Synergistic Antibacterial Activity of Azithromycin-Loaded Chitosan Nanoparticles Alone and in Combination with Cetirizine Dihydrochloride Against Resistant Isolates of Respiratory Tract Infections
Abstract
1. Introduction
2. Results
2.1. Distribution of Sputum Samples
2.2. Phenotypic and Genotypic Characterization of Klebsiella pneumoniae and MRSA
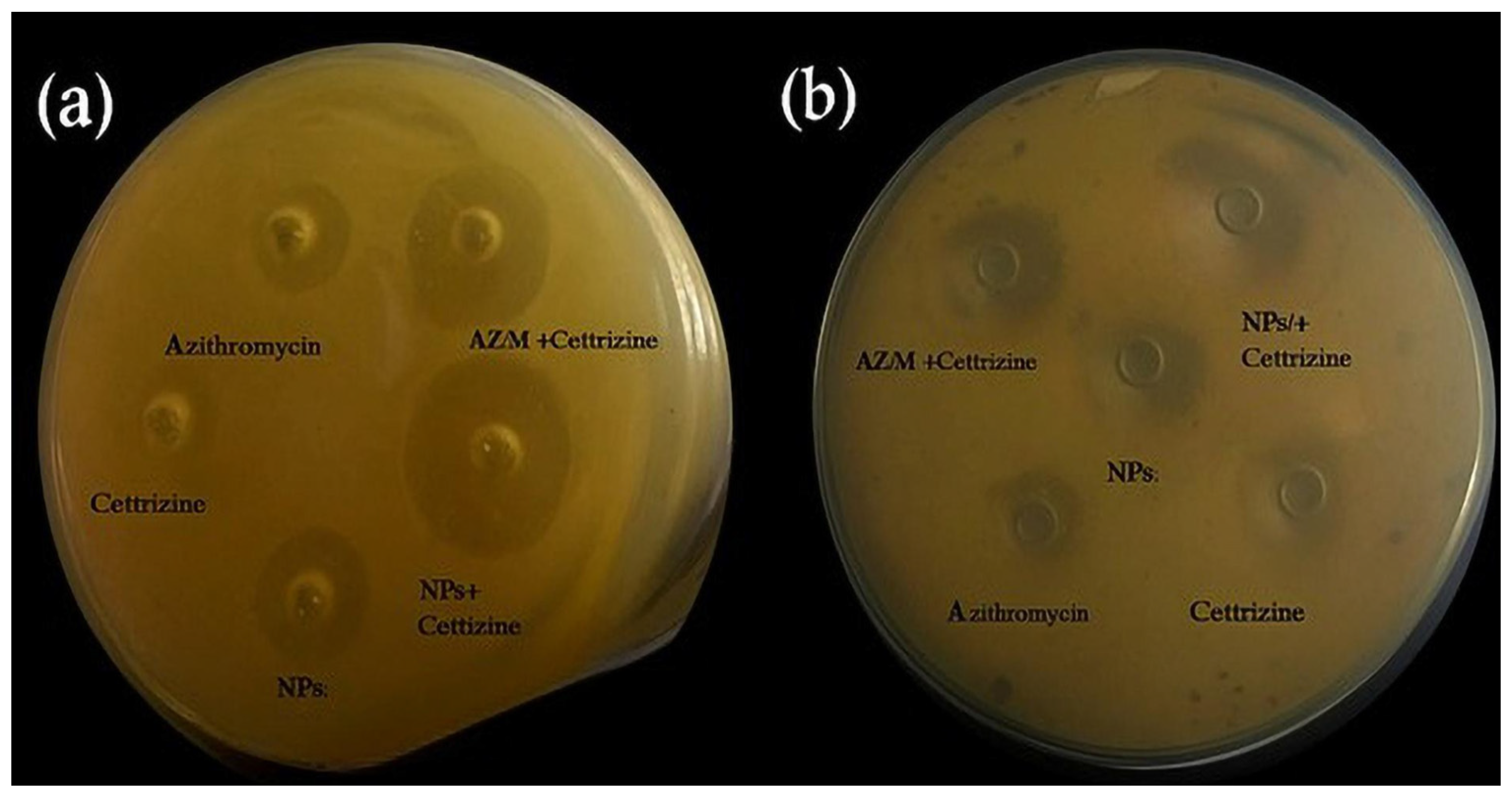
2.3. Antimicrobial Susceptibility Testing Using Agar Well Diffusion Method for MRSA
2.4. Zone of Inhibition by Agar Well Diffusion Method for Klebsiella pneumoniae
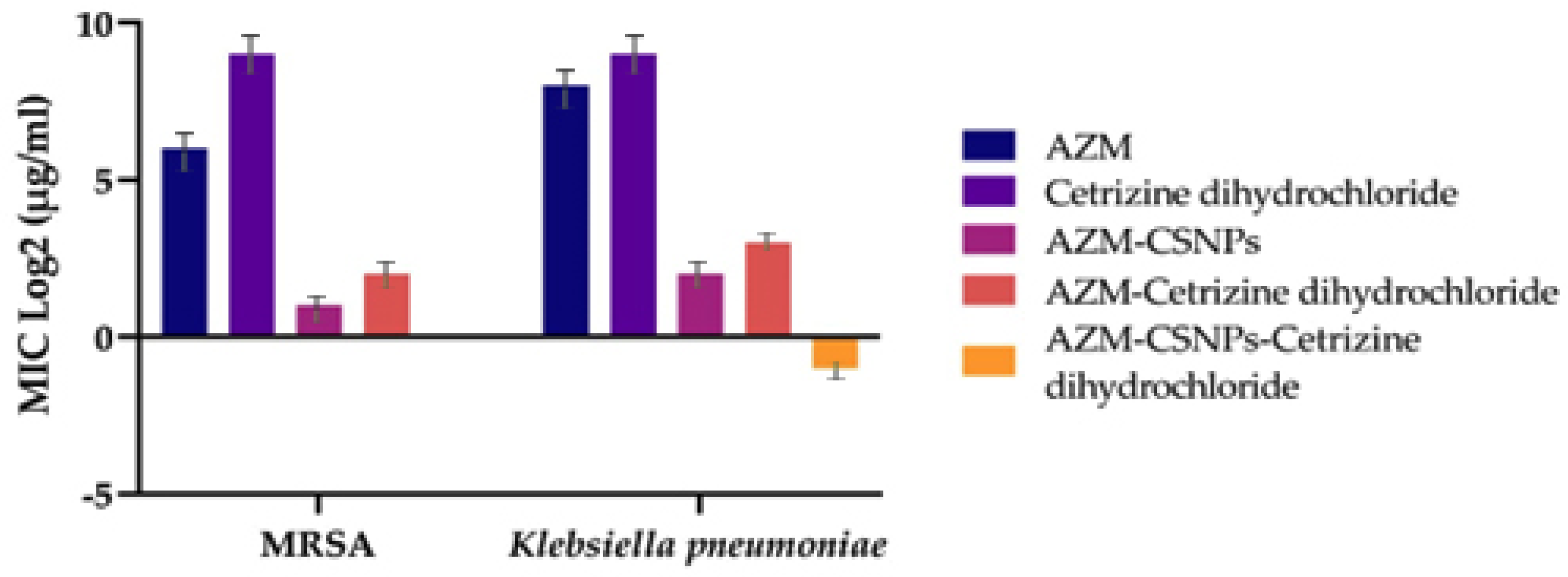
2.5. Micro Broth Dilution Method
2.6. Checkerboard Method
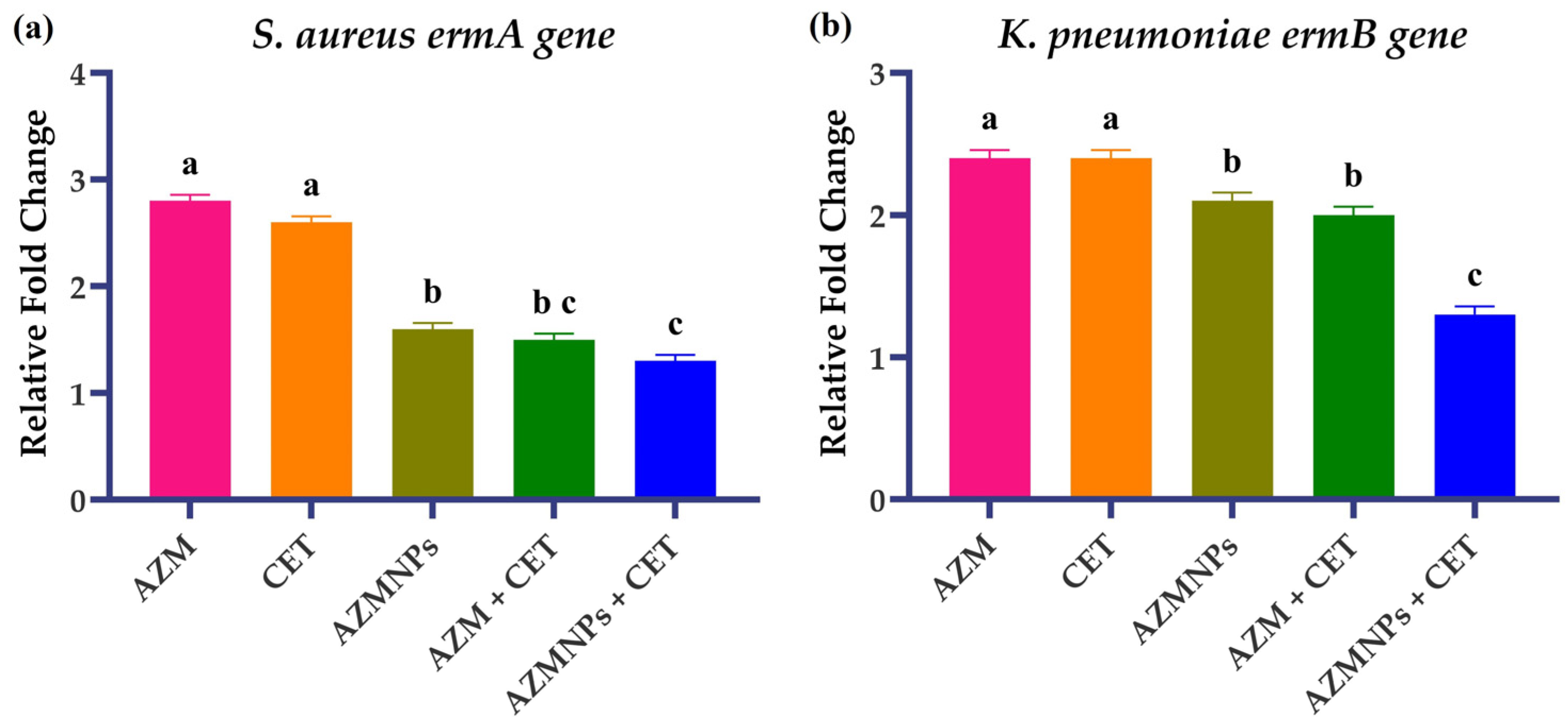
2.7. Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Collection of Nanoparticles
4.2. Sputum Samples Collection
4.3. Phenotypic and Biochemical Identification of Clinical Isolates
4.4. Genotypic Confirmation of Clinical Isolates
4.5. Antibiotic Susceptibility Testing
4.6. Evaluation of Antibacterial Activity by Agar Well Diffusion Method
4.7. Determination of Minimum Inhibitory Concentration by the Micro Broth Dilution Method
4.8. Determination of Synergistic Potential by Checkerboard Method
4.9. Identification of Gene Expression After Exposure to Various Drugs Alone and in Combination with Nanoparticles by qRT-PCR
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchan, B.W.; Windham, S.; Balada-Llasat, J.M.; Leber, A.; Harrington, A.; Relich, R.; Murphy, C.; Dien Bard, J.; Naccache, S.; Ronen, S.J. Practical comparison of the BioFire Film Array pneumonia panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. J. Clin. Microbiol. 2020, 58, e00135-00120. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C.J. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Hossain, M.; Jabin, N.; Ahmmed, F.; Sultana, A.; Abdur Rahman, S.; Islam, M. Irrational use of antibiotics and factors associated with antibiotic resistance: Findings from a cross-sectional study in Bangladesh. Health Sci. Rep. 2023, 6, e1465. [Google Scholar] [CrossRef]
- Sweileh, W. Global research publications on irrational use of antimicrobials: Call for more research to contain antimicrobial resistance. Glob. Health 2021, 17, 94. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Santella, B.; Serretiello, E.; De Filippis, A.; Folliero, V.; Iervolino, D.; Dell’Annunziata, F.; Manente, R.; Valitutti, F.; Santoro, E.; Pagliano, P. Lower Respiratory Tract Pathogens and Their Antimicrobial Susceptibility Pattern: A 5-Year Study. Antibiotics. J. Antibiot 2021, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. Chem. Med. Chem. 2021, 16, 65–80. [Google Scholar] [CrossRef]
- Miklasińska, M.J. Mechanisms of resistance to macrolide antibiotics among Staphylococcus aureus. J. Antibiot 2021, 10, 1406. [Google Scholar] [CrossRef]
- Pannewick, B.; Baier, C.; Schwab, F.; Vonberg, R. Infection control measures in nosocomial MRSA outbreaks—Results of a systematic analysis. PLoS ONE 2021, 16, e0249837. [Google Scholar] [CrossRef]
- Idrees, M.M.; Saeed, K.; Shahid, M.A.; Akhtar, M.; Qammar, K.; Hassan, J.; Khaliq, T.; Saeed, A.J. Prevalence of mecA-and mecC-Associated Methicillin-Resistant Staphylococcus aureus in Clinical Specimens, Punjab, Pakistan. Biomed 2023, 11, 878. [Google Scholar] [CrossRef]
- Ezeh, C.K. Antimicrobial resistance genes in Staphylococcus aureus isolates in Nigeria: A mini review. Eur. J. Microbiol. Infect. Dis. 2025, 2, 91–97. [Google Scholar] [CrossRef]
- González-Vázquez, R.; Córdova-Espinoza, M.G.; Escamilla-Gutiérrez, A.; Herrera-Cuevas, M.d.R.; González-Vázquez, R.; Esquivel-Campos, A.L.; López-Pelcastre, L.; Torres-Cubillas, W.; Mayorga-Reyes, L.; Mendoza-Pérez, F.; et al. Detection of mecA genes in hospital-acquired MRSA and Sosa strains associated with biofilm formation. Pathogens 2024, 13, 212. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Int. J. Antimicrob. Agents 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Asghari, B.; Goodarzi, R.; Mohammadi, M.; Nouri, F.; Taheri, M. Detection of mobile genetic elements in multidrug-resistant Klebsiella pneumoniae isolated from different infection sites in Hamadan, west of Iran. BMC research notes. Biomed 2021, 14, 1–6. [Google Scholar] [CrossRef]
- Hafiz, T.A.; Alanazi, S.; Alghamdi, S.S.; Mubaraki, M.A.; Aljabr, W.; Madkhali, N.; Alharbi, S.R.; Binkhamis, K.; Alotaibi, F. Klebsiella pneumoniae bacteraemia epidemiology: Resistance profiles and clinical outcome of King Fahad Medical City isolates, Riyadh, Saudi Arabia. BMC Infect. Dis. 2023, 23, 579. [Google Scholar] [CrossRef]
- Russo, A.; Fusco, P.; Morrone, H.L.; Trecarichi, E.M.; Torti, C. New advances in management and treatment of multidrug-resistant Klebsiella pneumoniae. Expert Rev. Anti-infect. Ther. 2023, 21, 41–55. [Google Scholar] [CrossRef]
- Ferreira, R.L.; da Silva, B.; Rezende, G.S.; Nakamura-Silva, R.; Pitondo-Silva, A. High prevalence of multidrug-resistant Klebsiella pneumoniae harboring several virulence and β-lactamase encoding genes in a Brazilian intensive care unit. Front. Microbol. 2019, 9, 3198. [Google Scholar] [CrossRef]
- Jabar, R.M.A.; Hassoon, A.H. The expression of efflux pump AcrAB in MDR Klebsiella pneumoniae isolated from Iraqi patients. JPSR 2019, 11, 423–428. [Google Scholar]
- Heidary, M.; Ebrahimi Samangani, A.; Kargari, A.; Kiani Nejad, A.; Yashmi, I.; Khoshnood, S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J. Clin. Lab. Anal. 2022, 36, e24427. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.K.; Lee, G.C.; Teng, C.; Frei, C.R. The in vitro activity of non-antibiotic drugs against S. aureus clinical strains. J. Glob. Antimicrob. Resist. 2021, 27, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Cederlund, H.; Mårdh, P.A. Antibacterial activities of non-antibiotic drugs. J. Antimicrob. Chemother. 1993, 32, 355–365. [Google Scholar] [CrossRef]
- Maji, H.; Maji, S.; Bhattacharya, M. An Exploratory Study on the Antimicrobial Activity of Cetirizine Dihydrochloride. Indian J. Pharm. Sci. 2017, 79, 751–757. [Google Scholar] [CrossRef]
- Lagadinou, M.; Onisor, M.O.; Rigas, A.; Musetescu, D.V.; Gkentzi, D.; Assimakopoulos, S.F.; Panos, G.; Marangos, M.J. Antimicrobial properties on non-antibiotic drugs in the era of increased bacterial resistance. J. Antibiot 2020, 9, 107. [Google Scholar] [CrossRef]
- Munir, T.; Mahmood, A.; Rasul, A.; Imran, M.; Fakhar-e-Alam, M. Biocompatible polymer functionalized magnetic nanoparticles for antimicrobial and anticancer activities. Mater. Chem. Phys 2023, 301, 127677. [Google Scholar] [CrossRef]
- Naskar, A.; Cho, H.; Lee, S.; Kim, K.S. Biomimetic nanoparticles coated with bacterial outer membrane vesicles as a new-generation platform for biomedical applications. Int. J. Pharm. 2021, 13, 1887. [Google Scholar] [CrossRef] [PubMed]
- Raouf, M.; Essa, S.; El Achy, S.; Essawy, M.; Rafik, S.; Baddour, M.J. Evaluation of Combined Ciprofloxacin and azithromycin free and nano formulations to control biofilm producing Pseudomonas aeruginosa isolated from burn wounds. Indian J. Med. Microbiol. 2021, 39, 81–87. [Google Scholar] [CrossRef] [PubMed]
- de Steenhuijsen Piters, W.A.; Binkowska, J.; Bogaert, D.J. Early life microbiota and respiratory tract infections. Cell Host Microbe 2020, 28, 223–232. [Google Scholar] [CrossRef]
- Zulfkar, Q.; Humaira, A.; Mohd, A.D.; Afshana, Q. The growing threat of antibiotic resistance: Mechanism, causes, consequences, and soilutions. Int. J. Cogn. Neurosci. Psychol. 2025, 3, 28–36. [Google Scholar]
- Muresu, N.; Deiana, G.; Dettori, M.; Palmieri, A.; Castiglia, P. Infection prevention control strategies of New Delhi Metallo-β-lactamase producing Klebsiella pneumoniae. Healthcare 2023, 11, 2592. [Google Scholar] [CrossRef]
- Almanaa, T.; Alyahya, S.; Khaled, J.; Shehu, M.; Alharbi, N.; Alzahrani, A.K. The extreme drug resistance (XDR) Staphylococcus aureus strains among patients: A retrospective study. Saudi J. Biol. Sci. 2020, 27, 1985–1992. [Google Scholar] [CrossRef]
- Priya, M.; Ramani, C.; Ravichandran, T. A cross-sectional study on clinical presentation with characteristics of etiological agents, including anti-microbial resistance in bacterial pneumonia. Int. J. Acad. Med. Pharm. 2024, 6, 1596–1605. [Google Scholar]
- Liu, X.; Yang, X.; Ye, L.; Chan, E.; Chen, S. Genetic characterization of a conjugative plasmid that encodes azithromycin resistance in Enterobacteriaceae. Microbol. Spectr. 2022, 10, e00788-22. [Google Scholar] [CrossRef] [PubMed]
- Kabeerdass, N.; Krishnamoorthy, S.; Anbazhagan, M.; Srinivasan, R.; Nachimuthu, S.; Rajendran, M.; Mathanmohun, M. Screening, detection and antimicrobial susceptibility of multi-drug resistant pathogens from the clinical specimens. Mater. Today 2021, 47, 461–467. [Google Scholar] [CrossRef]
- Yassin, A.; Albekairy, A.; Omer, M.; Almutairi, A.; Alotaibi, Y.; Althuwaini, S.; Halwani, M. Chitosan-coated azithromycin/ciprofloxacin-loaded polycaprolactone nanoparticles: A characterization and potency study. Nanotecnol. Sci. Appl. 2023, 16, 59–72. [Google Scholar] [CrossRef]
- Azhdarzadeh, M.; Lotfipour, F.; Zakeri-Milani, P.; Mohammadi, G.; Valizadeh, H. Anti-bacterial performance of azithromycin nanoparticles as colloidal drug delivery system against different gram-negative and gram-positive bacteria. Adv. Pharm. Bull. 2012, 2, 17. [Google Scholar]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fiber Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Farid, M.M.; Jaffar, M. Formulation and evaluation of ofloxacin loaded chitosan nanoparticles. WJPPS 2020, 10, 9. [Google Scholar]
- Ngan, L.T.; Wang, S.L.; Hiep, Đ.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Nat. Hum. Behav 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Patil, S.V.; Hajare, A.L.; Patankar, M.; Krishnaprasad, K.J. In vitro fractional inhibitory concentration (FIC) study of cefixime and azithromycin fixed dose combination (FDC) against respiratory clinical isolates. J. Clin. Diagn. Res. 2015, 9, DC13. [Google Scholar] [CrossRef]
- Brasil, M.S.; Filgueiras, A.L.; Campos, M.B.; Neves, M.S.; Eugênio, M.; Sena, L.A.; Sant’Anna, C.B.; Silva, V.L.; Diniz, C.G.; Sant’Ana, A.C. Synergism in the antibacterial action of ternary mixtures involving silver nanoparticles, chitosan and antibiotics. J. Braz. Chem. 2018, 29, 2026–2033. [Google Scholar] [CrossRef]
- Akram, F.E.; El-Tayeb, T.; Abou-Aisha, K.; El-Azizi, M.J. A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). WJPPS 2016, 15, 1–13. [Google Scholar] [CrossRef]
- Abo-Shama, U.H.; El-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F.; Abdeen, E.E.; Resistance, D. Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef]
- Singh, P.; Garg, A.; Pandit, S.; Mokkapati, V.; Mijakovic, I.J. Antimicrobial effects of biogenic nanoparticles. Nanomaterials 2018, 8, 1009. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Zhang, Y.; Li, Q.; Xie, X.; Zhao, D.; Tian, T.; Shi, S.; Meng, L.; Lin, Y.J. Tetrahedral framework nucleic acids loading ampicillin improve the drug susceptibility against methicillin-resistant Staphylococcus aureus. IE Interfaces 2020, 12, 36957–36966. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wei, C.; Zhou, C.; Zhao, J.; Liang, B.; Cui, J.; Wang, R.; Liu, Y.J. Tigecycline-amikacin combination effectively suppresses the selection of resistance in clinical isolates of KPC-producing Klebsiella pneumoniae. Front 2016, 7, 1304. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, T.; Sonbol, F.; El-Aziz, A.; Al-Fakharany, O. Modulation of antibiotic efficacy against Klebsiella pneumoniae by antihistaminic drugs. J. Med. Microb. Diagn. 2016, 5, 225. [Google Scholar] [CrossRef]
- Anwar, U.; Sattar, A.; Rasheed, M.; Shabbir, M.; Abbas, M. Preparation, characterization and toxicological evaluation of azithromycin-loaded chitosan nanoparticles alone and in combination with cetirizine dihydrochloride. AJAB 2025, 2025, 2024178. [Google Scholar]
- Khudhur, H.; Al-bassam, T.; Naser, S.; Kassim, N.; Alajeeli, F.; Salman, N.; Al-Jassani, M. Bacteriological analysis of respiratory tract infections: Identification and anti microbial sensitivity assessment. J. Exp. Zool. India 2025, 28, 1265. [Google Scholar]
- Singh, J.; Shah, A.; Parmar, H.; Duttaroy, B. Bacteriological Profile and Antibiotic Susceptibility Patterns in Lower Respiratory Tract Infection at a Tertiary Care Teaching Hospital. J. Med. Sci 2025, 5, 167–174. [Google Scholar]
- Archana, L.; Kumar, P.; Singh, A.; Kumar, M.; Abhishek, K. Comparative Assessment of Azithromycin and Erythromycin for Identifying Inducible Clindamycin resistance in Staphylococcus aureus. Cureus 2025, 17, e90166. [Google Scholar] [CrossRef]
- Kaabi, H.K.J.A.; AL-Yassari, A.K. 16SrRNA sequencing as tool for identification of Salmonella spp isolated from human diarrhea cases. J. Phys. Conf. Ser. 2019, 1294, 062041. [Google Scholar] [CrossRef]
- Chavez-Esquivel, G.; Cervantes-Cuevas, H.; Ybieta-Olvera, L.; Briones, M.C.; Acosta, D.; Cabello, J. Antimicrobial activity of graphite oxide doped with silver against Bacillus subtilis, Candida albicans, Escherichia coli, and Staphylococcus aureus by agar well diffusion test: Synthesis and characterization. Mater. Sci. Eng 2021, 123, 111934. [Google Scholar] [CrossRef]
- Dung, T.; Vinh, C.; Anh, P.; Linh, V.; Tuyen, H.; Tam, P. The bacterial etiology and antimicrobial susceptibility of lower respiratory tract infections in Vietnam. Ann. Clin. Microbiol. Antimicrob. 2025, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, J.; Yang, L.; Du, Y.; Zhu, Q.; Ma, C. Combination therapy strategies against multidrug resistant bacteria in vitro and in vivo. Lett. Appl. Microbiol. 2024, 77, ovae129. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, A.; Ashraf, M.; Omer, M.O.; Altaf, I. Synergistic antibacterial activity of carvacrol loaded chitosan nanoparticles with Topoisomerase inhibitors and genotoxicity evaluation. Saudi J. Biol. Sci. 2023, 30, 103765. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yun, S.; Nam, M.; Lee, C.; Cho, Y. Comparing sputum nasopharyngeal swabs combined samples for respiratory bacterial detection using multiplex, P.C.R. Microbiol. Spectr. 2025, 13, e02285-24. [Google Scholar] [CrossRef]
- Manna, S.; McAuley, J.; Jacobson, J.; Nguyen, C.D.; Ullah, M.A.; Sebina, I.; Satzke, C. Synergism and antagonism of bacterial-viral coinfection in the upper respiratory tract. Msphere 2022, 7, e00984-21. [Google Scholar] [CrossRef]

| Sr | Sensitivity | AZM | Cetirizine | AZM+ Cetirizine | AZM-CSNPs | AZM-CSNPs + Cetirizine | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | IQR | Mean | IQR | Mean | IQR | Mean | IQR | Mean | IQR | ||
| 1 | Susceptible | 0 | 0 (0–0) | 0 | 0 (0–0) | 14 | 14 (12–16) | 18 | 18 (16–20) | 28 | 28 (26–30) |
| 2 | Intermediately Susceptible | 6 | 6 (4–8) | 0 | 0 (0–0) | 11 | 11 (9–13) | 12 | 12 (10–14) | 4 | 4 (3–5) |
| 3 | Resistant | 26 | 26 (24–28) | 32 | 32 (30–34) | 7 | 7 (6–9) | 2 | 2 (1–3) | 0 | 0 (0–0) |
| Sr # | Sensitivity Pattern | Azithromycin | Cetirizine | Azithromycin + Cetirizine | Azithromycin NPs | Azithromycin NPs+ Cetirizine |
|---|---|---|---|---|---|---|
| 1 | Susceptible | 0 | 0 | 18 | 21 | 29 |
| 2 | Intermediately Susceptible | 0 | 0 | 0 | 8 | 0 |
| 3 | Resistant | 29 | 29 | 11 | 0 | 0 |
| MIC µg/mL (Combination) | MIC (A) | MIC (B) | FICI | Interpretation |
|---|---|---|---|---|
| 4 | 1024 | 512 | 0.011 | Synergism |
| 4 | 512 | 512 | 0.015 | Synergism |
| 4 | 256 | 512 | 0.023 | Synergism |
| 4 | 128 | 512 | 0.039 | Synergism |
| 4 | 64 | 512 | 0.070 | Synergism |
| MIC µg/mL (Combination) | MIC (A) | MIC (B) | FICI | Interpretation |
|---|---|---|---|---|
| 1 | 1024 | 512 | 0.0029 | Synergism |
| 1 | 512 | 512 | 0.0039 | Synergism |
| 1 | 256 | 512 | 0.0058 | Synergism |
| 1 | 128 | 512 | 0.0097 | Synergism |
| 1 | 64 | 512 | 0.0175 | Synergism |
| Sr # | MIC µg/mL (Combination) | MIC (A) | MIC (B) | FICI | Interpretation |
|---|---|---|---|---|---|
| 1 | 8 | 1024 | 512 | 0.0234 | Synergism |
| 2 | 8 | 512 | 512 | 0.0312 | Synergism |
| 3 | 8 | 256 | 512 | 0.0468 | Synergism |
| Sr # | MIC (µg/mL) Combination | MIC (A) | MIC (B) | FICI | Interpretation |
|---|---|---|---|---|---|
| 1 | 0.5 | 1024 | 512 | 0.0014 | Synergism |
| 2 | 0.5 | 512 | 512 | 0.0019 | Synergism |
| 3 | 0.5 | 256 | 512 | 0.0029 | Synergism |
| Target Gene | Initial Denaturation | Denaturation | Annealing | Extension | Final Extension |
|---|---|---|---|---|---|
| mecA | 94 °C/3 min | 94 °C/30 s | 54 °C/1 min | 72 °C/20 s | 72 °C/4 min |
| ermA | 94 °C/3 min | 94 °C/30 s | 56 °C/30 s | 72 °C/10 s | 72 °C/4 min |
| ermB | 95 °C/3 min | 95 °C/30 s | 57 °C/30 s | 72 °C/22 s | 78 °C/4 min |
| ermC | 95 °C/3 min | 95 °C/30 s | 58 °C/30 s | 72 °C/15 s | 77 °C/4 min |
| Target Genes | Primer Sequence | Amplicon Size |
|---|---|---|
| mecA (MRSA) | F-TCCAGATTACAACTTCACCAGG R-CCACTTCATATCTTGTAACG | 310 |
| ermA (MRSA) | F-TATCTTATCTTGAGAAGGGATT R-CTACACTTGGCTTAGGATGAAA | 139 |
| ermB (K. pneumoniae) | F-CCGTTTACGAAATTTGGAACAGGTAAAGGGC R-GAATCGAGACTTGAGTGTGC | 359 |
| ermC (K. pneumoniae) | F-ATCTTTGAAATCGGCTCAGG R-CAAACCCTCTATTTGGTGGT | 259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, U.; Sattar, A.; Rasheed, M.A.; Shabbir, M.A.B.; Abbas, M. Synergistic Antibacterial Activity of Azithromycin-Loaded Chitosan Nanoparticles Alone and in Combination with Cetirizine Dihydrochloride Against Resistant Isolates of Respiratory Tract Infections. Antibiotics 2025, 14, 992. https://doi.org/10.3390/antibiotics14100992
Anwar U, Sattar A, Rasheed MA, Shabbir MAB, Abbas M. Synergistic Antibacterial Activity of Azithromycin-Loaded Chitosan Nanoparticles Alone and in Combination with Cetirizine Dihydrochloride Against Resistant Isolates of Respiratory Tract Infections. Antibiotics. 2025; 14(10):992. https://doi.org/10.3390/antibiotics14100992
Chicago/Turabian StyleAnwar, Umbreen, Adeel Sattar, Muhammad Adil Rasheed, Muhammad Abu Bakr Shabbir, and Mateen Abbas. 2025. "Synergistic Antibacterial Activity of Azithromycin-Loaded Chitosan Nanoparticles Alone and in Combination with Cetirizine Dihydrochloride Against Resistant Isolates of Respiratory Tract Infections" Antibiotics 14, no. 10: 992. https://doi.org/10.3390/antibiotics14100992
APA StyleAnwar, U., Sattar, A., Rasheed, M. A., Shabbir, M. A. B., & Abbas, M. (2025). Synergistic Antibacterial Activity of Azithromycin-Loaded Chitosan Nanoparticles Alone and in Combination with Cetirizine Dihydrochloride Against Resistant Isolates of Respiratory Tract Infections. Antibiotics, 14(10), 992. https://doi.org/10.3390/antibiotics14100992








