Tourism and the Global Vectoring of Antimicrobial-Resistant Disease: What Countries Are Most Impacted?
Abstract
1. Background
2. Results
3. Discussion
4. Methods
4.1. Measuring AMR Bacteria Burden Generated by Travel
4.2. Global Travel Patterns
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendriksen, R.S.; Munk, P.; Njage, P.; van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.; Beggs, J.J. Socioeconomic Enablers for Contagion: Factors Impelling the Antimicrobial Resistance Epidemic. Antibiotics 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graham, D.W.; Collignon, P.; Davies, J.; Larsson, D.G.; Snape, J. Underappreciated role of regionally poor water quality on globally increasing antibiotic resistance. Environ. Sci. Technol. 2014, 48, 11746–11747. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.J.; McEwen, S.A. One Health-Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef]
- Fieldman, T.; Mossialos, E.; Anderson, M. Enhancing global insight into AMR spread and generation: Prospects and limitations of the WHO and quadripartite research agendas. J. Antimicrob. Chemother. 2024, 79, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Despotovic, M.; de Nies, L.; Busi, S.B.; Wilmes, P. Reservoirs of antimicrobial resistance in the context of One Health. Curr. Opin. Microbiol. 2023, 73, 102291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flach, C.F.; Kemper, M.; Marutescu, L.; Pircalabioru Gradisteanu, G.; Popa, M.; Spießberger, B.; Wengenroth, L.; Chifiriuc, M.C.; Larsson, D.G.J.; Nowak, D.; et al. International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli in a Large Sample of European Individuals-The AWARE Study. Int. J. Environ. Res. Public. Health 2022, 19, 4758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, K.; Collignon, P. Colonisation with Escherichia coli resistant to “critically important” antibiotics: A high risk for international travellers. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Stone, J.; Yakob, L.; Kirk, M.; Collignon, P.; Mills, D.J.; Lau, C.L. Risk factors for acquisition of multidrug-resistant Enterobacterales among international travellers: A synthesis of cumulative evidence. J. Travel. Med. 2020, 27, taz083. [Google Scholar] [CrossRef] [PubMed]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef]
- Campos-Madueno, E.I.; Aldeia, C.; Roumet, M.C.; Limacher, A.; Sendi, P.; Endimiani, A. Epidemiology and risk factors of expatriates returning to Switzerland colonized at the intestinal level with multidrug-resistant Enterobacterales. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haldorsen, B.C.; Samuelsen, Ø.; Janice, J.; Sare, M.; Molvik, M.; Sundsfjord, A.; The Norwegian Study Group On Cp-Pa; Pedersen, T. Import of global high-risk clones is the primary driver of carbapenemase-producing Pseudomonas aeruginosa in Norway. J. Med. Microbiol. 2025, 74, 001944. [Google Scholar] [CrossRef] [PubMed]
- Fifer, H.; Doumith, M.; Rubinstein, L.; Mitchell, L.; Wallis, M.; Singh, S.; Jagjit Singh, G.; Rayment, M.; Evans-Jones, J.; Blume, A.; et al. Ceftriaxone-resistant Neisseria gonorrhoeae detected in England, 2015–2024: An observational analysis. J. Antimicrob. Chemother. 2024, 79, 3332–3339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bokhary, H.; Pangesti, K.N.A.; Rashid, H.; Abd El Ghany, M.; Hill-Cawthorne, G.A. Travel-Related Antimicrobial Resistance: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- World Travel and Tourism Council. Economic Impact Research Annual Report. 2023. Available online: https://wttc.org/research/economic-impact (accessed on 15 October 2025).
- European Center for Disease Prevention and Control. Systematic Review of the Effectiveness of Infection Control Measures to Prevent the Transmission of Carbapenemase-Producing Enterobacteriaceae Through Cross-Border Transfer of Patients; ECDC: Stockholm, Sweden, 2014. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). Antimicrobial consumption and resistance in bacteria from humans and food-producing animals. Fourth joint inter-agency report on integrated analysis of antimicrobial agent consumption and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA. JIACRA IV—2019−2021. EFSA J. 2024, 22, e8589. [Google Scholar] [CrossRef]
- Jansen, W.; Müller, A.; Grabowski, N.T.; Kehrenberg, C.; Muylkens, B.; Al Dahouk, S. Foodborne diseases do not respect borders: Zoonotic pathogens and antimicrobial resistant bacteria in food products of animal origin illegally imported into the European Union. Vet. J. 2019, 244, 75–82. [Google Scholar] [CrossRef] [PubMed]
- CDC. Travelers’ Diarrhea. Available online: https://wwwnc.cdc.gov/travel/page/travelers-diarrhea (accessed on 15 October 2025).
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wailan, A.M.; Paterson, D.L. The spread and acquisition of NDM-1: A multifactorial problem. Expert. Rev. Anti Infect. Ther. 2014, 12, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roberts, M.J.; Bennett, H.Y.; Harris, P.N.; Holmes, M.; Grummet, J.; Naber, K.; Wagenlehner, F.M.E. Prostate Biopsy-related Infection: A Systematic Review of Risk Factors, Prevention Strategies, and Management Approaches. Urology 2017, 104, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Knaapila, J.; Kallio, H.; Hakanen, A.J.; Syvänen, K.; Ettala, O.; Kähkönen, E.; Lamminen, T.; Seppänen, M.; Jambor, I.; Rannikko, A.; et al. Antibiotic susceptibility of intestinal Escherichia coli in men undergoing transrectal prostate biopsies: A prospective, registered, multicentre study. BJU Int. 2018, 122, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Holmbom, M.; Forsberg, J.; Fredrikson, M.; Nilsson, M.; Nilsson, L.E.; Hanberger, H.; Hällgren, A. Fluoroquinolone-resistant Escherichia coli among the rectal flora is the predominant risk factor for severe infection after transrectal ultrasound-guided prostate biopsy: A prospective observational study. Scand. J. Urol. 2023, 58, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, T.; Kirveskari, J.; Johansson, S.; Väisänen, J.; Djupsjöbacka, A.; Nevalainen, A.; Kantele, A. Patients hospitalized abroad as importers of multiresistant bacteria-a cross-sectional study. Clin. Microbiol. Infect. 2017, 23, 673.e1–673.e8. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, M.C.; McEwen, S.A.; Pearl, D.L.; Lyytikäinen, O.; Jacobsson, G.; Collignon, P.; Gregson, D.B.; Valiquette, L.; Laupland, K.B. Mortality in Escherichia coli bloodstream infections: A multinational population-based cohort study. BMC Infect. Dis. 2021, 21, 606. [Google Scholar] [CrossRef]
- Bansal, N.; Sukhwani, K.S.; Ganta, V. Predictors of mortality in patients with Gram-Negative Bacilli (GNB) blood stream infections (BSI): Multicentre data from India. Infect. Dis. 2025, 57, 518–525. [Google Scholar] [CrossRef]
- Baquero, F.; Pérez-Cobas, A.E.; Aracil-Gisbert, S.; Coque, T.M.; Zamora, J. Selection versus transmission: Quantitative and organismic biology in antibiotic resistance. Infect. Genet. Evol. 2024, 121, 105606. [Google Scholar] [CrossRef] [PubMed]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; World Health Organisation: Geneva, Switzerland, 2021.
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- World Health Organization. Proportion of Bloodstream Infection Due to Escherichia coli Resistant to Third-Generation Cephalosporins. Appears in: SDG Target 3.d | National and Global Health Risks: Strengthen the Capacity of All Countries, in Particular Developing Countries, for Early Warning, Risk Reduction and Management of National and Global Health Risks. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/sdg-3.d.2--proportion-of-bloodstream-infections-due-to-selected-antimicrobial-resistant-organisms--median-(-) (accessed on 15 October 2025).
- Warda, Z.J.; Goldiea, S.J. Global Burden of Disease Study 2021 estimates: Implications for health policy and research. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Anderson, J.E. A theoretical foundation for the gravity equation. Am. Econ. Rev. 1979, 69, 106–116. [Google Scholar]
- Barrios, J.M.; Verstraeten, W.; Aerts, J.M.; Farifteh, J.; Coppin, P. Using the Gravity Model to Estimate the Spatial Spread of Vector-Borne Diseases. Int. J. Environ. Res. Public. Health 2012, 9, 4346–4364. [Google Scholar] [CrossRef]
- McKercher, B.; Chan, A.; Lam, C. The Impact of Distance on International Tourist Movements. J. Travel Res. 2008, 47, 208–224. [Google Scholar] [CrossRef]
- Urbonavicius, S.; Andruliene, R.; Adomaviciute, K.; Ozretic-Dosen, D. The role of travelling distances in tourism: Different planning motives. Bull. Geogr. Socio-Econ. Ser. 2023, 60, 145–156. [Google Scholar]
- Jørgensen, F.; Preston, J. The Relationship Between Fare and Travel Distance. J. Transp. Econ. Policy 2007, 41 Pt 3, 451–468. [Google Scholar]
- Nadal, J.R.; Gallego, M.S. Gravity models for tourism demand modeling: Empirical review and outlook. J. Econ. Surv. 2022, 36, 1358–1409. [Google Scholar] [CrossRef]
- Haversine Formula. Available online: https://en.wikipedia.org/wiki/Haversine_formula (accessed on 15 October 2025).
- Geographic Canonical Concepts. Available online: https://developers.google.com/public-data/docs/canonical/countries_csv (accessed on 15 October 2025).
- Zipf, G.K. The P1P2/D hypothesis: On the intercity movement of persons. Am. Sociol. Rev. 1946, 11, 677–686. [Google Scholar] [CrossRef]
- Tinbergen, J. Shaping the world economy: Suggestions for an International Economic Policy; The Twentieth Century Fund: New York, NY, USA, 1962. [Google Scholar]
- Anderson, J.E. The Gravity Model, Working Paper 16576; National Bureau of Economic Research: Cambridge, MA, USA, 2010; Available online: http://www.nber.org/papers/w16576 (accessed on 15 October 2025).
- Wilson, A.G. A statistical theory of spatial distribution models. Transp. Res. 1967, 1, 253–269. [Google Scholar] [CrossRef]
- Mathisen, T.A. Empirical Evidence on the Relationship between Fare and Travel Distance. Int. J. Transp. Econ. 2015, 42, 111–126. [Google Scholar]
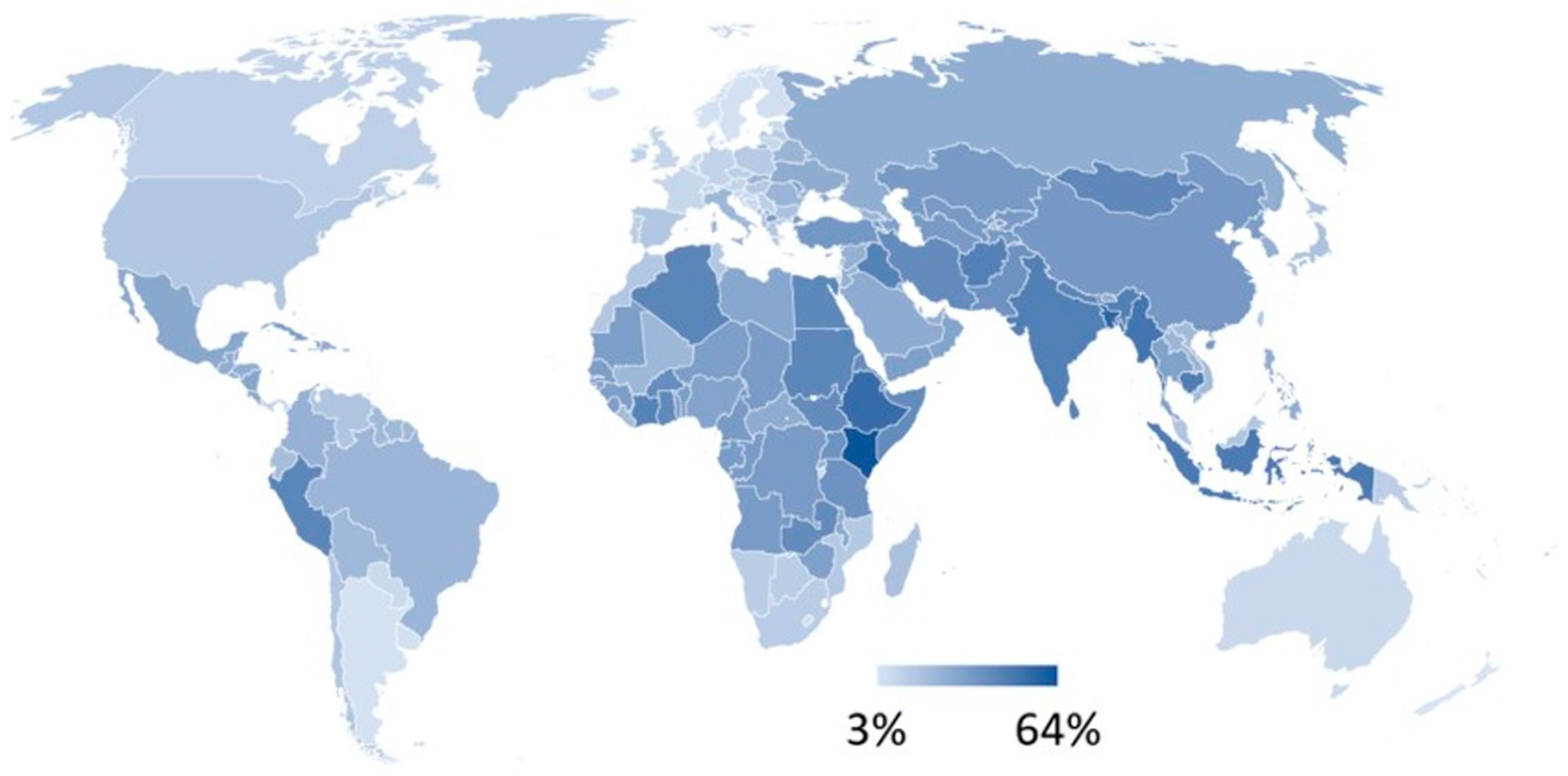
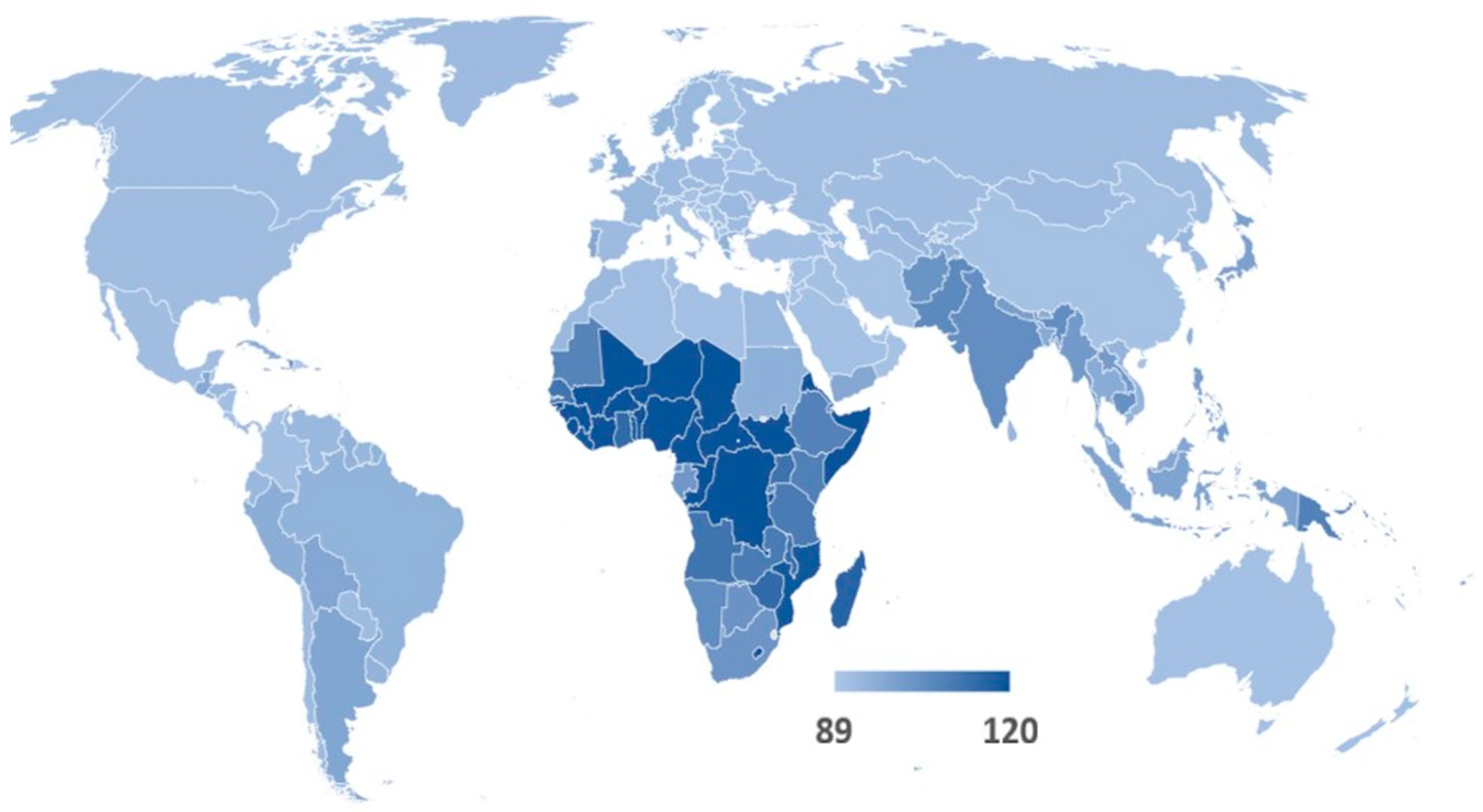
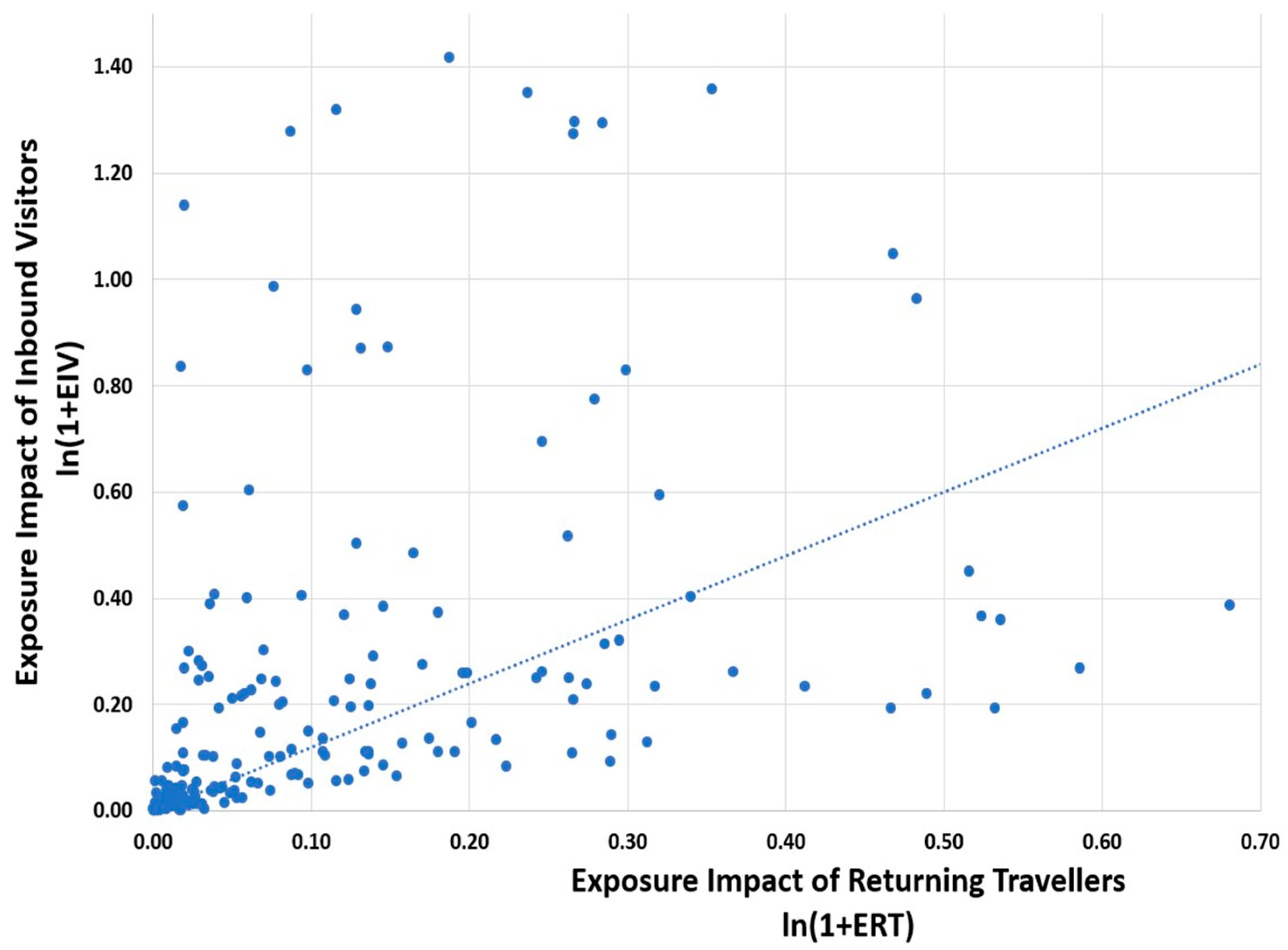
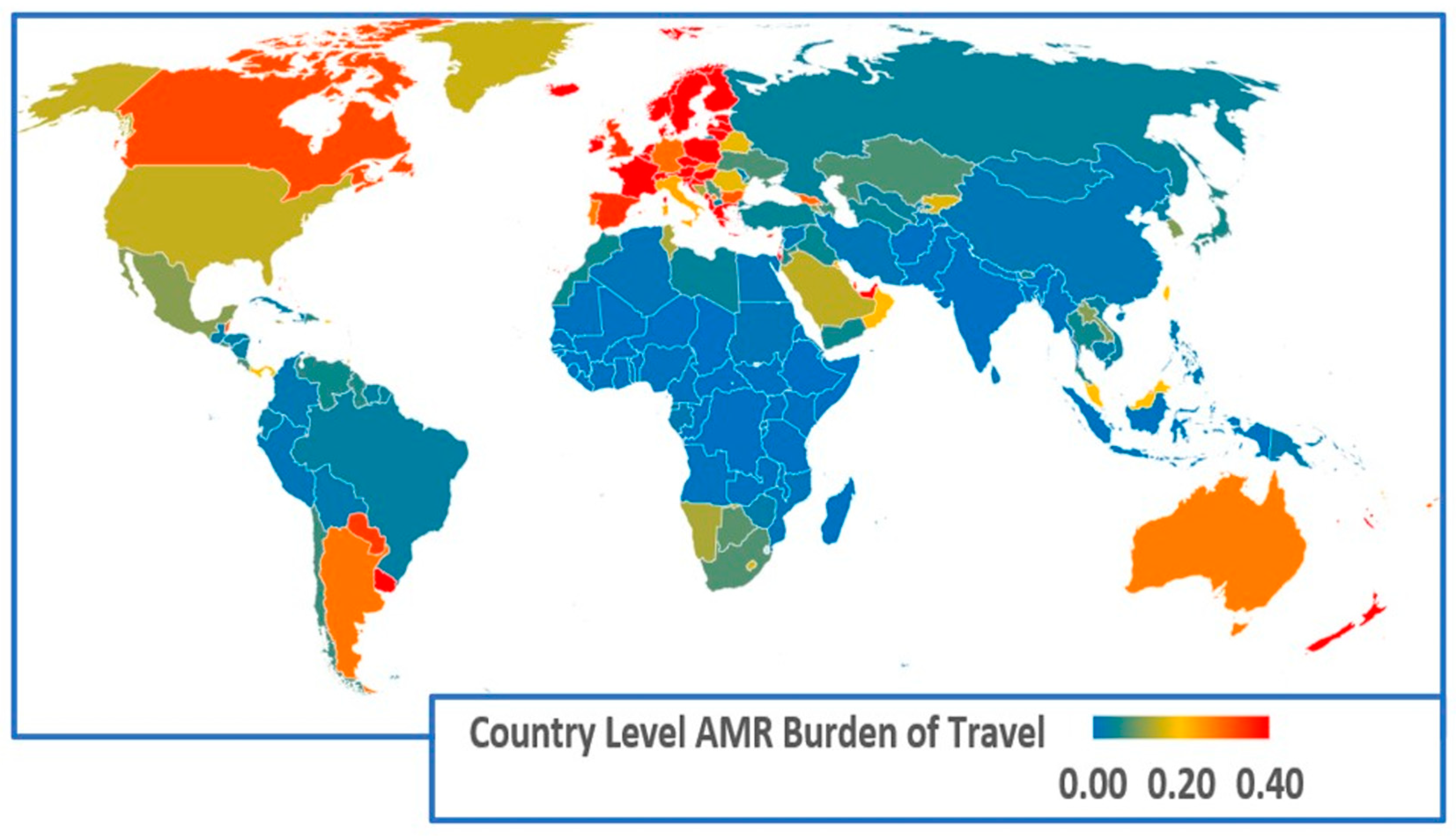
| Region | Regional Exposure Impact of Returning Travellers | Regional Exposure Impact of Visitors | Regional AMR Infection Burden of Travel |
|---|---|---|---|
| Caribbean | 0.05 | 0.42 | 0.45 |
| Central America | 0.03 | 0.07 | 0.11 |
| Central Asia | 0.03 | 0.04 | 0.07 |
| Eastern Africa | 0.01 | 0.02 | 0.03 |
| Eastern Asia | 0.27 | 0.32 | 0.48 |
| Eastern Europe | 0.11 | 0.19 | 0.27 |
| Middle Africa | 0.01 | 0.00 | 0.01 |
| Northern Africa | 0.01 | 0.03 | 0.04 |
| Northern America | 0.19 | 0.26 | 0.39 |
| Northern Europe | 0.36 | 0.42 | 0.68 |
| Oceania | 0.14 | 0.34 | 0.44 |
| South America | 0.06 | 0.04 | 0.10 |
| South-eastern Asia | 0.07 | 0.17 | 0.22 |
| Southern Africa | 0.08 | 0.10 | 0.17 |
| Southern Asia | 0.01 | 0.03 | 0.04 |
| Southern Europe | 0.14 | 0.58 | 0.67 |
| Western Africa | 0.00 | 0.01 | 0.01 |
| Western Asia | 0.11 | 0.11 | 0.20 |
| Western Europe | 0.37 | 0.35 | 0.62 |
| Country | Exposure Impact of Returning Travellers | Country | Exposure Impact of Inbound Visitors | Country | AMR Burden of Travel |
|---|---|---|---|---|---|
| SWEDEN | 0.59 | FRANCE | 0.50 | SWEDEN | 0.74 |
| BELGIUM | 0.31 | CZECH REPUBLIC | 0.40 | FRANCE | 0.58 |
| UNITED KINGDOM | 0.27 | GREECE | 0.37 | CZECH REPUBLIC | 0.47 |
| NETHERLANDS | 0.26 | SPAIN | 0.30 | NETHERLANDS | 0.46 |
| GERMANY | 0.22 | POLAND | 0.27 | GREECE | 0.45 |
| CANADA | 0.22 | SWEDEN | 0.27 | BELGIUM | 0.41 |
| ARGENTINA | 0.19 | NETHERLANDS | 0.25 | POLAND | 0.41 |
| AUSTRALIA | 0.18 | PORTUGAL | 0.22 | SPAIN | 0.35 |
| POLAND | 0.17 | MALAYSIA | 0.15 | UNITED KINGDOM | 0.35 |
| TAIWAN | 0.13 | ITALY | 0.14 | CANADA | 0.32 |
| FRANCE | 0.13 | CANADA | 0.13 | GERMANY | 0.29 |
| ROMANIA | 0.12 | BELGIUM | 0.13 | ARGENTINA | 0.28 |
| GREECE | 0.12 | AUSTRALIA | 0.11 | PORTUGAL | 0.28 |
| ITALY | 0.11 | ARGENTINA | 0.11 | AUSTRALIA | 0.27 |
| SAUDI ARABIA | 0.10 | UNITED KINGDOM | 0.11 | ITALY | 0.23 |
| CZECH REPUBLIC | 0.09 | TUNISIA | 0.10 | MALAYSIA | 0.21 |
| UNITED STATES | 0.09 | GERMANY | 0.08 | TAIWAN | 0.20 |
| SOUTH KOREA | 0.07 | TAIWAN | 0.07 | ROMANIA | 0.17 |
| SPAIN | 0.07 | UNITED STATES | 0.07 | UNITED STATES | 0.15 |
| MALAYSIA | 0.07 | ROMANIA | 0.06 | SAUDI ARABIA | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collignon, P.; Beggs, J.J. Tourism and the Global Vectoring of Antimicrobial-Resistant Disease: What Countries Are Most Impacted? Antibiotics 2025, 14, 1055. https://doi.org/10.3390/antibiotics14111055
Collignon P, Beggs JJ. Tourism and the Global Vectoring of Antimicrobial-Resistant Disease: What Countries Are Most Impacted? Antibiotics. 2025; 14(11):1055. https://doi.org/10.3390/antibiotics14111055
Chicago/Turabian StyleCollignon, Peter, and John J. Beggs. 2025. "Tourism and the Global Vectoring of Antimicrobial-Resistant Disease: What Countries Are Most Impacted?" Antibiotics 14, no. 11: 1055. https://doi.org/10.3390/antibiotics14111055
APA StyleCollignon, P., & Beggs, J. J. (2025). Tourism and the Global Vectoring of Antimicrobial-Resistant Disease: What Countries Are Most Impacted? Antibiotics, 14(11), 1055. https://doi.org/10.3390/antibiotics14111055






