1. Introduction
According to the latest Global Research on Antimicrobial Resistance (GRAM) report, bacterial antimicrobial resistance was directly responsible for approximately 1.14 million deaths in 2021 and associated with 4.95 million deaths globally in 2019, highlighting the urgent need for effective therapies [
1]. The prevalence of infections caused particularly by Gram-negative bacteria, such as multidrug-resistant
Acinetobacter baumannii,
Klebsiella pneumoniae, and
Pseudomonas aeruginosa, has led to the re-emergence of polymyxins in clinical practice because they have proven to be effective against resistant Gram-negative strains [
2,
3]. Polymyxin B (PMB), which is a polypeptide antibiotic, predominantly targets the lipid A component of lipopolysaccharides (LPS) within the outer membrane of Gram-negative bacteria, thereby compromising the structural integrity of the membrane. Its self-uptake mechanism is critically reliant on its amphipathic properties that facilitate increased membrane permeability and result in leakage of intracellular contents [
4].
Nephrotoxicity remains a significant challenge for PMB. The prevalence of nephrotoxicity ranges from 10% to 60% despite the effectiveness of PMB in treating multidrug-resistant Gram-negative bacterial infections [
5,
6]. The amphipathic properties of polymyxins are also responsible for their nephrotoxicity where the cationic diaminobutyric acid (Dab) residues of PMB interact with anionic phospholipids (e.g., phosphatidylinositol, cardiolipin) on the membranes of renal tubular cells. This electrostatic interaction, combined with the insertion of the lipophilic tail into the lipid bilayer, disrupts membrane integrity, increases permeability, and leads to cell swelling, necrosis, and ultimately, nephrotoxicity [
7,
8]. In contrast, PMB nonapeptide (PMB without the fatty acyl tail) was found to uniformly distribute in the kidney [
9]. The selective uptake with high tubular reabsorption was shown in low urinary recovery (<5%) from animal models [
7,
10]. Studies suggest that the toxicity to renal tubular cells is indicated by rising creatinine (Cr) levels in the blood. The proposed mechanisms include oxidative stress, apoptosis, cell cycle arrest, and autophagy. Oxidative stress disrupts redox balance and causes lipid peroxidation, protein damage, and mitochondrial dysfunction, while apoptosis leads to tubular cell loss via caspase activation and cytochrome c release [
11,
12].
There is a high demand for new antimicrobial agents to combat resistant bacteria; however, the complex processes, regulatory challenges, and financial risks have made research into nanotechnology-based antimicrobial drugs more preferable [
13,
14,
15]. The development of a suitable delivery system would aim to optimize the pharmacokinetics and pharmacodynamics of PMB to reduce systemic exposure and deliver the drug more precisely to infection sites to mitigate the toxicity of PMB and enhance antimicrobial performance [
16]. The chemical structure and properties of polymyxins are important factors to consider in designing a delivery system. The five primary aliphatic amino groups in polymyxins facilitate their efficient binding with anionic molecules and polyanions. Additionally, the combination of a positively charged structure, a lipophilic tail, and hydrophobic amino acids in the peptide chain enables the use of amphiphilic and hydrophobic low-molecular-weight compounds and polymers as carriers in drug delivery systems [
17,
18,
19].
Micelle-based delivery systems have shown significant promise in enhancing the therapeutic efficacy of peptide antibiotics. These nanosized carriers, which are formed by the self-assembly of amphiphilic molecules, can encapsulate antimicrobial peptides to improve solubility, stability, and bioavailability [
20]. Sodium deoxycholate sulfate (SDCS) is an amphiphilic carrier that is synthesized from deoxycholic acid [
21]. SDCS is particularly suitable for delivering PMB due to its unique structural properties. As an anionic surfactant, it can form stable micellar complexes with the cationic PMB molecule via electrostatic and hydrophobic interactions. Its safety profile has been demonstrated in previous in vitro and in vivo studies, which showed that SDCS formulations of nephrotoxic drugs like amphotericin B and colistin significantly reduced cytotoxicity and organ damage without compromising antimicrobial efficacy [
22,
23,
24,
25]. For instance, PMB-SDCS formulations demonstrated significantly reduced cytotoxicity in human proximal tubule (HK-2) cells while retaining potent antimicrobial activity against carbapenem-resistant
Acinetobacter baumannii and
Pseudomonas aeruginosa [
23,
26]. Rats that were administered amphotericin B and SDCS formulations via intratracheal instillation showed no evidence of toxicity, especially in lung and kidney tissue, with significantly lower drug accumulation in the kidney compared with a commercial formulation [
25]. A prior study showed that PMB-SDCS formulations facilitated PMB release for effective penetration into lipid membranes or micelles. The complex LPS micelles or membranes were disrupted while neutralizing LPSs through hydrogen bonding and electrostatic interactions between PMB amine residues and the phosphate and glucosamine groups of LPSs, as well as salt bridge formation with SDCS sulfonate groups [
27]. The PMB-SDCS complex is formed primarily through electrostatic interactions between the cationic Dab residues of PMB and the anionic sulfate groups of SDCS, supplemented by hydrophobic interactions between the fatty acyl tail of PMB and the steroid backbone of SDCS. This results in the encapsulation of PMB within the SDCS micelles, enhancing its aqueous stability and altering its interaction with biological membranes [
27,
28].
The objective of the present study was to investigate PMB-SDCS formulation treatment effects in a rat model. The treatments caused histological alterations in the kidney and several other organs. Biochemical markers that indicated the treatments’ nephrotoxicity were measured. PMB concentrations in the serum and tissue were also analyzed to further investigate the formulation’s effect in vivo, while using molecular docking to analyze the human serum albumin (HSA) interaction. The binding affinity of PMB to HSA is a key pharmacokinetic parameter, as extensive binding can reduce the free, pharmacologically active drug concentration, potentially necessitating higher doses that increase the risk of nephrotoxicity. Investigating this interaction helps elucidate the formulation’s mechanism for increasing/decreasing serum drug availability.
3. Discussion
Polymyxins continue to gain interest with the rise of the antimicrobial resistance pandemic. Addressing the nephrotoxicity issue of polymyxins remains a challenge that limits the use of polymyxins in clinical therapy [
29]. The therapeutic window of polymyxins is narrow; for example, clinical PK/PD targets for PMB often aim for average steady-state plasma concentrations of 2–4 µg/mL, which overlaps with concentrations known to cause nephrotoxicity [
30]. Dosing optimization becomes more important through detailed findings about polymyxin-induced nephrotoxicity to overcome the narrow therapeutic window of polymyxins by increasing its antimicrobial potency and decreasing its nephrotoxicity [
31]. Previous studies showed incorporating PMB into SDCS micelles showed increased viability of normal human primary renal proximal tubule epithelial cell line while providing equivalent antimicrobial activity when tested against carbapenem-resistant
Acinetobacter baumannii and resistant
Pseudomonas aeruginosa [
23,
26].
In this study, the PMB was formulated with SDCS, with a 1:2 ratio selected based on previous work by Madhumanchi et al. and Temboot et al., demonstrating that SDCS micelles significantly reduced PMB-induced cytotoxicity and hemolysis in human renal epithelial and erythrocyte models [
23,
26]. The PMB-SDCS formulation produced in this research has hydrodynamic properties similar to those in the previous report and produces a clear solution when suspended in water. The formulations were subcutaneously administered to a group of rats on a 7-day treatment course at a dosage of 6 mg/kg/day of PMB equivalent and compared to PMB, SDCS (carrier), and normal saline. In our rat model, after seven days of treatment, the group subjected to PMB treatment experienced a significantly reduced body weight and body weight progression for some individual rats. The observed weight loss in the PMB group likely reflects multiple interacting mechanisms, including renal tubular injury and altered metabolism. The elevated BUN and creatinine levels support impaired renal function, while histopathological evidence of tubular degeneration corroborates tissue-level damage. These findings align with decreased nutrient assimilation and possible anorexic effects secondary to systemic inflammation, explaining the reduced body weight gain in PMB-treated rats. In contrast, PMB-SDCS mitigated these alterations, consistent with normal renal biomarkers and preserved tissue integrity [
32].
The nephrotoxicity of polymyxin can be detected or defined by elevated serum Cr and BUN, which are commonly used to measure glomerular damage [
33]. The rat groups administered with 6 mg/kg/day of PMB showed elevated BUN and Cr after seven days of administration. This level might not be significantly elevated depending on the degree of injury to renal glomeruli [
34]. Polymyxin-induced kidney injury is strongly associated with oxidative stress, which is driven by increased reactive oxygen species (ROS) production after exposure [
35,
36]. In the PMB group, the serum SOD and CAT levels showed reduced oxidative stress enzyme activity, which was consistent with previous findings [
37]. Deficiencies in key antioxidant enzymes, such as SOD and CAT, have been shown to exacerbate kidney damage. SOD deficiency aggravates renal dysfunction, promotes tubulointerstitial fibrosis, induces inflammation, and increases apoptosis in kidney tissues. Similarly, CAT deficiency, as observed in a mouse model of unilateral ureteral obstruction, worsens tubulointerstitial fibrosis and elevates lipid peroxidation products that further contribute to tubulointerstitial injury [
38]. PMB formulated with SDCS significantly mitigated nephrotoxicity and did not hinder kidney function as evidenced by comparable BUN and Cr levels. Furthermore, lower ROS production after exposure was indicated by higher activity of SOD and CAT.
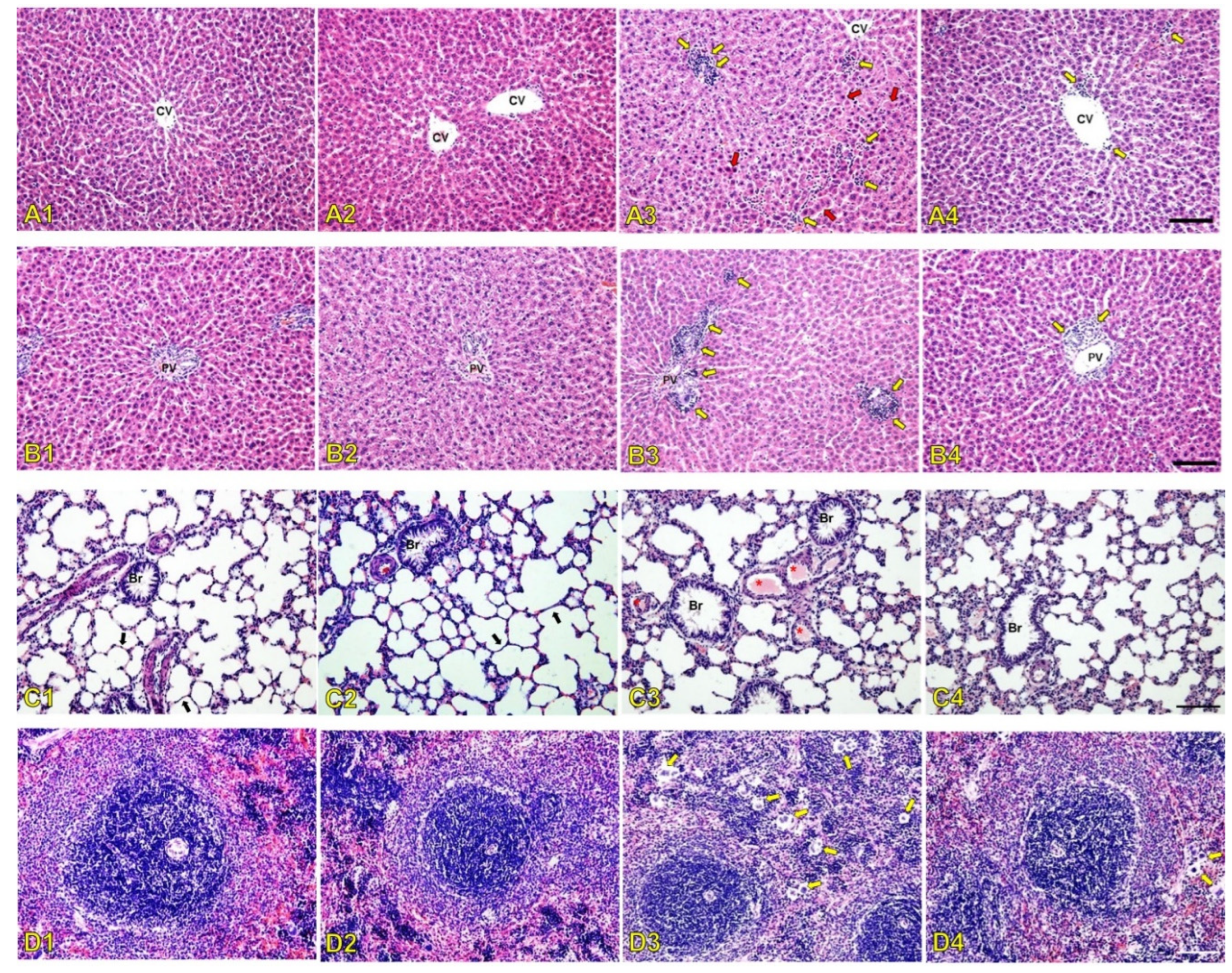
The serum biochemistry results were in line with the histopathological changes in rat kidneys after seven days of treatment with 6 mg/kg/day of PMB. The results showed signs of congested blood vessels due to inflammation indicated by inflammatory cells present from the histology of the glomerulus in the renal cortex. The affected glomerulus exhibited signs of hyperemia characterized by dilated capillaries engorged with red blood cells. This finding suggests increased blood flow to the glomerulus that is potentially part of an inflammatory response or hemodynamic alteration where oxidative stress can trigger transcription factors that activate genes driving inflammatory pathways [
39,
40]. The administration of SDCS showed no histological damage on the rat kidney with alteration that was similar to the control group. The PMB formulation attenuated kidney damage from PMB exposure with mild alternations without evidence of renal glomerular injury. The inflammation caused by increased ROS production was also apparent from the histological alteration of the liver, lung, and spleen tissues. PMB-treated animals showed significant liver damage that included monocyte infiltration, necrotic foci near blood vessels, and disrupted hepatocyte architecture. In contrast, the PMB formulation group exhibited only mild monocyte infiltration with preserved liver structure. The PMB-treated rats showed increased small pulmonary venules with fibrin thrombi that indicated endothelial damage along with a higher number of multinucleated giant cells in the spleen. In contrast, the PMB formulation-treated animals exhibited fewer thrombi and multinucleated giant cells in both the lungs and spleen.
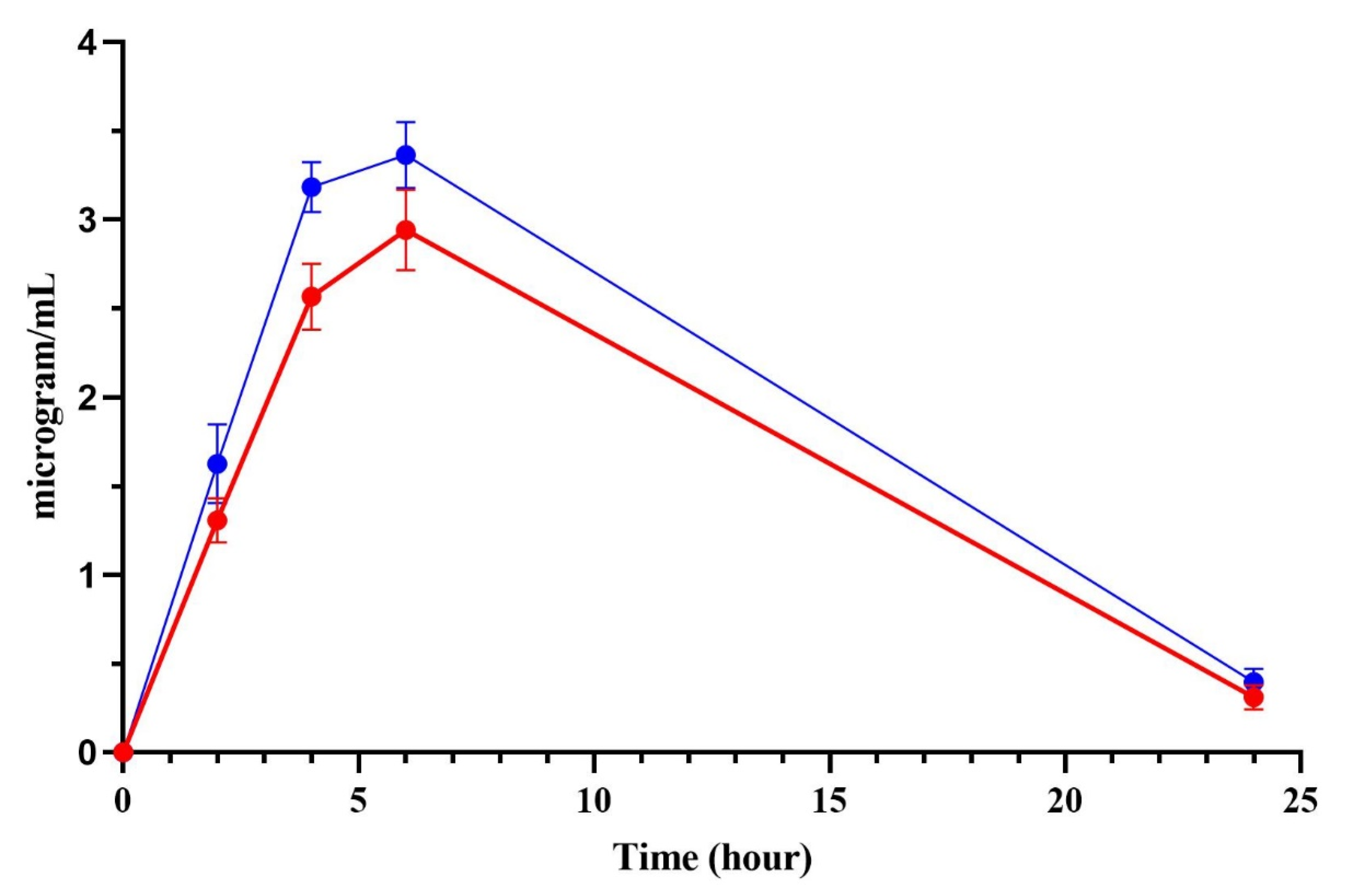
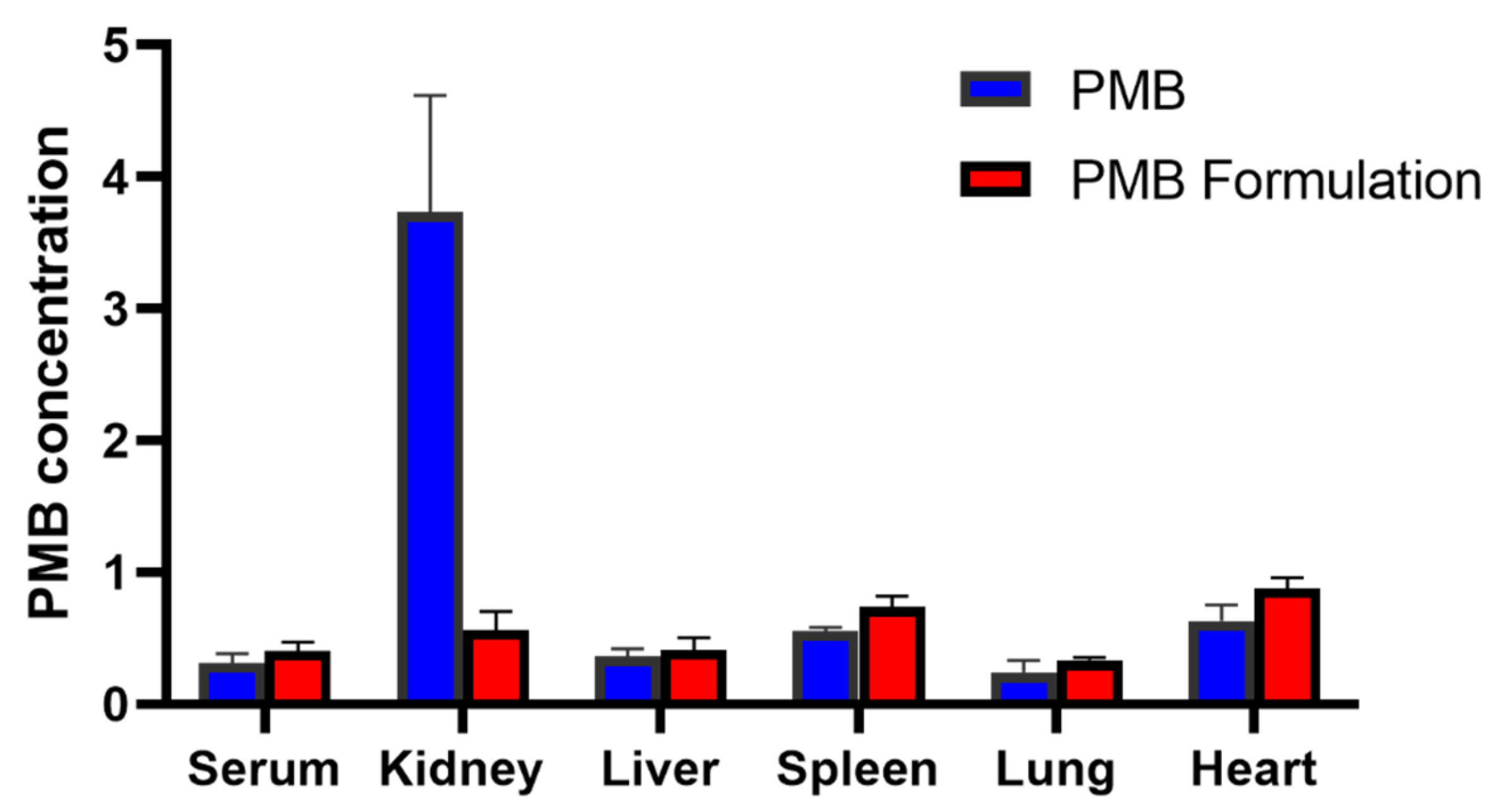
PMB undergoes extensive reabsorption in the renal tubule, and a very high concentration of PMB is found in human renal tubular cells compared to the extracellular concentration. The increased accumulation of PMB leads to cell swelling and lysis by disrupting the permeability of the renal tubular epithelial cell membrane [
41,
42]. In our results, the administration of the PMB formulation increased the serum concentration profile of PMB. The results showed a higher onset concentration compared to pure PMB. The higher free-PMB from formulation in the serum is in line with a significantly lower concentration of PMB in the kidney tissue after 24 h resulting in more free-PMB available from the loss of PMB renal uptake. The accumulation of PMB in the kidney might be higher depending on the timing of sample collection. In another study, the PMB kidney/serum concentration ratio was 18-fold [
7]. The decrease in kidney accumulation in this current study might be attributed to the altered physicochemical properties of the PMB-SDCS complex. The overall negative surface charge of the SDCS micelles may reduce the electrostatic attraction to anionic phospholipids on the brush-border membrane of renal tubular cells, a key mechanism for the high renal reabsorption and accumulation of free, cationic PMB. This is consistent with findings for other negatively charged nanocarriers, such as colistin liposomes, which also demonstrated reduced renal accumulation [
43]. The molecular docking results provide a plausible mechanism for the observed increase in serum total PMB concentration. The shielding of PMB by SDCS, leading to a highly unfavorable (positive) binding energy with HSA, suggests that less PMB is bound to serum albumin in the circulation. While our assay measured total PMB, a shift towards the unbound fraction could alter the drug’s distribution and clearance kinetics. The increased unbound fraction, coupled with the reduced affinity for renal tubular cells due to the complex’s negative charge, collectively explains the higher serum levels and lower renal accumulation. Whereas another study suggests that the electrostatic interaction played a major role, mainly from the close contact of the protein with dab residues of polymyxins [
44]. Unlike PMB, which preferentially accumulates in renal cortex tissue, PMB nonapeptide distributes more uniformly throughout the kidney [
9]. The PMB-SDCS formulation mimics this favorable distribution pattern by minimizing localized cortical accumulation, possibly through electrostatic repulsion and reduced endocytic uptake. The PMB-SDCS interaction showed that the main interaction from dab residues was shielded by SDCS, with different binding sites also contributing to significantly lower binding affinity with the HSA. The shielding effect of the higher availability of free PMB in serum and the reduced kidney accumulation may potentially increase the therapeutic window of PMB.
Although this study focused on safety and biodistribution, previous reports confirmed that SDCS formulations preserve PMB’s antibacterial potency in vitro [
23,
26]. Future in vivo infection studies are warranted to confirm that the safety improvement does not compromise antimicrobial efficacy. A limitation of this work is that the molecular docking was performed using human serum albumin, whereas the in vivo studies used rats. Structural differences between HSA and rat serum albumin may lead to minor variations in binding affinity; however, the overall interaction trends are expected to be consistent. This study was limited by its short treatment duration and use of a healthy rat model, which may not fully represent infected physiological conditions. In addition, only preliminary pharmacokinetic and histological endpoints were assessed, without direct in vivo antimicrobial efficacy data. Nevertheless, the observed reduction in nephrotoxicity and improved serum exposure suggest a promising foundation for translational development. Future studies should evaluate PMB-SDCS in infection models and explore long-term safety and pharmacodynamic correlations to support potential clinical application.
4. Materials and Methods
4.1. Materials
Polymyxin B sulfate and deoxycholic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). SDCS was synthesized in-house (Gangadhar et al., 2014) [
21]. Superoxide dismutase and catalase kits were obtained from Cayman Chemical (Ann Arbor, MI, USA). Polyamide membranes with pore sizes of 0.22 µm and 0.45 µm were obtained from Sartorius (Göttingen, Germany). All chemicals, except tetrahydrofuran, were used as received without further purification. All other reagents and chemicals were of analytical grade.
4.2. Preparation and Characterization of the PMB-SDCS Formulation
The PMB-SDCS micelles were prepared using 80 mg of PMB (0.06 mmol) and 58 mg of SDCS (0.12 mmol), corresponding to a 1:2 molar ratio (PMB:SDCS) based on their molecular weights (PMB ≈ 1302 g/mol; SDCS ≈ 480 g/mol). This ratio was optimized in previous studies [
23,
26]. The mixtures were stirred until complete dissolution. To these solutions, 5 mL of a sodium hydroxide solution (0.2 M) was added slowly dropwise at room temperature to obtain a clear solution. The pH of the solution was about 9.5, which was adjusted to 7.4 using phosphoric acid (0.2 M) for an in situ phosphate buffer. The final volume of the solution was made by adding deionized water. The solution was lyophilized, and the reconstituted formulation particle size and zeta potential were measured using a Zetasizer Nano ZS (Malvern, Worcestershire, UK). The drug content was also measured using liquid chromatography-mass spectrometry (LC-MS). The osmolarity of the formulation in normal saline was measured using a K-7400S freezing point osmometer (Knauer GmbH, Berlin, Germany).
4.3. Animals
The animal study was approved by the Animal Ethics Committee of Prince of Songkla University (Project License No. MHESI 68014/675, Ref. AR042/2024). The animals were housed in a controlled environment with a temperature of 22 ± 2 °C, relative humidity of 50 ± 10%, and a 12/12-h light/dark cycle. They were provided with a standard laboratory rodent diet and water ad libitum. The study was conducted on male outbred Sprague-Dawley rats (5–7 weeks old, 130–170 g) that were obtained from the National Laboratory Animal Center, Mahidol University, Nakorn Pathom, Thailand. The rats were fed the standard feed protocol by the Southern Laboratory Animal Facility, Faculty of Sciences, Prince of Songkla University. Rats were allowed to acclimate for 7 days prior to the experiment. Animals that did not reach a body weight of 110 g by the end of the acclimatization period were planned to be excluded; however, all rats met the inclusion criteria and were included in the study.
4.4. Study Design
The nephrotoxicity experiment of the PMB formulation was performed on 36 male rats that were randomly divided into four groups (
n = 9). Randomization was performed using computer-generated random sequences in Microsoft Excel with a fixed seed value to ensure reproducibility. Block randomization was used to balance the number of animals per group. Each group was treated subcutaneously for ease of administration and controlled dosing with 6 mg/kg/day of polymyxin B sulfate (Group 1: positive control group), PMB-SDCS formulation (Group 2), SDCS (Group 3: carrier), and normal saline solution (Group 4: normal control) for seven consecutive days. The PMB dose of 6 mg/kg/day (expressed as PMB equivalent) was selected based on previous pharmacokinetic and toxicity studies in rats, where this dose consistently produced measurable nephrotoxicity without lethality [
7,
34]. All formulations were administered subcutaneously in a constant injection volume of 1 mL/kg using normal saline as the vehicle, ensuring comparable dosing across groups. The average weight gain of each group was recorded for the experiment. We randomized the treatment order and controlled the timing of measurements, and all researchers involved in the study were blinded to the intervention. The animals were euthanized 24 h after the last treatment with an intraperitoneal lethal dose of sodium pentobarbital (200 mg/kg). Blood samples were collected by cardiac puncture, and the serum was separated by centrifugation (3000×
g for 15 min) and stored at −80 °C until assayed. Samples of the kidney, liver, spleen, lung, and heart tissues were carefully collected, weighed, and sectioned for histopathological analysis. Histological observation was performed to assess potential tissue damage and evaluate the extent of toxicity following the treatment.
4.5. Biochemical Analysis
The serum BUN and Cr levels were measured using an autoanalyzer to investigate the effects of the PMB-SDCS formulation and PMB after seven days of consecutive treatments on the physiology of the kidneys and liver. The serum levels of SOD and CAT were measured after the 7-day treatment period to investigate the oxidative properties of the treatments. The measurements were carried out using commercial kits according to the manufacturer’s instructions.
4.6. Histopathological Evaluation
Excess fat from the kidney, liver, spleen, lung, and heart tissues was removed through dissection and trimming. All samples were preserved in 10% buffered formalin using a fixative volume at least 10 times the tissue volume for three days. Subsequently, the tissues were dehydrated through a graded series of ethanol (70%, 80%, 95%, and 100%) using a tissue processor (LEICA TP 1020, Leica Microsystems GmbH, Wetzlar, Germany), with each concentration step lasting for 60 min. Following this, the tissues were embedded in Paraplast blocks using a tissue embedder (LEICA EG 1160, Leica Microsystems GmbH, Wetzlar, Germany). Thin sections that measured 5 μm in thickness were obtained using an automatic microtome. The slides were stained with Harris’ hematoxylin and eosin (H&E) and examined under an Olympus DP73 microscope (Olympus, Tokyo, Japan) equipped with cellSens software version 6.1.4.2. Histopathological analysis was performed by an investigator who was blinded to the treatment groups.
4.7. Biodistribution and Serum Concentration of PMB
For the biodistribution study, a sample size of four rats per group was chosen as a preliminary exploratory analysis to determine major organ distribution trends. This sample size was consistent with previous biodistribution studies involving polymyxins and sufficient to identify statistically meaningful differences in renal accumulation [
7]. Two groups of rats (
n = 4) were subcutaneously administered either PMB or the PMB-SDCS formulation as a single administration group. At predetermined time points (2, 4, 6, and 24 h post-dose), approximately 150 µL of blood was collected from the lateral tail vein using a sterile 25-gauge needle and capillary tubes under gentle manual restraint. The tail was warmed to dilate the vein, and alternating sides were used for repeated sampling. Blood was collected into serum separator tubes, allowed to clot at room temperature for 30 min, and centrifuged at 3000×
g for 10 min at 4 °C. The resulting serum was stored at −80 °C until analysis. The rats were euthanized at 24 h to collect the kidney, liver, spleen, lung, and heart samples. PMB concentrations were measured in LC-MS for free (unbound) fraction of serum and tissue homogenates after protein precipitation.
4.8. PMB Assay on Serum and Organ Using LC-MS
The PMB was quantified using an LC-MS/ion trap time-of-flight system (Shimadzu, Tokyo, Japan) equipped with a C18 column (2.1 mm id × 150 mm, 2.7 µm particle size; Thermo Fisher Scientific, Scoresby, Australia). Mass spectrometry was employed with mass-to-charge ratio detection conducted from m/z 200–1200 in positive ionization mode (ESI+). The column temperature was set at 35 °C. The tuning voltage was fixed to 1.68 kV. The interface, curved desolvation line, and heat block temperature were maintained at 200 °C. Nitrogen gas flowed at a rate of 1.5 L/min. The mobile phase was a mixture of 0.1% formic acid and 0.01% trifluoroacetate (TFA) in acetonitrile/water (25:75 v/v) and pumped at 0.2 mL/min for a run time of 10 min with an injection volume of 10 µL of standard PMB. The organ samples were weighed and homogenized with water (2 times their weight), and 200 µL of organ and serum were added to 400 µL of 0.1% TFA in acetonitrile to precipitate the protein. The mixture was vortexed for 30 s and centrifuged at 7200× g for 5 min. A quantity of 400 µL of supernatant was added to 600 µL of the mobile phase. A known amount of standard PMB in blank serum was prepared using the same method to produce a calibration curve of PMB and quantification was performed using colistin sulfate (1 µg/mL) as the internal standard. The monitored mass transitions were m/z 602.4 → 101.1 for PMB and m/z 585.5 → 101.1 for colistin. The calibration curve was linear over the range of 0.05–10 µg/mL (R2 = 0.998). The recovery of PMB from serum and tissue homogenates was greater than 85%, and the matrix effect was found to be negligible under the used chromatographic conditions.
4.9. PMB Binding on HSA by Molecular Docking
PMB and PMB-SDCS binding with HSA was simulated using AutoDock 4.2 from the Scripps Research Institute (
http://autodock.scripps.edu/, accessed on 9 September 2025) [
18]. The PMB structure was obtained from Chemspider database while the SDCS drawn in Avogadro and joined with PMB to simulate the shielding effect of SDCS. The ligand then docked to HSA obtained from rscb.org (PDB ID: 5TGZ). The pdb file obtained was converted to pdbqt (a molecule with partial charges and atom type). The docking grid box was centered at coordinates (x = 29.551, y = 31.855, z = 23.559) with dimensions 250 × 250 × 250 Å
3 to cover the entire HSA molecule position to perform blind docking with no specific binding site target. The Lamarckian Genetic Algorithm (LGA) was used with 50 independent runs, a population size of 200, and each docking run involved 200 energy evaluations. The docking was then processed in AutoDockTools using the pdbqt file of the receptor and ligand grid parameter file, and the docking parameter file to produce the docking log file (.dlg). The resultant files were then analyzed and the lowest docking score conformations were analyzed and visualized in Discovery Studio Visualizer Software Version 25.1.0.24284 (BIOVIA, Dassault Systèmes
®, San Diego, CA, USA). The docking protocol was validated by re-docking the native co-crystallized ligand, which yielded a root-mean-square deviation (RMSD) of <2.0 Å, confirming the reliability of the docking parameters.
4.10. Statistical Analysis
The sample size was calculated using the MINITAB Statistical Analysis Package (Minitab 18, Minitab Inc., State College, PA, USA). The sample size was determined using a one-way ANOVA with α = 0.05, an assumed standard deviation of 1.5, and four levels of the factor. With a target power of 0.8, the calculated sample size was 9, achieving an actual power of 0.806. The results were analyzed and expressed as mean ± SD. The data were evaluated using one-way or repeated measures ANOVA, as appropriate, followed by Tukey’s post hoc test for multiple comparisons. The level of significance was set at p < 0.05. All statistical comparisons were determined using GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA, USA).