance in the European Union [
18]. While the overall resistance incidence in
E. coli declined compared to 2019, levels have been rising since 2021 and may soon return to previous highs. Conversely, AMR incidence in
K. pneumoniae has already surpassed 2019 levels, showing a consistent upward trend from 2019 to 2023. Notably, carbapenem resistance in
K. pneumoniae remains a primary concern, with higher average resistance rates than those observed for
E. coli, despite the latter’s higher infection incidence. Other Gram-negative pathogens, including
P. aeruginosa and
Acinetobacter spp., exhibited lower overall incidence rates but demonstrated worrisome resistance trends, with a significant upward trend in resistance to carbapenems, piperacillin-tazobactam, and ceftazidime in
P. aeruginosa. While some antimicrobial resistance trends appear to be stabilizing or even declining, particularly in Northern and Western Europe, countries in Southern and Eastern Europe consistently report higher rates of AMR and associated bloodstream infections. These geographic disparities underscore the need for regionally tailored surveillance and infection control strategies to effectively contain the spread of MDR bacteria across the continent [
15,
16,
17,
18]. In Romania, AMR is a major public health challenge, mainly due to excessive and often inappropriate use of antimicrobials. Romania ranks as the second-highest country among EU/EEA countries in total consumption (community and hospital sectors combined) of antibiotics for systemic use with 27.4 DDD per 1000 inhabitants per day. This led to high resistance rates in clinically relevant pathogens, such as
S. aureus,
K. pneumoniae,
P. aeruginosa, and
A. baumannii [
18]. According to the most recent EU/EEA annual report on antimicrobial resistance, Romania has reported concerning trends between 2019 and 2023. An increasing trend in carbapenem resistance was observed for
E. coli and
K. pneumoniae; however, the resistance rates remained low for
E. coli (<2%), while for
K. pneumoniae, resistance rates for carbapenems reached 52.8%.
K. pneumoniae also shows alarmingly high resistance rates for third-generation cephalosporins (69.4%), fluoroquinolones (64%) and aminoglycosides (57.1%). In
Acinetobacter spp., resistance rates exceed 80% for carbapenems, fluoroquinolones, and aminoglycosides, although a declining trend has been observed for fluoroquinolones. Similarly, MRSA has shown a downward trend but remains prevalent at 39.4%.
P. aeruginosa continues to display high resistance rates to piperacillin & tazobactam (50%), ceftazidime (48.6%), carbapenems (52.7%), fluoroquinolones (49.2%), and aminoglycosides (44.2%). Moreover, combined resistance to ≥3 antimicrobial groups remain widespread, particularly in
K. pneumoniae (54.4%),
P. aeruginosa (49.5%), and
Acinetobacter spp. (81.6%) [
17,
18]. These data underscore the significant burden of MDR bacteria in Romania and raise concerns about therapeutic limitations and infection control strategies.
Despite these worrying trends, important surveillance gaps remain. National AMR data are frequently incomplete, with insufficient details regarding regional variation, hospital context, or specific patient groups. Routine surveillance in Romania tends to underrepresent colonization data, and information linking colonization with subsequent infection is especially scarce. This gap in knowledge hampers the implementation of effective infection prevention and control measures and limits the ability of stewardship programs to adapt to local resistance patterns. Given the country’s high antibiotic consumption and high rates of resistance in critical pathogens, local ICU-focused surveillance is essential. Generating such data not only informs patient management at the institutional level but also contributes to the broader understanding of regional resistance dynamics. The aim of the study is therefore to evaluate the antibiotic susceptibility patterns of MDR bacteria involved in colonization and/or infection among patients admitted to the ICU at a tertiary care hospital in Northeastern Romania, and to investigate if there is a relationship between MDR bacterial colonization and subsequent infection. By addressing local surveillance gaps, this work provides much-needed insights into the burden of MDR bacteria in Romanian ICUs and strengthens the evidence base for antimicrobial stewardship and infection prevention strategies in high-risk clinical settings.
3. Discussion
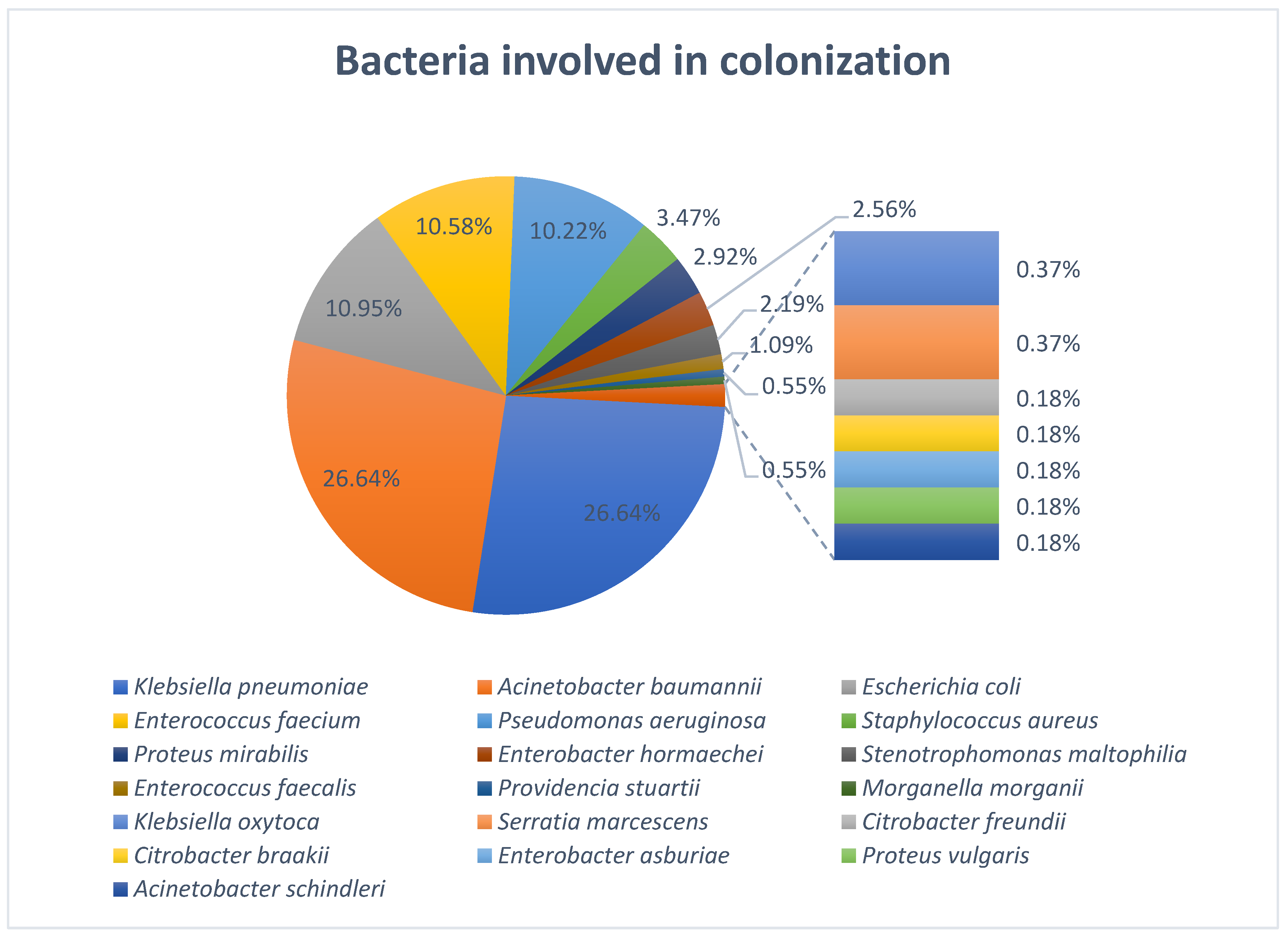
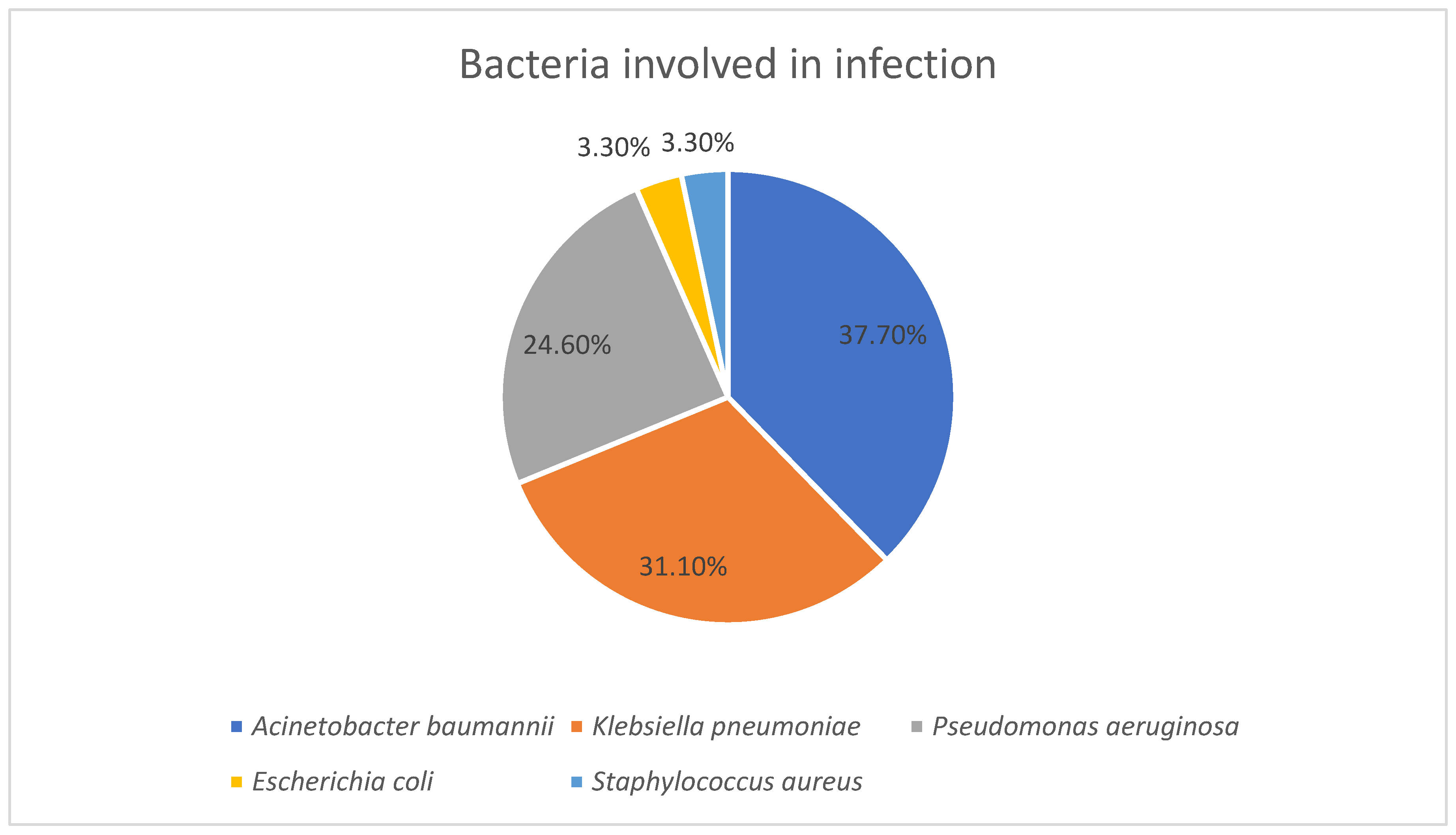
This study provides an evaluation of bacterial colonization and subsequent healthcare-associated infections among ICU patients in a tertiary care hospital from Northeastern Romania, with a particular focus on MDR bacteria and their antibiotic resistance patterns. A total of 609 strains were isolated from 118 patients, highlighting an important microbial burden in this category of high-risk patients. Our study highlighted a predominance of Gram-negative bacilli involved in both colonization and infection and a poor representation of Gram-positive bacteria which may indicate a reduced pathogenic role in our cohort. The predominance of Gram-negative bacilli in both colonization and infection likely reflects the combined effects of antibiotic selection pressure, which favors MDR Gram-negative strains, and the ecological advantage of these organisms in the hospital environment, where they persist on equipment and in the gastrointestinal tract. Their numerous virulence factors may also facilitate progression from colonization to infection, explaining their overrepresentation among infecting strains. In contrast, the lower proportion of Gram-positive bacteria may suggest a reduced pathogenic role in this cohort, possibly due to stricter infection control measures or a lower adaptation to the hospital environment in this clinical context. These findings align with other studies across several geographical regions which also showed a predominant colonization of ICU patients with Gram-negative bacteria [
19,
20,
21,
22,
23].
A. baumannii was the predominant organism isolated from pharyngeal and nasal swabs, whereas
K. pneumoniae,
E. coli, and
E. faecium were more frequently found in rectal swabs. The preferential colonization of different anatomical sites reflects the ecological adaptation of the organisms.
K. pneumoniae,
E. coli, and
E. faecium are enteric bacteria and therefore predominate in rectal swabs, whereas
A. baumannii shows a greater affinity for pharyngeal and nasal mucosa, where it can survive and adhere to epithelial cells. These site-specific patterns are clinically relevant, as colonization of the upper airways by
A. baumannii and other Gram-negative bacilli may serve as an important reservoir for subsequent lower respiratory tract infections, consistent with our finding of a strong association between pharyngeal/nasal colonization and respiratory infection. This significant association between pharyngeal/nasal colonization and subsequent lower respiratory tract infections was also demonstrated in other studies [
24,
25], while Huang et al. showed no relationship between respiratory colonization and subsequent development of respiratory tract infections [
26]. However, in our study there was a higher prevalence of
A. baumannii colonization and combined with poorer infection control measures can explain the discrepancies observed between the studies. Furthermore,
K. pneumoniae and
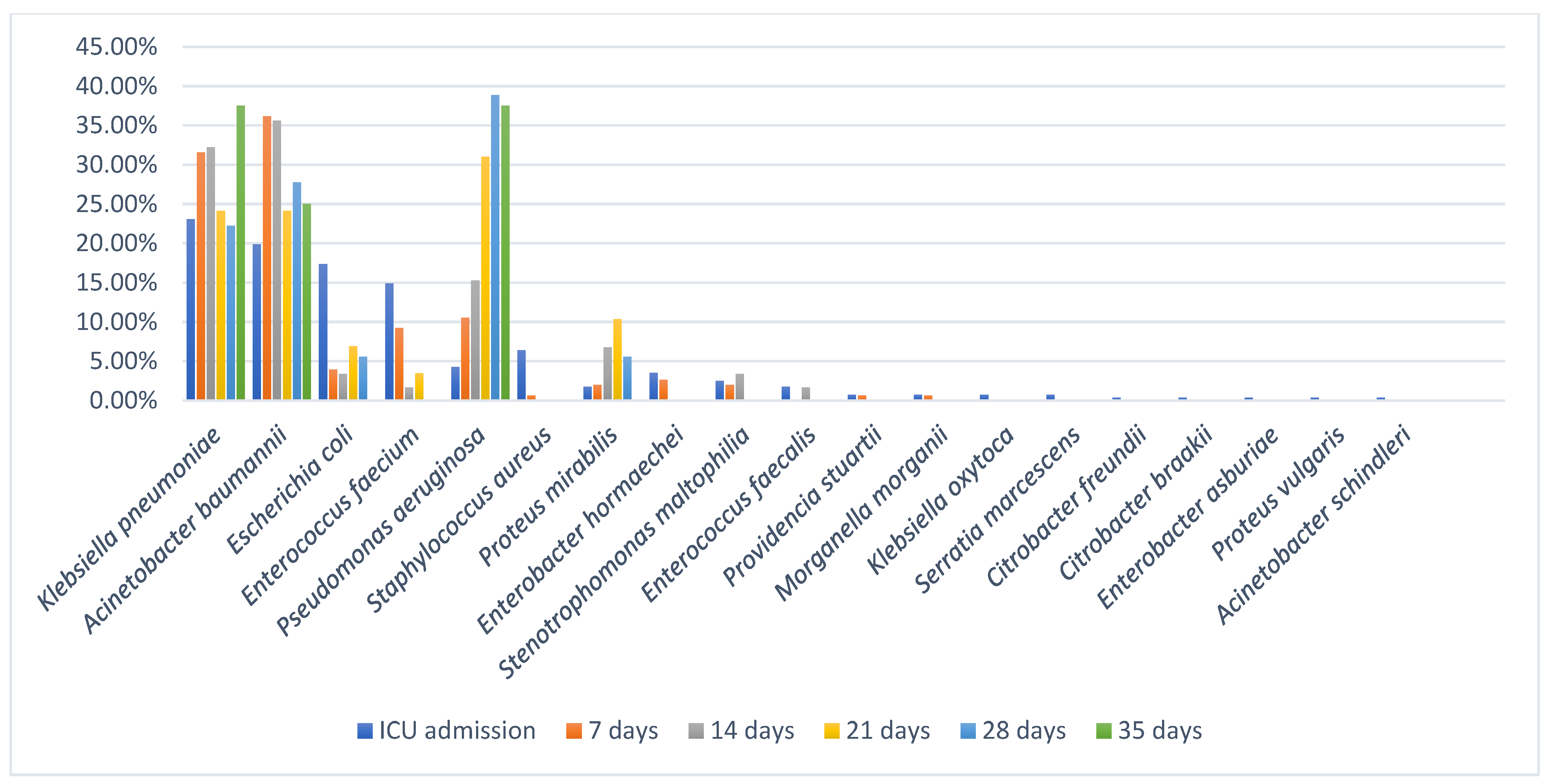
A. baumannii were among the most frequently isolated organisms on ICU admission, which may suggest that some patients were colonized before admission. This finding may reflect previous healthcare contact or the growing dissemination of MDR pathogens within the community. Although follow-up surveillance beyond day 7 was limited, our findings provide important insights into colonization dynamics in ICU patients. All 42 patients with extended follow-up acquired at least one additional MDR bacteria during their ICU stay, highlighting the rapid and ongoing risk of colonization in this setting. Moreover, the two patients that were screened at 28 days after ICU admission carried a total of 18 colonizing strains, and although the number may appear unusually high, both patients had a long period of ICU stay which was associated with prolonged exposure to invasive procedures, broad-spectrum antibiotics, and a high-risk environment for cross-transmission of MDR organisms. Extended ICU stays are known to promote colonization by multiple bacterial species due to selective antibiotic pressure and frequent contact with healthcare personnel and medical devices. Therefore, the large number of strains isolated from these two patients likely reflects the cumulative impact of prolonged hospitalization and intensive medical care.
A. baumannii and
K. pneumoniae remained the predominant colonizers, with
P. aeruginosa consistently present at later time points. The persistence of these bacteria over time, even if the number of patients under surveillance decreased, suggests that they are well adapted to the ICU environment, potentially due to their intrinsic ability to survive on surfaces, form biofilms, and resist antibiotic pressure. These findings align with other studies demonstrating the ability of these bacteria to persist in ICU environments and act as reservoirs for infection [
27,
28,
29]. The repeated acquisition of new MDR bacteria also indicates ongoing cross-transmission or endogenous expansion of resistant strains during prolonged ICU stay. Environmental contamination and selective antibiotic pressure have been identified as key drivers for MDR bacteria acquisition, particularly for
A. baumannii and
P. aeruginosa [
28,
30]. Clinically, these findings underscore the need for continued surveillance throughout the ICU stay and reinforce the importance of strict infection control practices, including environmental cleaning, hand hygiene, and targeted antimicrobial stewardship, to prevent colonization from progressing to infection and limit the spread of high-risk pathogens.
From the antibiotype point of view, we observed a high phenotypic diversity among colonizing isolates. Among K. pneumoniae strains, a high degree of phenotypic resistance diversity was observed which indicate a widespread circulation of various strains within the population. While most antibiotypes were represented by only 1–3 strains, four major antibiotypes accounted for the majority of isolates. The distribution of these antibiotypes revealed distinct epidemiological and clinical patterns with important implications for infection control and patient management.
The most prevalent profile (antibiotype 1) corresponded to an XDR phenotype resistant to all tested antibiotics except colistin. Its predominance in colonization, with only a few cases progressing to infection, highlights the role of colonized patients as reservoirs. The detection of these strains both at ICU admission and during ICU stay points to a dual origin: importation from external healthcare settings and ongoing nosocomial spread. This observation underscores the importance of admission screening and rigorous in-hospital infection prevention, particularly since prior studies showed that colonization with XDR bacteria substantially increases the risk of subsequent infection in critically ill patients [
31,
32]. Antibiotype 2 exhibited a more favorable susceptibility profile, and unlike antibiotype 1, this phenotype was more often acquired during ICU stay, suggesting that antibiotic selective pressure within the hospital environment contribute to its emergence [
33]. Although predominantly associated with colonization, its involvement in infections, especially those linked to prior colonization, underlines its clinical significance, however considering the broad therapeutic options available for this phenotype, infections caused by this antibiotype may be more manageable if they are appropriately treated. Antibiotype 3 was associated only with colonization and had a higher prevalence at ICU admission which suggests that its often imported rather than acquired during ICU stay. This finding emphasizes the importance of screening upon ICU admission, since colonized patients may serve as sources for cross-transmission even if they are not at immediate risk of infection [
34]. While less clinically alarming than antibiotypes 1 and 4, its potential to evolve under antimicrobial pressure remains concerning, especially given the limited therapeutic options available in the ICU. Finally, antibiotype 4 represented the most concerning phenotype, displaying resistance to all tested antibiotics, including colistin. Its involvement in both colonization and infection, and both its presence upon ICU admission and its acquisition during ICU stay highlights the dual challenge of external introduction and nosocomial dissemination. The clinical implications are particularly severe, as infections with such strains are essentially untreatable, a problem well documented in recent reports of infections with colistin-resistant
K. pneumoniae [
35,
36]. The emergence of colistin resistance was also reported in recent surveillance data from countries such as Romania, Greece and Italy reporting elevated rates (25.8%, 19.9% and 15.4%, respectively) [
15,
16,
17,
18]. Another study from Northeastern Romania also showed that
K. pneumoniae exhibited a high prevalence of colistin resistance over the last 5 years, with annual resistance rates fluctuating between 12.97% and 21.64% [
37]. The presence of this phenotype emphasizes the urgent need for strict infection control measures, active surveillance, and strict antimicrobial stewardship to limit the spread of these high-risk clones.
For
E. coli, we identified a considerable phenotypic diversity in antibiotic resistance. No predominant antibiotypes were identified, with many antibiotypes being represented by 1–2 strains which implies high variability in antibiotic resistance. Notably, only one strain of
E. coli was not present at ICU admission and was acquired during ICU stay, suggesting a limited cross-transmission of
E. coli in ICU, being more often introduced from external sources. However, the two strains involved in infections were also involved in colonization and had the same antibiotype, highlighting the role of colonization as a precursor to infection. These findings underscore the importance of screening to identify patients at risk, support targeted antimicrobial therapy based on susceptibility profiles, and suggest that infection control measures are effective in limiting in-ICU transmission. Other studies highlighted the complexity of
E. coli colonization, its phenotypic diversity, and the dynamics of infection in ICU settings, emphasizing the importance of genomic surveillance to establish the complexity of
E. coli colonization and the potential for strain evolution within ICU settings [
38,
39].
For
A. baumannii, we observed a marked predominance of a single phenotype which was characterized by resistance to all tested antibiotics except colistin. This highlights the therapeutic limitations clinicians face, as colistin remains one of the last active agents against XDR
A. baumannii. Similar findings regarding increased rates of resistance and an alarming reliance on colistin for effective therapy have been reported [
40,
41,
42]. The clinical relevance of this finding is underscored by the fact that all strains involved in infections were part of this antibiotype and moreover, all strains involved in infections were also involved in colonization suggesting a strong correlation between colonization and infection with
A. baumannii. This finding aligns with previous studies demonstrating that colonization is a prerequisite for
A. baumannii infection, particularly in ICU patients [
24,
43]. Out of the 137 strains involved in colonization, 44 were acquired during ICU stay, which emphasize that cross-transmission within the ICU is concerning. This is consistent with outbreak investigations demonstrating that clonal spread within the ICU occurs via contaminated surfaces, shared equipment, and healthcare worker hands.
A. baumannii is well known for its ability to survive desiccation and persist on inanimate surfaces, facilitating environmental reservoirs that sustain transmission [
44,
45,
46].
Regarding
P. aeruginosa strains, our study revealed a marked phenotypic diversity. Colonization was more common than infection, consistent with the opportunistic nature of this pathogen in ICU settings, also stated by Harris et al. [
47]. The predominance of a few major antibiotypes highlights the emergence of highly resistant clones. Notably, antibiotype 1, resistant to all antibiotics except colistin, was the most frequent and concerning, indicating limited therapeutic options although most isolates were associated with colonization. Antibiotypes 2 and 3, also showing extensive resistance, were exclusively acquired during ICU stay, suggesting in-hospital selection pressures and possible cross-transmission. This pattern highlights the critical role of the ICU environment in driving the emergence of MDR
P. aeruginosa and the need for strict infection control measures. In contrast, antibiotype 4 displayed broader susceptibility and a lower prevalence, indicating that less resistant strains persist but are less competitive in the hospital environment. Interestingly, all patients who developed an infection with
P. aeruginosa were colonized with the same pathogen, however the antibiotypes did not match in most cases. Out of the 15 strains involved in infections, 9 strains had a different antibiotype than the one involved in colonization. This finding may suggest that the infecting strain may have replaced the initial colonizing strain during the ICU stay, reflecting dynamic shifts in the patient’s microbial flora under selective pressure from antibiotics on environmental exposure, or horizontal gene transfer or rapid mutational events may have altered the resistance phenotype between colonization and infection, particularly under strong antimicrobial pressure. Several studies observed the mismatch between colonizing and infecting
P. aeruginosa antibiotypes [
48,
49,
50,
51,
52]. One possibility for this mismatch is strain replacement, where new, often more resistant strains displace resident colonizing populations under antibiotic selective pressure [
49]. Within-host diversity can also accelerate resistance evolution. For example, patients colonized by multiple strains tend to select for pre-existing resistant strains, while patients with single-strain colonization acquire resistance more sporadically through novel mutations. Additionally, within-host evolution generates phenotypic diversity, enabling initially colonizing strains to acquire resistance mutations or virulence traits that facilitate transition to infection [
49,
50]. Environmental and patient factors, such as immune status and ICU exposure, further influence which strains persist and which cause infection [
51,
52]. Clinically, these dynamics have several implications. The divergence between colonizing and infecting strains complicates empiric therapy, as the antibiotic susceptibility of colonizing strains may not reliably predict the susceptibility profile of infecting strains. This underscores the need for continuous surveillance and molecular typing to detect emergent resistant clones and guide antimicrobial stewardship [
51]. Also, the potential for rapid evolution and strain mixing highlights the importance of strict infection control measures, including grouping patients colonized or infected with the same pathogen together, environmental decontamination, and hand hygiene, to limit in-hospital transmission [
49]. Recognizing colonization as a reservoir for MDR
P. aeruginosa can therefore inform both preventive strategies and treatment decisions, ultimately reducing ICU morbidity and mortality. Another notable finding regarding
P. aeruginosa was the very high rates of resistance to carbapenems, compared to cephalosporins such as ceftazidime and cefepime, where resistance rates were somewhat lower. Mechanisms for carbapenem resistance likely result from the loss of
OprD porin, efflux pump overexpression, and carbapenemase production, which offers high-level, often complete resistance to carbapenems, while for cephalosporins, resistance is offered by an overexpression of
AmpC β-lactamase or altered membrane permeability, which reduce but do not fully eliminate drug activity. This can explain why cephalosporins showed more intermediate results than carbapenems, despite both being β-lactam agents [
53,
54,
55].
All isolates (100%) of both
P. aeruginosa and
A. baumannii were classified as susceptible to colistin, which emphasizes the critical role of this last-line antibiotic in treating infections caused by non-fermentative Gram-negative bacilli. Although in our study no strain was resistant to colistin, others reported resistant strains of
P. aeruginosa and
A. baumannii [
56,
57,
58] emphasizing the need for development of new agents for these critical pathogens.
When looking at resistance patterns for Gram-positive bacteria, we observed the same alarming resistance profiles observed in Gram-negative bacteria. For
Enterococcus spp. we observed key differences between the two species. All
E. faecium isolates displayed a very high resistance to both vancomycin and teicoplanin, but a low resistance to linezolid and no resistance to tigecycline, thus preserving some treatment options. HLAR was frequent, significantly limiting the potential for effective synergistic combination therapy. In contrast, all
E. faecalis isolates remained susceptible to ampicillin, tigecycline, and linezolid. Glycopeptide resistance was less common, with only one isolate resistant to vancomycin and teicoplanin, suggesting sporadic occurrence of VRE in this species. All isolates of
S. aureus were methicillin-resistant (MRSA), with 100% resistance to both penicillin and oxacillin, however all isolates were susceptible to anti-MRSA agents. Notably, linezolid resistance was detected in 63.2% of isolates which is an alarming finding compared to other studies who showed very low rates of resistance to linezolid (<5%) [
59,
60]. Several factors may explain these findings, including local clonal expansion or outbreak of resistant MRSA strains, selective pressure from prior use of linezolid as a therapeutic choice, and horizontal transfer of resistance genes such as
cfr or
optrA [
61]. Patient-related risk factors such as prolonged ICU stay or invasive devices, and potential lapses in infection control may have further contributed. These observations underscore the importance of enhanced surveillance, molecular typing to track resistant clones, strict infection prevention measures, and antimicrobial stewardship to mitigate the emergence and spread of linezolid-resistant MRSA in critical care settings. VRE and MRSA remain important challenges in ICU settings, considering the high prevalence of MDR profiles reported by several studies [
21,
23,
62,
63].
Recent studies conducted in Romanian ICUs also highlight the high burden and evolving dynamics of antimicrobial resistance among Gram-negative pathogens. Golli et al. (2019) reported high MDR rates for several pathogens, such as
P. aeruginosa (64.7%), MRSA (62.7%), and
Klebsiella spp. (53.3%) [
64]. Also, they highlighted the impact of the COVID-19 pandemic, showing a post-pandemic rise in MDR and PDR strains, particularly among
Klebsiella and
Acinetobacter, including resistance to last-resort agents such as colistin and tigecycline [
65,
66]. Another study by Apetroaei et al. (2025) demonstrated that
A. baumannii and
P. aeruginosa continue to display the highest resistance levels, particularly in high-risk units such as the ICU and transplant wards [
67]. Collectively, these findings underscore the persistent and evolving threat of MDR and PDR Gram-negative pathogens in ICU settings, emphasizing the urgent need for continuous surveillance and robust antimicrobial stewardship strategies to mitigate the spread of these high-risk organisms [
64,
65,
66,
67]. Finally, when looking at the MAR index for all bacterial strains included in our study we observed an alarming trend. A MAR index > 0.2 indicates that the bacteria come from a high-risk source where antibiotics are frequently used, while a MAR index ≤ 0.2 indicates that the isolate is from an environment with infrequent or less intensive antibiotic exposure. In our study, all strains had a MAR index > 0.2, with the majority of strains having a MAR index > 0.8. This suggests that every strain originated from high-risk environments with frequent antibiotic exposure, such as hospital wards, especially ICUs, where antimicrobial use is intensive. MAR values exceeding 0.8 suggest repeated or prolonged exposure to diverse antimicrobials, allowing resistant clones to thrive and spread. Others showed that MAR values of bacterial strains isolated from ICUs tend to be higher than those isolated from surgical/medical wards [
68].
Our study has several limitations regarding dynamics between colonization and infection. Only 42 patients were followed-up beyond ICU admission, with a decreasing number of patients screened at later time points, which may have led to underestimation of late-onset colonization or infection. Also, information regarding patients’ prior hospitalizations, antibiotic exposure, or invasive medical procedures was not available, so it is plausible that some patients were colonized before admission. Further studies should incorporate detailed patient history to more accurately distinguish between community and healthcare associated colonization. Another key limitation is the absence of molecular typing methods to confirm the relatedness of isolates involved in colonization and infection. Although we used antibiotypes to assess the correlations between colonization and infection, future studies integrating genotypic methods are needed to provide more robust insights into transmission dynamics and strain relatedness.