Mechanism, Efficacy, and Safety of Natural Antibiotics
Abstract
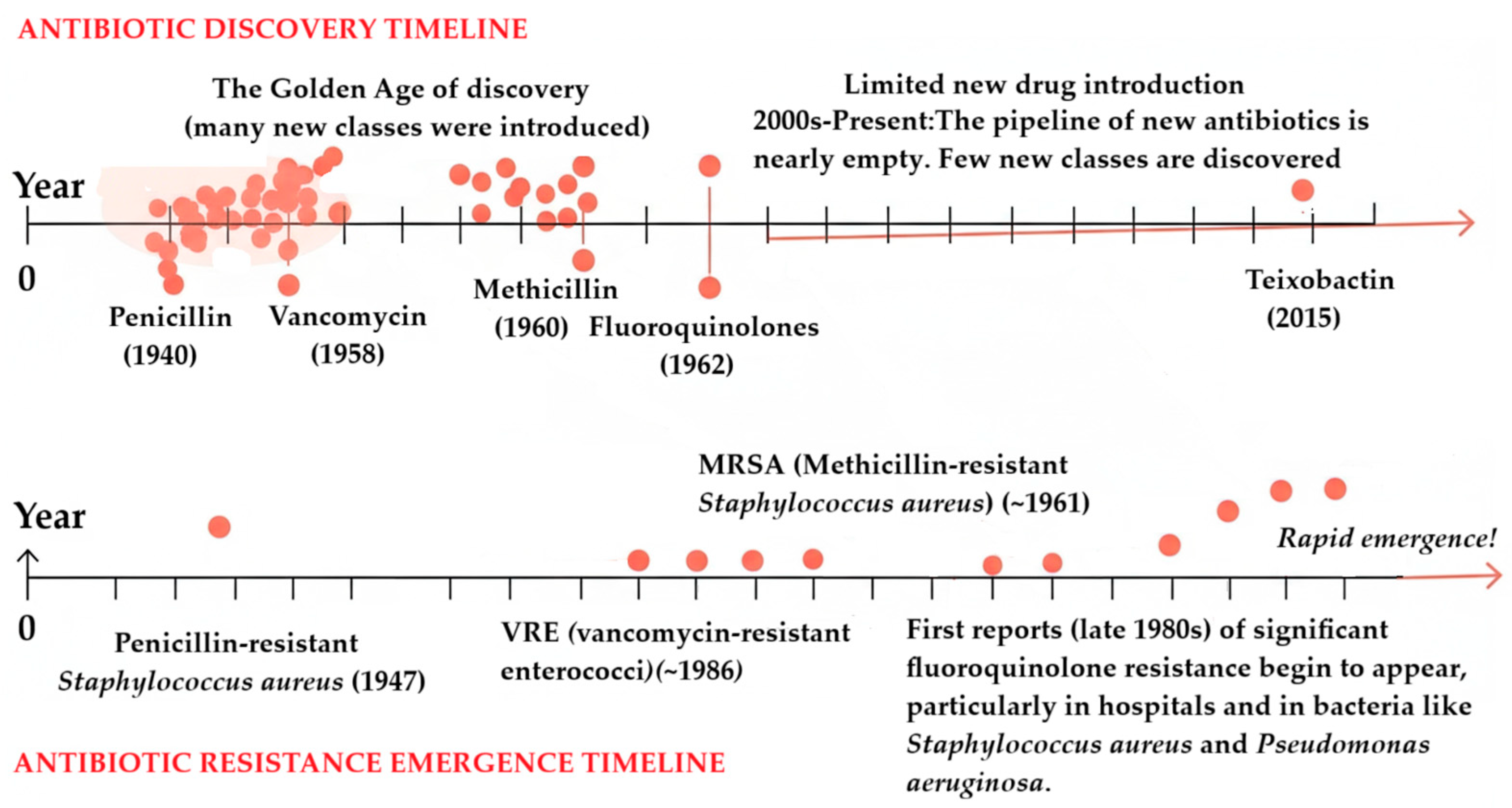
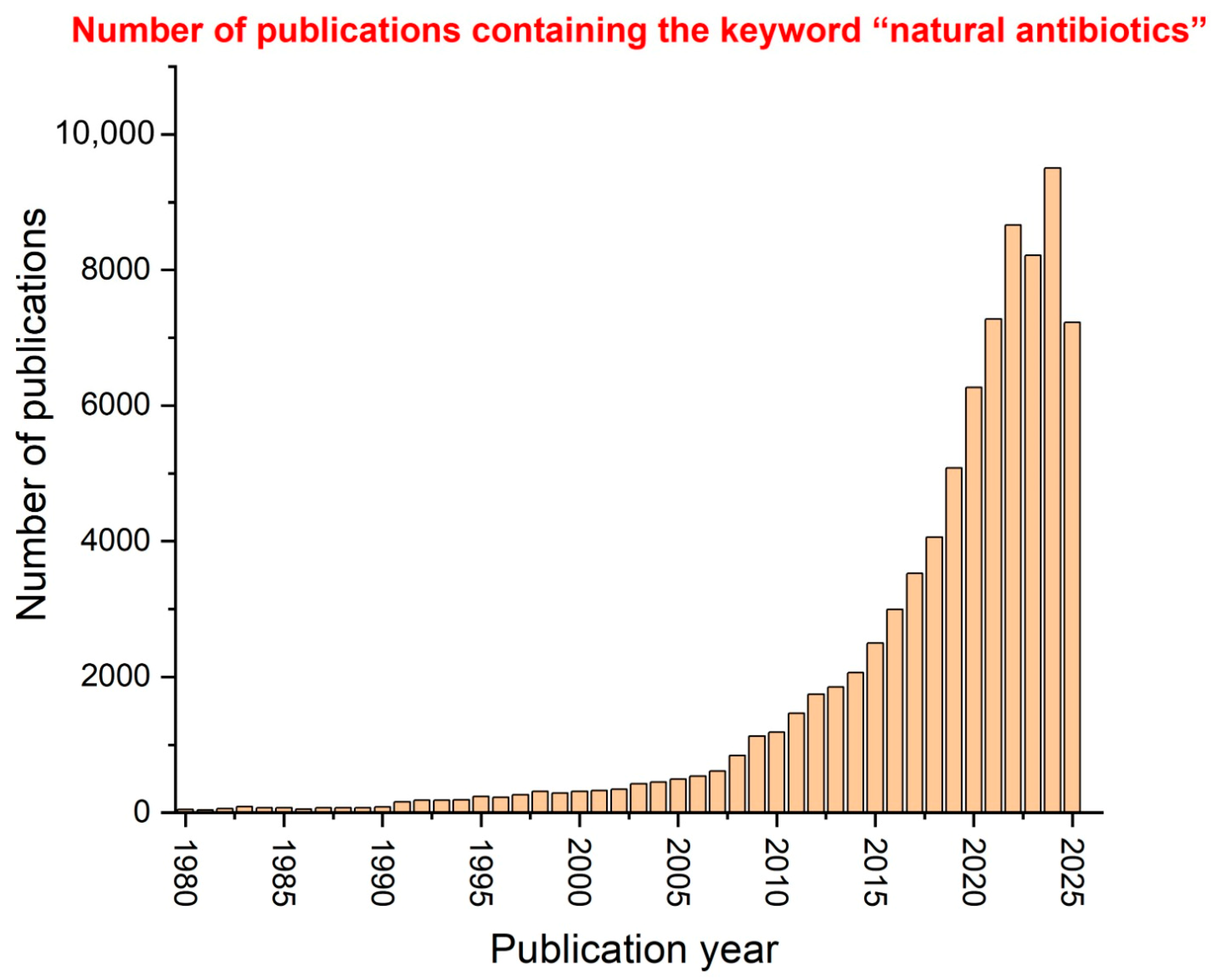
1. Introduction
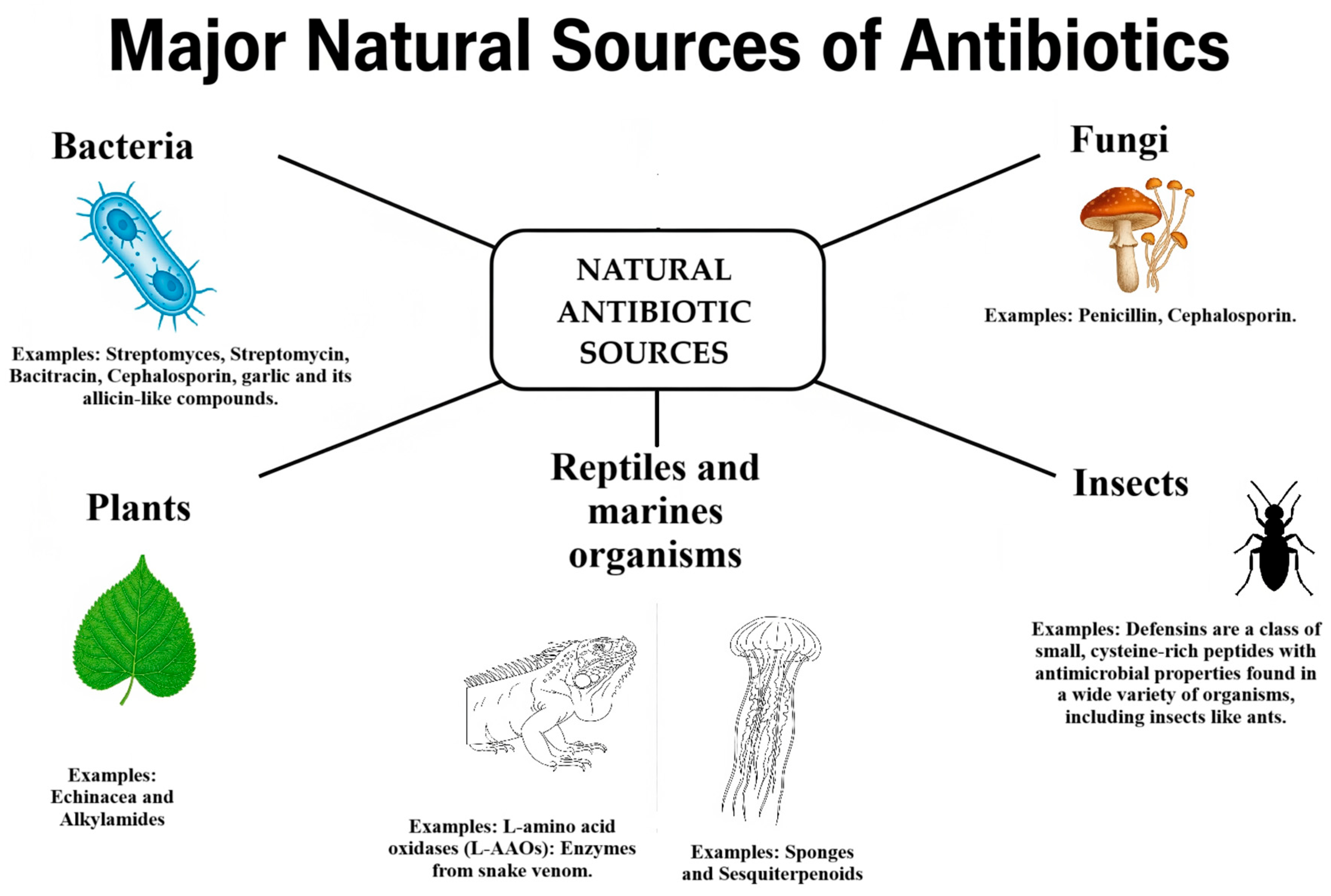
2. Natural Antibiotics Classification
2.1. Animal-Derived Antimicrobials
2.1.1. Insect-Derived Compounds
2.1.2. Bee Products
2.1.3. Reptile and Marine Animal Compounds
2.2. Bacterial Antimicrobials
2.3. Fungal Antimicrobials
2.4. Plant-Derived Antimicrobials
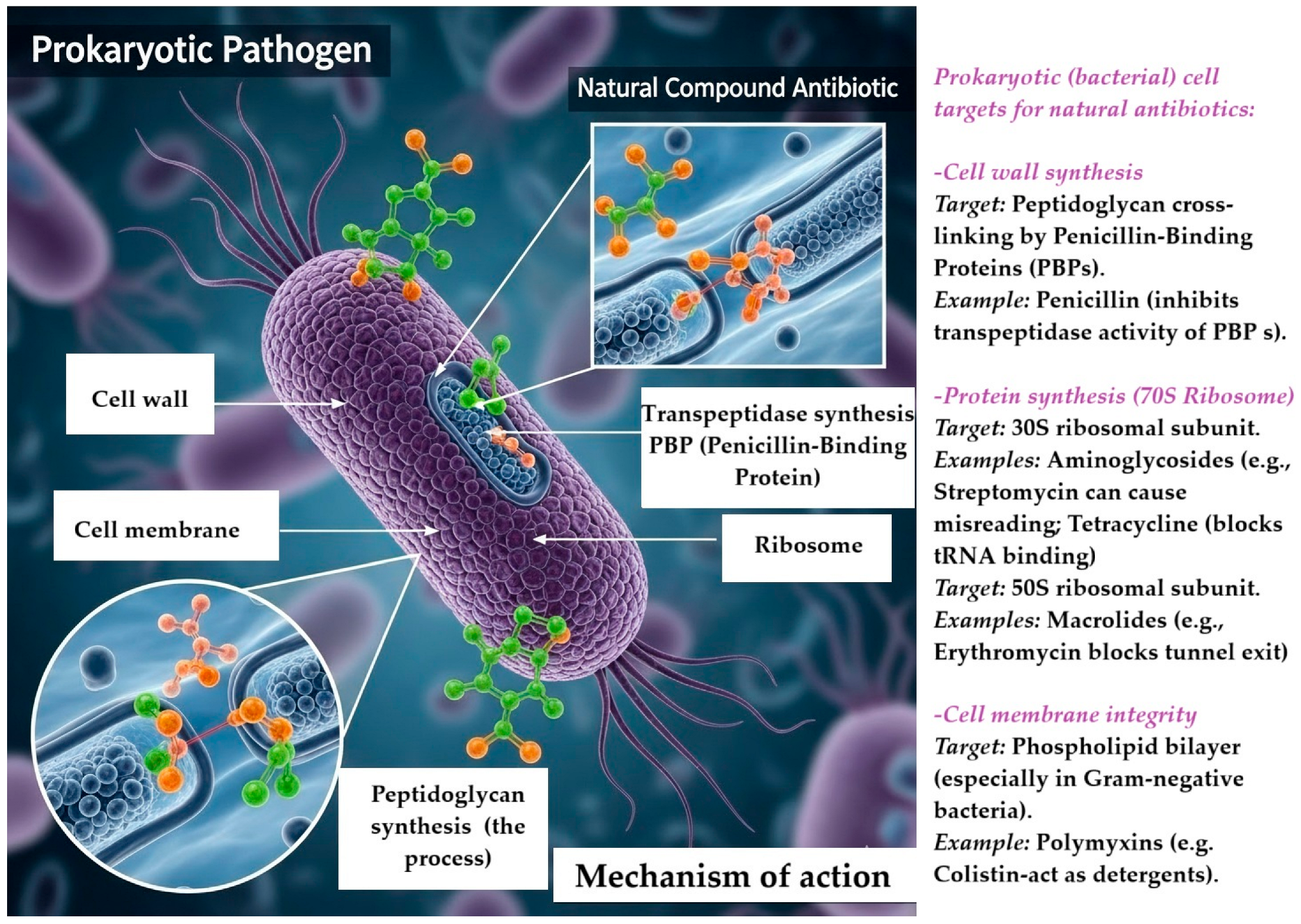
3. Mechanism of Action
Synergistic Strategies
4. Incorporation of Natural Antibiotics in Drug-Delivery Systems
- The Effect of Carrier Matrices on Nanoparticle Antimicrobial Activity
4.1. Nanoparticles
4.2. Hydrogels
4.3. Liposomes
4.4. Solid Lipid Nanoparticles (SLNs)
5. Safety, Efficacy, and Toxicity of Natural Antibiotics
6. Current Limitations and Challenges
The Clinical Trial Pipeline
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| WHO | World Health Organization |
| MDR | multidrug-resistant |
| ESKAPE pathogens | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| AMPs | antimicrobial peptides |
| 3D | three-dimensional |
| NPs | nanoparticles |
| MRSA | methicillin-resistant Staphylococcus aureus |
| H2O2 | hydrogen peroxide |
| 10-HDA | 10-hydroxy-2-decenoic acid |
| CaTx-II | Crotalus adamanteus toxin-II |
| MIC | minimum inhibitory concentrations |
| MBC | minimum bactericidal concentrations |
| VRE | Vancomycin-resistant Enterococci |
| MTN | mesoporous titanium nanoparticles |
| PEI | ethylene imine polymer |
| BH | berberine hydrochloride |
| BER | berberine chloride |
| GBM | glioblastoma |
| PLGA | poly lactic-co-glycolic acid |
| ALG | blending alginate |
| MC | methylcellulose |
| LAP | laponite |
| Gen | gentamicin |
| PVA | Polyvinyl alcohol |
| MVLs | multivesicular liposomes |
| VAN HL | vancomycin hydrochloride |
| SLNs | solid lipid nanoparticles |
| NRG | naringenin |
| PM | paromomycin ulphate |
| VM-FB | vancomycin base |
| MSSA | methicillin-sensitive Staphylococcus aureus |
| PTA | target attainment |
| PK | pharmacokinetic |
| PD | pharmacodynamic |
| OHA-PBA | acid-grafted oxidized hyaluronic acid |
| SpsB | signal peptidase type IB |
References
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Price, R. O’Neill Report on Antimicrobial Resistance: Funding for Antimicrobial Specialists Should Be Improved. Eur. J. Hosp. Pharm. 2016, 23, 245–247. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016; 80p. [Google Scholar]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- MacLean, R.C.; San Millan, A. The Evolution of Antibiotic Resistance. Science 2019, 365, 1082–1083. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Durão, P.; Balbontín, R.; Gordo, I. Evolutionary Mechanisms Shaping the Maintenance of Antibiotic Resistance. Trends Microbiol. 2018, 26, 677–691. [Google Scholar] [CrossRef]
- Sultan, I.; Rahman, S.; Jan, A.T.; Siddiqui, M.T.; Mondal, A.H.; Haq, Q.M.R. Antibiotics, Resistome and Resistance Mechanisms: A Bacterial Perspective. Front. Microbiol. 2018, 9, 2066. [Google Scholar] [CrossRef]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Lin, J.; Nishino, K.; Roberts, M.C.; Tolmasky, M.; Aminov, R.I.; Zhang, L. Mechanisms of Antibiotic Resistance. Front. Microbiol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic Action and Resistance: Updated Review of Mechanisms, Spread, Influencing Factors, and Alternative Approaches for Combating Resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Shady, N.H.; Abdeljawad, K.M.; Elzamkan, M.B.; Helmy, H.H.; Tarshan, E.A.; Adly, A.N.; Hussien, Y.H.; Sayed, N.G.; Zayed, A.; et al. Antimicrobial Potentials of Natural Products against Multidrug Resistance Pathogens: A Comprehensive Review. RSC Adv. 2022, 12, 29078–29102. [Google Scholar] [CrossRef]
- Gajic, I.; Kekic, D.; Jankovic, M.; Tomic, N.; Skoric, M.; Petrovic, M.; Mitic Culafic, D.; Opavski, N.; Ristivojevic, P.; Krstic Ristivojevic, M.; et al. Nature’s Arsenal: Uncovering Antibacterial Agents Against Antimicrobial Resistance. Antibiotics 2025, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms To Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef] [PubMed]
- Ankri, S.; Mirelman, D. Antimicrobial Properties of Allicin from Garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A Systematic Review of the Anticancer Properties of Berberine, a Natural Product from Chinese Herbs. Anticancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Mao, C.; Jin, L.; Wu, S.; Zheng, Y.; Cui, Z.; Li, Z.; Zhang, Y.; Zhu, S.; et al. Natural Extracts for Antibacterial Applications. Small 2024, 20, 2306553. [Google Scholar] [CrossRef] [PubMed]
- Unnam, S.; Gayasuddin Mouid, M.; Thota, R.D.; Bantaram, J.; Sulthana, N.; Pilli, G.D.; Gudise, V. Natural Products as Antimicrobials: An Exploratory Overview of Current Research and Future Perspectives. J. Appl. Pharm. Sci. 2025, 15, 048–066. [Google Scholar] [CrossRef]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas Systems for the Detection and Control of Antibiotic-Resistant Infections. J. Nanobiotechnol. 2021, 19, 401. [Google Scholar] [CrossRef]
- Sahana, S.; Sarkar, J.; Mandal, S.; Chatterjee, I.; Dhar, S.; Datta, S.; Mondal, S. Multi-Omics Approaches: Transforming the Landscape of Natural Product Isolation. Funct. Integr. Genom. 2025, 25, 132. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from January 1981 to September 2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Buchmann, K. Evolution of Innate Immunity: Clues from Invertebrates via Fish to Mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef]
- Izadpanah, A.; Gallo, R.L. Antimicrobial Peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Bahar, A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- Vilcinskas, A. Yellow Biotechnology I: Insect Biotechnologie in Drug Discovery and Preclinical Research; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 135, ISBN 978-3-642-39862-9. [Google Scholar]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial Peptides (AMPs): Ancient Compounds That Represent Novel Weapons in the Fight against Bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Buonocore, F.; Fausto, A.M.; Della Pelle, G.; Roncevic, T.; Gerdol, M.; Picchietti, S. Attacins: A Promising Class of Insect Antimicrobial Peptides. Antibiotics 2021, 10, 212. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H.G. Sequence and Specificity of Two Antibacterial Proteins Involved in Insect Immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Hetru, C. Insect Defensins: Inducible Antibacterial Peptides. Immunol. Today 1992, 13, 411–415. [Google Scholar] [CrossRef]
- Vitali, A. Proline-Rich Peptides: Multifunctional Bioactive Molecules as New Potential Therapeutic Drugs. Curr. Protein Pept. Sci. 2015, 16, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Bang, K.; Park, S.; Yoo, J.Y.; Cho, S. Characterization and Expression of Attacin, an Antibacterial Protein-Encoding Gene, from the Beet Armyworm, Spodoptera Exigua (Hübner) (Insecta: Lepidoptera: Noctuidae). Mol. Biol. Rep. 2012, 39, 5151–5159. [Google Scholar] [CrossRef]
- Raveendran, S.; Chauhan, N.; Palaninathan, V.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Extremophilic Polysaccharide for Biosynthesis and Passivation of Gold Nanoparticles and Photothermal Ablation of Cancer Cells. Part. Part. Syst. Charact. 2015, 32, 54–64. [Google Scholar] [CrossRef]
- Fakoorziba, M.R.; Eghbal, F.; Hassanzadeh, J.; Moemenbellah-Fard, M.D. Cockroaches (Periplaneta americana and Blattella germanica) as Potential Vectors of the Pathogenic Bacteria Found in Nosocomial Infections. Ann. Trop. Med. Parasitol. 2010, 104, 521–528. [Google Scholar] [CrossRef]
- Ravi, J.; Renuka, D. Antimicrobial Peptides from Insects: An Overview. Res. Biotechnol. 2011, 2, 1–7. [Google Scholar]
- Shamma, M. The Isoquinoline Alkaloids: Chemistry and Pharmacology; Organic Chemistry; A Series of Monographs; Academic Press: New York, NY, USA, 1972; ISBN 978-0-12-638250-1. [Google Scholar]
- Najmanová, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The Pharmacokinetics of Flavanones. Crit. Rev. Food Sci. Nutr. 2020, 60, 3155–3171. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E. Sulfonamide Antibiotics. Prim. Care Update Ob/Gyns 1998, 5, 32–35. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Arora, S.; Sing, L.C.; Baptista, C. Antibacterial Activity of Lucilia Cuprina Maggot Extracts and Its Extraction Techniques. Int. J. Integr. Biol. 2010, 9, 43–48. [Google Scholar]
- Chernysh, S.I.; Gordja, N.A. The Immune System of Maggots of the Blow Fly (Calliphora vicina) as a Source of Medicinal Drugs. J. Evol. Biochem. Physiol. 2011, 47, 524–533. [Google Scholar] [CrossRef]
- Preußer, D.; Fischer, T.; Juretzek, T. Effects of Antibiotics Ceftriaxone and Levofloxacin on the Growth of Protophormia terraenovae (Diptera: Calliphoridae). Forensic Sci. Med. Pathol. 2024, 20, 1318–1330. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Lupia, C.; Poerio, G.; Liguori, G.; Lombardi, R.; Naturale, M.D.; Bulotta, R.M.; Biondi, V.; Passantino, A.; et al. Hive Products: Composition, Pharmacological Properties, and Therapeutic Applications. Pharmaceuticals 2024, 17, 646. [Google Scholar] [CrossRef]
- Brudzynski, K. A Current Perspective on Hydrogen Peroxide Production in Honey. A Review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-Derived Antibacterial Peptide, Defensin-1, Promotes Wound Re-Epithelialisation In Vitro and In Vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef]
- Taïbi, N.; Ameraoui, R.; Kaced, A.; Abou-Mustapha, M.; Bouchama, A.; Djafri, A.; Taïbi, A.; Mellahi, K.; Hadjadj, M.; Touati, S.; et al. Multifloral White Honey Outclasses Manuka Honey in Methylglyoxal Content: Assessment of Free and Encapsulated Methylglyoxal and Anti-Microbial Peptides in Liposomal Formulation against Toxigenic Potential of Bacillus subtilis Subsp spizizenii Strain. Food Funct. 2022, 13, 7591–7613. [Google Scholar] [CrossRef]
- Bava, R.; Puteo, C.; Lombardi, R.; Garcea, G.; Lupia, C.; Spano, A.; Liguori, G.; Palma, E.; Britti, D.; Castagna, F. Antimicrobial Properties of Hive Products and Their Potential Applications in Human and Veterinary Medicine. Antibiotics 2025, 14, 172. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Lima, W.G.; Brito, J.C.M.; Verly, R.M.; Lima, M.E.D. Jelleine, a Family of Peptides Isolated from the Royal Jelly of the Honey Bees (Apis mellifera), as a Promising Prototype for New Medicines: A Narrative Review. Toxins 2024, 16, 24. [Google Scholar] [CrossRef]
- İzol, E.; Yapici, İ.; Gülçin, I. 10-Hydroxy-2-Decenoic Acid (10-Hda) and Bioactive Components in Royal Jelly. In Biological Activities of Honeybee Products; Orient Publications: Delhi, India, 2023; pp. 1–9. ISBN 978-625-6893-17-7. [Google Scholar]
- Ratajczak, M.; Kaminska, D.; Matuszewska, E.; Hołderna-Kedzia, E.; Rogacki, J.; Matysiak, J. Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products. Molecules 2021, 26, 4007. [Google Scholar] [CrossRef]
- Sagheer, M.; Siddiqui, R.; Iqbal, J.; Khan, N.A. Black Cobra (Naja naja karachiensis) Lysates Exhibit Broad-Spectrum Antimicrobial Activities. Pathog. Glob. Health 2014, 108, 129–136. [Google Scholar] [CrossRef]
- Rangsipanuratn, W.; Sandee, A.; Daduang, J.; Janwithayanuchit, I. Antibacterial Activity of Snake Venoms against Bacterial Clinical Isolates. Pharm. Sci. Asia 2019, 46, 80–87. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Karimi, A.; Tohidpour, A.; Abbasi, N.; Fallah, F.; Akhavan, M.M. The Antimicrobial Potential of a New Derivative of Cathelicidin from Bungarus Fasciatus against Methicillin-Resistant Staphylococcus Aureus. J. Microbiol. 2018, 56, 128–137. [Google Scholar] [CrossRef]
- Samy, R.P.; Kandasamy, M.; Gopalakrishnakone, P.; Stiles, B.G.; Rowan, E.G.; Becker, D.; Shanmugam, M.K.; Sethi, G.; Chow, V.T.K. Wound Healing Activity and Mechanisms of Action of an Antibacterial Protein from the Venom of the Eastern Diamondback Rattlesnake (Crotalus adamanteus). PLoS ONE 2014, 9, e80199. [Google Scholar] [CrossRef]
- Chen, C.; Liang, C.-S.; Wang, T.; Shen, J.-L.; Ling, F.; Jiang, H.-F.; Li, P.-F.; Wang, G.-X. Antiviral, Antioxidant, and Anti-Inflammatory Activities of Rhein against White Spot Syndrome Virus Infection in Red Swamp Crayfish (Procambarus clarkii). Microbiol. Spectr. 2023, 11, e01047-23. [Google Scholar] [CrossRef]
- Philpott, D.J.; Edgeworth, J.D.; Sansonetti, P.J. The Pathogenesis of Shigella flexneri Infection: Lessons from In Vitro and In Vivo Studies. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 575–586. [Google Scholar] [CrossRef]
- Sun, C.; Xu, W.-T.; Zhang, H.-W.; Dong, L.-P.; Zhang, T.; Zhao, X.-F.; Wang, J.-X. An Anti-Lipopolysaccharide Factor from Red Swamp Crayfish, Procambarus Clarkii, Exhibited Antimicrobial Activities In Vitro and In Vivo. Fish Shellfish Immunol. 2011, 30, 295–303. [Google Scholar] [CrossRef]
- Mendes, L.C.; Viana, G.M.M.; Nencioni, A.L.A.; Pimenta, D.C.; Beraldo-Neto, E. Scorpion Peptides and Ion Channels: An Insightful Review of Mechanisms and Drug Development. Toxins 2023, 15, 238. [Google Scholar] [CrossRef]
- Atakuziev, B.U.; Wright, C.E.; Graudins, A.; Nicholson, G.M.; Winkel, K.D. Efficacy of Australian Red-Back Spider (Latrodectus Hasselti) Antivenom in the Treatment of Clinical Envenomation by the Cupboard Spider Steatoda Capensis (Theridiidae). Toxicon 2014, 86, 68–78. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The Immunology of Host Defence Peptides: Beyond Antimicrobial Activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing Antimicrobial Peptides: Form Follows Function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- De Simeis, D.; Serra, S. Actinomycetes: A Never-Ending Source of Bioactive Compounds—An Overview on Antibiotics Production. Antibiotics 2021, 10, 483. [Google Scholar] [CrossRef]
- Jakubiec-Krzesniak, K.; Rajnisz-Mateusiak, A.; Guspiel, A.; Ziemska, J.; Solecka, J. Secondary Metabolites of Actinomycetes and Their Antibacterial, Antifungal and Antiviral Properties. Pol. J. Microbiol. 2018, 67, 259–272. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R. Developing a New Resource for Drug Discovery: Marine Actinomycete Bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef]
- Butler, M.S.; Hansford, K.A.; Blaskovich, M.A.T.; Halai, R.; Cooper, M.A. Glycopeptide Antibiotics: Back to the Future. J. Antibiot. 2014, 67, 631–644. [Google Scholar] [CrossRef]
- Levine, D.P. Vancomycin: A History. Clin. Infect. Dis. 2006, 42, S5–S12. [Google Scholar] [CrossRef]
- Kim, N.; Sengupta, S.; Lee, J.; Dash, U.; Kim, S.; Kim, H.J.; Song, C.; Sim, T. Synthesis and Antibacterial Activities of Baulamycin A Inspired Derivatives. Eur. J. Med. Chem. 2023, 259, 115592. [Google Scholar] [CrossRef]
- Tripathi, A.; Schofield, M.M.; Chlipala, G.E.; Schultz, P.J.; Yim, I.; Newmister, S.A.; Nusca, T.D.; Scaglione, J.B.; Hanna, P.C.; Tamayo-Castillo, G.; et al. Baulamycins A and B, Broad-Spectrum Antibiotics Identified as Inhibitors of Siderophore Biosynthesis in Staphylococcus Aureus and Bacillus Anthracis. J. Am. Chem. Soc. 2014, 136, 1579–1586. [Google Scholar] [CrossRef]
- Kemung, H.M.; Tan, L.T.-H.; Khan, T.M.; Chan, K.-G.; Pusparajah, P.; Goh, B.-H.; Lee, L.-H. Streptomyces as a Prominent Resource of Future Anti-MRSA Drugs. Front. Microbiol. 2018, 9, 2221. [Google Scholar] [CrossRef]
- Hover, B.M.; Kim, S.-H.; Katz, M.; Charlop-Powers, Z.; Owen, J.G.; Ternei, M.A.; Maniko, J.; Estrela, A.B.; Molina, H.; Park, S.; et al. Culture-Independent Discovery of the Malacidins as Calcium-Dependent Antibiotics with Activity against Multidrug-Resistant Gram-Positive Pathogens. Nat. Microbiol. 2018, 3, 415–422. [Google Scholar] [CrossRef]
- Kiani, F.A.; Fischer, S. Catalytic Strategy Used by the Myosin Motor to Hydrolyze ATP. Proc. Natl. Acad. Sci. USA 2014, 111, E2947–E2956. [Google Scholar] [CrossRef]
- Phillips, J.W.; Goetz, M.A.; Smith, S.K.; Zink, D.L.; Polishook, J.; Onishi, R.; Salowe, S.; Wiltsie, J.; Allocco, J.; Sigmund, J.; et al. Discovery of Kibdelomycin, A Potent New Class of Bacterial Type II Topoisomerase Inhibitor by Chemical-Genetic Profiling in Staphylococcus Aureus. Chem. Biol. 2011, 18, 955–965. [Google Scholar] [CrossRef]
- Hartkoorn, R.C.; Sala, C.; Neres, J.; Pojer, F.; Magnet, S.; Mukherjee, R.; Uplekar, S.; Boy-Röttger, S.; Altmann, K.; Cole, S.T. Towards a New Tuberculosis Drug: Pyridomycin—Nature’s Isoniazid. EMBO Mol. Med. 2012, 4, 1032–1042. [Google Scholar] [CrossRef]
- Sasse, F.; Steinmetz, H.; Schupp, T.; Petersen, F.; Memmert, K.; Hofmann, H.; Heusser, C.; Brinkmann, V.; Matt, P.V.; Höfle, G.; et al. Argyrins, Immunosuppressive Cyclic Peptides from Myxobacteria. I. Production, Isolation, Physico-Chemical and Biological Properties. J. Antibiot. 2002, 55, 543–551. [Google Scholar] [CrossRef]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Smith, P.A.; Koehler, M.F.T.; Girgis, H.S.; Yan, D.; Chen, Y.; Chen, Y.; Crawford, J.J.; Durk, M.R.; Higuchi, R.I.; Kang, J.; et al. Optimized Arylomycins Are a New Class of Gram-Negative Antibiotics. Nature 2018, 561, 189–194. [Google Scholar] [CrossRef]
- Bracegirdle, J.; Hou, P.; Nowak, V.V.; Ackerley, D.F.; Keyzers, R.A.; Owen, J.G. Skyllamycins D and E, Non-Ribosomal Cyclic Depsipeptides from Lichen-Sourced Streptomyces anulatus. J. Nat. Prod. 2021, 84, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Ebrahimi Samangani, A.; Kargari, A.; Kiani Nejad, A.; Yashmi, I.; Motahar, M.; Taki, E.; Khoshnood, S. Mechanism of Action, Resistance, Synergism, and Clinical Implications of Azithromycin. J. Clin. Lab. Anal. 2022, 36, e24427. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting Virulence: A New Paradigm for Antimicrobial Therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The Biology and Future Prospects of Antivirulence Therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal Secondary Metabolism: Regulation, Function and Drug Discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- King, A.M.; Reid-Yu, S.A.; Wang, W.; King, D.T.; De Pascale, G.; Strynadka, N.C.; Walsh, T.R.; Coombes, B.K.; Wright, G.D. Aspergillomarasmine A Overcomes Metallo-β-Lactamase Antibiotic Resistance. Nature 2014, 510, 503–506. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Dussart, F. Selected Fungal Natural Products with Antimicrobial Properties. Molecules 2020, 25, 911. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xu, L.; Chen, Z.; Guo, S.; Liao, J.; Ren, M.; Wang, Y.; Chen, Y.; Wan, C.; et al. The Broad-spectrum Antimicrobial Peptide BMAP-27B Potentiates Carbapenems against NDM-producing Pathogens in Food Animals. mLife 2025, 4, 275–293. [Google Scholar] [CrossRef]
- Mohan, S.; Ajay Krishna, M.S.; Chandramouli, M.; Keri, R.S.; Patil, S.A.; Ningaiah, S.; Somappa, S.B. Antibacterial Natural Products from Microbial and Fungal Sources: A Decade of Advances. Mol. Divers. 2023, 27, 517–541. [Google Scholar] [CrossRef]
- Karaman, M.; Jovin, E.; Malbaša, R.; Matavuly, M.; Popović, M. Medicinal and Edible Lignicolous Fungi as Natural Sources of Antioxidative and Antibacterial Agents. Phytother. Res. 2010, 24, 1473–1481. [Google Scholar] [CrossRef]
- Spížek, J.; Řezanka, T. Lincomycin, Clindamycin and Their Applications. Appl. Microbiol. Biotechnol. 2004, 64, 455–464. [Google Scholar] [CrossRef]
- Ondeyka, J.G.; Smith, S.K.; Zink, D.L.; Vicente, F.; Basilio, A.; Bills, G.F.; Polishook, J.D.; Garlisi, C.; Mcguinness, D.; Smith, E.; et al. Isolation, Structure Elucidation and Antibacterial Activity of a New Tetramic Acid, Ascosetin. J. Antibiot. 2014, 67, 527–531. [Google Scholar] [CrossRef]
- Wei, M.-Y.; Li, D.; Shao, C.-L.; Deng, D.-S.; Wang, C.-Y. (±)-Pestalachloride D, an Antibacterial Racemate of Chlorinated Benzophenone Derivative from a Soft Coral-Derived Fungus Pestalotiopsis sp. Mar. Drugs 2013, 11, 1050–1060. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Marine Compounds and Their Antimicrobial Activities. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 51, 1293–1306. [Google Scholar]
- Sułkowska-Ziaja, K.; Trepa, M.; Olechowska-Jarząb, A.; Nowak, P.; Ziaja, M.; Kała, K.; Muszyńska, B. Natural Compounds of Fungal Origin with Antimicrobial Activity—Potential Cosmetics Applications. Pharmaceuticals 2023, 16, 1200. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kinoshita, T.; Masukawa, H. Mechanism of Protein Synthesis Inhibition by Fusidic Acid and Related Antibiotics. Biochem. Biophys. Res. Commun. 1968, 30, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; Tu, M.M.; Brown, E.D. Important Challenges to Finding New Leads for New Antibiotics. Curr. Opin. Microbiol. 2025, 83, 102562. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, Antiviral and Antimicrobial Activities of Alkaloids, Flavonoids, and Phenolic Acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Marsoul, A.; Ijjaali, M.; Oumous, I.; Bennani, B.; Boukir, A. Determination of Polyphenol Contents in Papaver rhoeas L. Flowers Extracts (Soxhlet, Maceration), Antioxidant and Antibacterial Evaluation. Mater. Today Proc. 2020, 31, S183–S189. [Google Scholar] [CrossRef]
- Nolasco-González, Y.; Anaya-Esparza, L.M.; Aguilar-Hernández, G.; López-Romero, B.A.; Montalvo-González, E. Extraction, Encapsulation, and Biological Activity of Phenolic Compounds, Alkaloids, and Acetogenins from the Annona Genus. In Advances in Plant Biotechnology; CRC Press: Boca Raton, FL, USA, 2023; pp. 133–160. ISBN 978-1-003-16653-5. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in Flavonoid Research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, P.; Rai, D.K. Quercetin: A Versatile Flavonoid. Internet J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. BioMed Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Teng, Y.; Ou, L.; Xi, Y.; Chen, S.; Duan, G. The Resistance Mechanism of Escherichia Coli Induced by Ampicillin in Laboratory. Infect. Drug Resist. 2019, 12, 2853–2863. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Lee, H.-Y.; Tseng, L.-Y.; Chen, C.-L.; Chia, J.-H.; Su, L.-H.; Liu, S.-Y. Mechanisms of Resistance to Ciprofloxacin, Ampicillin/Sulbactam and Imipenem in Acinetobacter Baumannii Clinical Isolates in Taiwan. Int. J. Antimicrob. Agents 2010, 35, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, M.G.; Brusch, J.L.; Barza, M.; Weinstein, L. Bactericidal Activity and Pharmacology of Cefazolin. Antimicrob. Agents Chemother. 1973, 4, 396–401. [Google Scholar] [CrossRef]
- Barradell, L.B.; Bryson, H.M. Cefepime: A Review of Its Antibacterial Activity, Pharmacokinetic Properties and Therapeutic Use. Drugs 1994, 47, 471–505. [Google Scholar] [CrossRef]
- Clissold, S.P.; Todd, P.A.; Campoli-Richards, D.M. Imipenem/Cilastatin: A Review of Its Antibacterial Activity, Pharmacokinetic Properties and Therapeutic Efficacy. Drugs 1987, 33, 183–241. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Niezgódka, P.; Szewczyk, W.; Wińska, K. The Impact of Plant-Derived Polyphenols on Combating Efflux-Mediated Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 4030. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, J.; Fan, Y.; Liu, Y. Synergistic Effects of Unripe Raspberry Extracts (Rubus chingii) and Antibiotics against Three Bacteria. Food Sci. Technol. 2021, 41, 482–488. [Google Scholar] [CrossRef]
- Ang, S.-T.; Kim, T.H.; Cheesman, M.J.; Cock, I.E. Antibacterial and Synergistic Effects of Terminalia Citrina Leaf Extracts Against Gastrointestinal Pathogens: Insights from Metabolomic Analysis. Antibiotics 2025, 14, 593. [Google Scholar] [CrossRef]
- Ashraf, M.V.; Pant, S.; Khan, M.A.H.; Shah, A.A.; Siddiqui, S.; Jeridi, M.; Alhamdi, H.W.S.; Ahmad, S. Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance. Pharmaceuticals 2023, 16, 881. [Google Scholar] [CrossRef]
- Saraf, A.; Quereshi, S.; Sharma, K.; Khan, N.A. Antimicrobial Activity of Lantana camara L. J. Exp. Sci. 2011, 2, 50–54. [Google Scholar]
- Subramani, R.; Narayanasamy, M.; Feussner, K.-D. Plant-Derived Antimicrobials to Fight against Multi-Drug-Resistant Human Pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of Natural Antimicrobials to Increase Antibiotic Susceptibility of Drug Resistant Bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Haddis, T.; Khalfin, B.; Dahan, A.; Ben-Shabat, S. Natural Antimicrobial Compounds as Promising Preservatives: A Look at an Old Problem from New Perspectives. Molecules 2024, 29, 5830. [Google Scholar] [CrossRef]
- Davidson, P.M.; Critzer, F.J.; Taylor, T.M. Naturally Occurring Antimicrobials for Minimally Processed Foods. Annu. Rev. Food Sci. Technol. 2013, 4, 163–190. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Otero-González, A.; Ghattas, M.; Ständker, L. Discovery, Optimization, and Clinical Application of Natural Antimicrobial Peptides. Biomedicines 2021, 9, 1381. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, S.; Islam, R.; Hossain, E.; Mannan Mithi, F.; Ahmed, M.; Saldías, M.; Akkol, E.K.; Sobarzo-Sánchez, E. Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics 2021, 10, 1076. [Google Scholar] [CrossRef]
- Das, A.; Satyaprakash, K. Antimicrobial Properties of Natural Products: A Review. Pharma. Innovat. J. 2018, 7, 532–537. [Google Scholar]
- Eshboev, F.; Mamadalieva, N.; Nazarov, P.; Hussain, H.; Katanaev, V.; Egamberdieva, D.; Azimova, S. Antimicrobial Action Mechanisms of Natural Compounds Isolated from Endophytic Microorganisms. Antibiotics 2024, 13, 271. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, J.; Xing, J.; He, L.; Xu, C.; Wu, A.; Li, J. Unlocking the Potential of Antimicrobial Peptides: Cutting-Edge Advances and Therapeutic Potential in Combating Bacterial Keratitis. Bioconjugate Chem. 2025, 36, 311–331. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How Antibiotics Kill Bacteria: From Targets to Networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Grayson, M.L.; Cosgrove, S.; Crowe, S.; Hope, W.; McCarthy, J.; Mills, J.; Mouton, J.W.; Paterson, D. (Eds.) Kucers’ the Use of Antibiotics; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-4796-7. [Google Scholar]
- Wilson, D.N. The A–Z of Bacterial Translation Inhibitors. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 393–433. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.-K.; Li, J. Novel Antimicrobial Agents for Combating Antibiotic-Resistant Bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Villain-Guillot, P.; Bastide, L.; Gualtieri, M.; Leonetti, J.-P. Progress in Targeting Bacterial Transcription. Drug Discov. Today 2007, 12, 200–208. [Google Scholar] [CrossRef]
- Bremner, J. Multiple Action-Based Design Approaches to Antibacterials; Springer: Singapore, 2021; ISBN 978-981-16-0998-5. [Google Scholar]
- Rocha, B.M.; Pinto, E.; Sousa, E.; Resende, D.I.S.P. Targeting Siderophore Biosynthesis to Thwart Microbial Growth. Int. J. Mol. Sci. 2025, 26, 3611. [Google Scholar] [CrossRef]
- Ymele-Leki, P.; Cao, S.; Sharp, J.; Lambert, K.G.; McAdam, A.J.; Husson, R.N.; Tamayo, G.; Clardy, J.; Watnick, P.I. Correction: A High-Throughput Screen Identifies a New Natural Product with Broad-Spectrum Antibacterial Activity. PLoS ONE 2012, 7, e31307. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-Biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a Medicinal Plant: Antimicrobial Action of Berberine Potentiated by 5′-Methoxyhydnocarpin, a Multidrug Pump Inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef]
- Andrae-Marobela, K.; Ghislain, F.W.; Okatch, H.; Majinda, R.R.T. Polyphenols: A Diverse Class of Multi-Target Anti-HIV-1 Agents. Curr. Drug Metab. 2013, 14, 392–413. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic Resistance and Its Cost: Is It Possible to Reverse Resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Tam, V.H.; Louie, A.; Deziel, M.R.; Liu, W.; Leary, R.; Drusano, G.L. Bacterial-Population Responses to Drug-Selective Pressure: Examination of Garenoxacin’s Effect on Pseudomonas aeruginosa. J. Infect. Dis. 2005, 192, 420–428. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta BBA—Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus Aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Zhang, S.; Sun, X.; Chang, W.; Dai, Y.; Ma, X. Systematic Review and Meta-Analysis of the Epidemiology of Vancomycin-Intermediate and Heterogeneous Vancomycin-Intermediate Staphylococcus Aureus Isolates. PLoS ONE 2015, 10, e0136082. [Google Scholar] [CrossRef]
- Appelbaum, P.C. The Emergence of Vancomycin-Intermediate and Vancomycin-Resistant Staphylococcus Aureus. Clin. Microbiol. Infect. 2006, 12, 16–23. [Google Scholar] [CrossRef]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef]
- Götz, F.; Heilmann, C.; Stehle, T. Functional and Structural Analysis of the Major Amidase (Atl) in Staphylococcus. Int. J. Med. Microbiol. 2014, 304, 156–163. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.; Simoes, M. Insights on Antimicrobial Resistance, Biofilms and the Use of Phytochemicals as New Antimicrobial Agents. Curr. Med. Chem. 2015, 22, 2590–2614. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Abass, S.; Parveen, R.; Irfan, M.; Jan, B.; Husain, S.A.; Ahmad, S. Synergy Based Extracts of Medicinal Plants: Future Antimicrobials to CombatMultidrug Resistance. Curr. Pharm. Biotechnol. 2022, 23, 1527–1540. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Lotfalizadeh, M.; Badpeyma, M.; Shakeri, A.; Soheili, V. From Plants to Antimicrobials: Natural Products against Bacterial Membranes. Phytother. Res. 2022, 36, 33–52. [Google Scholar] [CrossRef]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas Aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside. Indian J. Med. Res. 2019, 149, 129–145. [Google Scholar] [CrossRef]
- Ohene-Agyei, T.; Mowla, R.; Rahman, T.; Venter, H. Phytochemicals Increase the Antibacterial Activity of Antibiotics by Acting on a Drug Efflux Pump. Microbiologyopen 2014, 3, 885–896. [Google Scholar] [CrossRef]
- Kakarla, P.; Floyd, J.; Mukherjee, M.; Devireddy, A.R.; Inupakutika, M.A.; Ranweera, I.; Kc, R.; ‘Shrestha, U.; Cheeti, U.R.; Willmon, T.M.; et al. Inhibition of the Multidrug Efflux Pump LmrS from Staphylococcus Aureus by Cumin Spice Cuminum cyminum. Arch. Microbiol. 2017, 199, 465–474. [Google Scholar] [CrossRef]
- Zhou, T.; Li, Z.; Kang, O.-H.; Mun, S.-H.; Seo, Y.-S.; Kong, R.; Shin, D.-W.; Liu, X.-Q.; Kwon, D.-Y. Antimicrobial Activity and Synergism of Ursolic Acid 3-O-α-L-Arabinopyranoside with Oxacillin against Methicillin-Resistant Staphylococcus Aureus. Int. J. Mol. Med. 2017, 40, 1285–1293. [Google Scholar] [CrossRef]
- Ahmed, A.; Salih, F.; Yousef, M. Rhus Coriaria Extracts Inhibit Quorum Sensing Related Virulence and Biofilm Production in Drug-Resistant Pseudomonas Aeruginosa Recovered from Burn Wounds. Iran. J. Basic Med. Sci. 2022, 25, 1349. [Google Scholar] [CrossRef]
- Sudano Roccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-Gallate Enhances the Activity of Tetracycline in Staphylococci by Inhibiting Its Efflux from Bacterial Cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Zha, J.; Guleria, S.; Koffas, M.A.G. Recent Advances in the Recombinant Biosynthesis of Polyphenols. Front. Microbiol. 2017, 8, 2259. [Google Scholar] [CrossRef]
- Tsuchiya, H. Membrane Interactions of Phytochemicals as Their Molecular Mechanism Applicable to the Discovery of Drug Leads from Plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef]
- Upadhyay, A.; Karumathil, D.P.; Upadhyaya, I.; Bhattaram, V.; Venkitanarayanan, K. Controlling Bacterial Antibiotic Resistance Using Plant-Derived Antimicrobials. In Antibiotic Resistance; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–226. ISBN 978-0-12-803642-6. [Google Scholar]
- Zhao, W.-H.; Hu, Z.-Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing Natural Products as Potential Anti-Biofilm Agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; Van Hoek, M.L. Cranberry Proanthocyanidins Have Anti-Biofilm Properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef]
- Malik, F.Z.A.; Allaudin, Z.N.; Loh, H.S.; Nee, T.K.; Hani, H.; Abdullah, R. Antiviral and Virucidal Activities of Duabanga grandiflora Leaf Extract against Pseudorabies Virus In Vitro. BMC Complement. Altern. Med. 2016, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Maggini, V.; Semenzato, G.; Gallo, E.; Nunziata, A.; Fani, R.; Firenzuoli, F. Antimicrobial Activity of Syzygium Aromaticum Essential Oil in Human Health Treatment. Molecules 2024, 29, 999. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, M.; Bozic, D.; Slavkovska, V.; Lakusic, B. Synergistic Effects of Salvia officinalis L. Essential Oils and Antibiotics against Methicillin-Resistant Staphylococcus Aureus. Arch. Biol. Sci. 2015, 67, 949–956. [Google Scholar] [CrossRef]
- Yang, Z.-C.; Wang, B.-C.; Yang, X.-S.; Wang, Q.; Ran, L. The Synergistic Activity of Antibiotics Combined with Eight Traditional Chinese Medicines against Two Different Strains of Staphylococcus Aureus. Colloids Surf. B Biointerfaces 2005, 41, 79–81. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Hoffman, P.S. Antibacterial Discovery: 21st Century Challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef]
- Karpecki, P.; Paterno, M.R.; Comstock, T.L. Limitations of Current Antibiotics for the Treatment of Bacterial Conjunctivitis. Optom. Vis. Sci. 2010, 87, 908–919. [Google Scholar] [CrossRef]
- Nazir, F.; Tabish, T.A.; Tariq, F.; Iftikhar, S.; Wasim, R.; Shahnaz, G. Stimuli-Sensitive Drug Delivery Systems for Site-Specific Antibiotic Release. Drug Discov. Today 2022, 27, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Loupias, P.; Dassonville-Klimpt, A.; Sonnet, P. Drug Delivery Systems Designed to Overcome Antimicrobial Resistance. Med. Res. Rev. 2019, 39, 2343–2396. [Google Scholar] [CrossRef] [PubMed]
- Guedes, B.N.; Krambeck, K.; Durazzo, A.; Lucarini, M.; Santini, A.; Oliveira, M.B.P.P.; Fathi, F.; Souto, E.B. Natural Antibiotics against Antimicrobial Resistance: Sources and Bioinspired Delivery Systems. Braz. J. Microbiol. 2024, 55, 2753–2766. [Google Scholar] [CrossRef]
- Noel, S.P.; Courtney, H.; Bumgardner, J.D.; Haggard, W.O. Chitosan Films: A Potential Local Drug Delivery System for Antibiotics. Clin. Orthop. 2008, 466, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Based Local Antimicrobial Drug Delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Brunetti, J.; Falciani, C.; Roscia, G.; Pollini, S.; Bindi, S.; Scali, S.; Arrieta, U.C.; Gómez-Vallejo, V.; Quercini, L.; Ibba, E.; et al. In Vitro and In Vivo Efficacy, Toxicity, Bio-Distribution and Resistance Selection of a Novel Antibacterial Drug Candidate. Sci. Rep. 2016, 6, 26077. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, N.D.; Ouimet, M.A.; Uhrich, K.E. Antibiotic-Containing Polymers for Localized, Sustained Drug Delivery. Adv. Drug Deliv. Rev. 2014, 78, 77–87. [Google Scholar] [CrossRef]
- Yah, C.S.; Simate, G.S. Nanoparticles as Potential New Generation Broad Spectrum Antimicrobial Agents. DARU J. Pharm. Sci. 2015, 23, 43. [Google Scholar] [CrossRef]
- Polívková, M.; Hubáček, T.; Staszek, M.; Švorčík, V.; Siegel, J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int. J. Mol. Sci. 2017, 18, 419. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Sharaf, S.S.; El Shafei, A.; Hegazy, A.A. Using Chitosan Nanoparticles as Drug Carriers for the Development of a Silver Sulfadiazine Wound Dressing. Carbohydr. Polym. 2017, 158, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Omerović, N.; Djisalov, M.; Živojević, K.; Mladenović, M.; Vunduk, J.; Milenković, I.; Knežević, N.Ž.; Gadjanski, I.; Vidić, J. Antimicrobial Nanoparticles and Biodegradable Polymer Composites for Active Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2428–2454. [Google Scholar] [CrossRef]
- Varma, M.V.S.; Kaushal, A.M.; Garg, A.; Garg, S. Factors Affecting Mechanism and Kinetics of Drug Release from Matrix-Based Oral Controlled Drug Delivery Systems. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Narasimha Reddy, M.; Cheralathan, K.K.; Sasikumar, S. In Vitro Bioactivity and Drug Release Kinetics Studies of Mesoporous Silica-Biopolymer Composites. J. Porous Mater. 2015, 22, 1465–1472. [Google Scholar] [CrossRef]
- Khalbas, A.H.; Albayati, T.M.; Ali, N.S.; Salih, I.K. Drug Loading Methods and Kinetic Release Models Using of Mesoporous Silica Nanoparticles as a Drug Delivery System: A Review. S. Afr. J. Chem. Eng. 2024, 50, 261–280. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wang, H.; Ducheyne, P. Polymer-Coated Mesoporous Silica Nanoparticles for the Controlled Release of Macromolecules. Acta Biomater. 2012, 8, 3429–3435. [Google Scholar] [CrossRef]
- Paris, J.L.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M.; Vallet-Regí, M. Tuning Mesoporous Silica Dissolution in Physiological Environments: A Review. J. Mater. Sci. 2017, 52, 8761–8771. [Google Scholar] [CrossRef]
- Andersson, J.; Rosenholm, J.; Areva, S.; Lindén, M. Influences of Material Characteristics on Ibuprofen Drug Loading and Release Profiles from Ordered Micro- and Mesoporous Silica Matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Gómez Chabala, L.; Cuartas, C.; López, M. Release Behavior and Antibacterial Activity of Chitosan/Alginate Blends with Aloe Vera and Silver Nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, C.; Che, S. Amino and Quaternary Ammonium Group Functionalized Mesoporous Silica: An Efficient Ion-Exchange Method to Remove Anionic Surfactant from AMS. Microporous Mesoporous Mater. 2008, 116, 299–307. [Google Scholar] [CrossRef]
- Osman, N.; Devnarain, N.; Omolo, C.A.; Fasiku, V.; Jaglal, Y.; Govender, T. Surface Modification of Nano-drug Delivery Systems for Enhancing Antibiotic Delivery and Activity. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1758. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial Hydrogels: Promising Materials for Medical Application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Dorotkiewicz-Jach, A. Liposomes as Delivery Systems for Antibiotics. Int. J. Pharm. 2010, 387, 187–198. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Ganji, F.; Taghizadeh, S.M.; Vasheghani-Farahani, E.; Mohiti-Asli, M. Drug in Adhesive Transdermal Patch Containing Antibiotic-Loaded Solid Lipid Nanoparticles. J. Biosci. Bioeng. 2022, 134, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Pal, I.; Brahmkhatri, V.P.; Bera, S.; Bhattacharyya, D.; Quirishi, Y.; Bhunia, A.; Atreya, H.S. Enhanced Stability and Activity of an Antimicrobial Peptide in Conjugation with Silver Nanoparticle. J. Colloid Interface Sci. 2016, 483, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Rananaware, P.; Bauri, S.; Keri, R.; Mishra, M.; Brahmkhatri, V. Polymeric Curcumin Nanospheres for Lysozyme Aggregation Inhibition, Antibacterial, and Wound Healing Applications. Environ. Sci. Pollut. Res. 2023, 31, 46625–46640. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Ballesteros Monrreal, M.G.; Mendez-Encinas, M.A.; Valencia, D.; Ortiz, B.; González-Davis, O.; Cadena-Nava, R.D. Nanoparticles in Antibacterial Therapy: A Systematic Review of Enhanced Efficacy against Intracellular Bacteria. ACS Omega 2025, 10, 17070–17086. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Ben Thomas, M.; Elango, M.; Raichur, A.M.; Chakravortty, D. Chitosan-Dextran Sulphate Nanocapsule Drug Delivery System as an Effective Therapeutic against Intraphagosomal Pathogen Salmonella. J. Antimicrob. Chemother. 2013, 68, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Akkineni, A.R.; Spangenberg, J.; Geissler, M.; Reichelt, S.; Buechner, H.; Lode, A.; Gelinsky, M. Controlled and Local Delivery of Antibiotics by 3D Core/Shell Printed Hydrogel Scaffolds to Treat Soft Tissue Infections. Pharmaceutics 2021, 13, 2151. [Google Scholar] [CrossRef]
- Rai, A.; Comune, M.; Ferreira, L. Nanoparticle-Based Drug Delivery Systems: Promising Approaches Against Bacterial Infections. In Antibacterial Drug Discovery to Combat MDR; Ahmad, I., Ahmad, S., Rumbaugh, K.P., Eds.; Springer: Singapore, 2019; pp. 605–633. ISBN 978-981-13-9870-4. [Google Scholar]
- Buzisa Mbuku, R.; Poilvache, H.; Maigret, L.; Vanbever, R.; Van Bambeke, F.; Cornu, O. Targeting Staphylococcus Aureus Biofilm-Related Infections on Implanted Material with a Novel Dual-Action Thermosensitive Hydrogel Containing Vancomycin and a Tri-Enzymatic Cocktail: In Vitro and In Vivo Studies. Biofilm 2025, 9, 100288. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. Silver Nanoparticles as Multi-Functional Drug Delivery Systems. In Nanomedicines; Akhyar Farrukh, M., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-283-7. [Google Scholar]
- Kotrange, H.; Najda, A.; Bains, A.; Gruszecki, R.; Chawla, P.; Tosif, M.M. Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics. Int. J. Mol. Sci. 2021, 22, 9596. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, B.; Wang, L.; He, J.; Qiu, X. UV-Triggered Drug Release from Mesoporous Titanium Nanoparticles Loaded with Berberine Hydrochloride: Enhanced Antibacterial Activity. Molecules 2024, 29, 1607. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Gungor Ak, A.; Turan, I.; Sayan Ozacmak, H.; Karatas, A. Chitosan Nanoparticles as Promising Tool for Berberine Delivery: Formulation, Characterization and In Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2023, 80, 104203. [Google Scholar] [CrossRef]
- Comincini, S.; Manai, F.; Sorrenti, M.; Perteghella, S.; D’Amato, C.; Miele, D.; Catenacci, L.; Bonferoni, M.C. Development of Berberine-Loaded Nanoparticles for Astrocytoma Cells Administration and Photodynamic Therapy Stimulation. Pharmaceutics 2023, 15, 1078. [Google Scholar] [CrossRef] [PubMed]
- Mensah, A.; Rodgers, A.M.; Larrañeta, E.; McMullan, L.; Tambuwala, M.; Callan, J.F.; Courtenay, A.J. Treatment of Periodontal Infections, the Possible Role of Hydrogels as Antibiotic Drug-Delivery Systems. Antibiotics 2023, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Fu, M.; Gan, Y.; Bu, C.; Guo, Z.; Lv, X.; Ding, X. A Multifunctional Hydrogel Dressing Loaded with Antibiotics for Healing of Infected Wound. Int. J. Pharm. 2024, 666, 124770. [Google Scholar] [CrossRef]
- Smith, A. Biofilms and Antibiotic Therapy: Is There a Role for Combating Bacterial Resistance by the Use of Novel Drug Delivery Systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Wang, Y. Liposome as a Delivery System for the Treatment of Biofilm-mediated Infections. J. Appl. Microbiol. 2021, 131, 2626–2639. [Google Scholar] [CrossRef]
- Pande, S. Liposomes for Drug Delivery: Review of Vesicular Composition, Factors Affecting Drug Release and Drug Loading in Liposomes. Artif. Cells Nanomed. Biotechnol. 2023, 51, 428–440. [Google Scholar] [CrossRef]
- Makhlouf, Z.; Ali, A.A.; Al-Sayah, M.H. Liposomes-Based Drug Delivery Systems of Anti-Biofilm Agents to Combat Bacterial Biofilm Formation. Antibiotics 2023, 12, 875. [Google Scholar] [CrossRef]
- Alzahrani, N.M.; Booq, R.Y.; Aldossary, A.M.; Bakr, A.A.; Almughem, F.A.; Alfahad, A.J.; Alsharif, W.K.; Jarallah, S.J.; Alharbi, W.S.; Alsudir, S.A.; et al. Liposome-Encapsulated Tobramycin and IDR-1018 Peptide Mediated Biofilm Disruption and Enhanced Antimicrobial Activity against Pseudomonas Aeruginosa. Pharmaceutics 2022, 14, 960. [Google Scholar] [CrossRef]
- Vatankhah, M.; Dadashzadeh, S.; Mahboubi, A.; Haeri, A.; Jandaghi Alaee, K.; Mostafavi Naeini, S.B.; Abbasian, Z. Preparation of Multivesicular Liposomes for the Loco-Regional Delivery of Vancomycin Hydrochloride Using Active Loading Method: Drug Release and Antimicrobial Properties. J. Liposome Res. 2024, 34, 77–87. [Google Scholar] [CrossRef]
- Erdene, E.; Munkhjargal, O.; Batnasan, G.; Dorjbal, E.; Oidov, B.; Byambaa, A. Evaluation of Liposome-Encapsulated Vancomycin Against Methicillin-Resistant Staphylococcus Aureus. Biomedicines 2025, 13, 378. [Google Scholar] [CrossRef]
- Seyfoddin, A.; Shaw, J.; Al-Kassas, R. Solid Lipid Nanoparticles for Ocular Drug Delivery. Drug Deliv. 2010, 17, 467–489. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Mellace, S.; Cassano, R. Solid Lipid Nanoparticles for Antifungal Drugs Delivery for Topical Applications. Ther. Deliv. 2016, 7, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- De Gaetano, F.; Caridi, F.; Totaro, N.; Celesti, C.; Venuti, V.; Ginestra, G.; Nostro, A.; Tommasini, S.; Ventura, C.A.; Stancanelli, R. Naringenin-Loaded Solid Lipid Nanoparticles: Physical–Chemical Characterization and In Vitro Antibacterial Activity. Pharmaceuticals 2025, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Kharaji, M.; Rodrigues, P.; Petersen, C. Solid Lipid Nanoparticles Encapsulated With Paromomycin: An Effective Oral Formulation Against Leishmania major in Mouse Model. Parasite Immunol. 2025, 47, e70002. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Sikwal, D.R.; Rambharose, S.; Mocktar, C.; Singh, S.; Bester, L.; Oh, J.K.; Renukuntla, J.; Govender, T. Enhancing Targeted Antibiotic Therapy via pH Responsive Solid Lipid Nanoparticles from an Acid Cleavable Lipid. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2067–2077. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef]
- Raza, A.; Sime, F.B.; Cabot, P.J.; Maqbool, F.; Roberts, J.A.; Falconer, J.R. Solid Nanoparticles for Oral Antimicrobial Drug Delivery: A Review. Drug Discov. Today 2019, 24, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Pancu, D.F.; Scurtu, A.; Macasoi, I.G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics: Conventional Therapy and Natural Compounds with Antibacterial Activity—A Pharmaco-Toxicological Screening. Antibiotics 2021, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Leite Dos Santos, C.A.; Silva Pereira, R.L.D.; Pereira-de-Morais, L.; Santos, G.J.G.; Coutinho, H.D.M.; Assis Bezerra Da Cunha, F.; Rodrigues, F.F.G.; Sousa Júnior, D.L.D.; Silva, J.C. Antibacterial Activity of Ethanolic Extract of Ocimum gratissimum L. (BASIL) and Its Toxicity against Drosophila Melanogaster. Pharmacol. Res.—Nat. Prod. 2025, 7, 100212. [Google Scholar] [CrossRef]
- Fung, J.S.; Cook, V.J.; Johnston, J.; Connors, W.J. Bedaquiline, Pretomanid, Linezolid, and Moxifloxacin Treatment for Multi-Drug Resistant Extrapulmonary Tuberculosis in Canada: A Report. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2025, 10, 203–208. [Google Scholar] [CrossRef]
- Fox, S.J.; Park, M.A. Penicillin Skin Testing Is a Safe and Effective Tool for Evaluating Penicillin Allergy in the Pediatric Population. J. Allergy Clin. Immunol. Pract. 2014, 2, 439–444. [Google Scholar] [CrossRef]
- Macy, E.; Vyles, D. Who Needs Penicillin Allergy Testing? Ann. Allergy Asthma Immunol. 2018, 121, 523–529. [Google Scholar] [CrossRef]
- Vyles, D.; Antoon, J.W.; Norton, A.; Stone, C.A.; Trubiano, J.; Radowicz, A.; Phillips, E.J. Children with Reported Penicillin Allergy. Ann. Allergy Asthma Immunol. 2020, 124, 558–565. [Google Scholar] [CrossRef]
- Ham, Y.; Sukerman, E.S.; Lewis, J.S.; Tucker, K.J.; Yu, D.L.; Joshi, S.R. Safety and Efficacy of Direct Two-Step Penicillin Challenges with an Inpatient Pharmacist-Driven Allergy Evaluation. Allergy Asthma Proc. 2021, 42, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zamoner, W.; Prado, I.R.S.; Balbi, A.L.; Ponce, D. Vancomycin Dosing, Monitoring and Toxicity: Critical Review of the Clinical Practice. Clin. Exp. Pharmacol. Physiol. 2019, 46, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, N.; Xu, J.; Yang, T.; Yin, H.; Cai, Y. Efficacy and Safety of Vancomycin for the Treatment of Staphylococcus Aureus Bacteraemia: A Systematic Review and Meta-Analysis. Int. J. Antimicrob. Agents 2023, 62, 106946. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Wang, J.; Che, H.; Wang, R.; Cai, Y. The Clinical Efficacy and Safety of Vancomycin Loading Dose: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e17639. [Google Scholar] [CrossRef]
- Ghoneim, R.H.; Thabit, A.K.; Lashkar, M.O.; Ali, A.S. Optimizing Gentamicin Dosing in Different Pediatric Age Groups Using Population Pharmacokinetics and Monte Carlo Simulation. Ital. J. Pediatr. 2021, 47, 167. [Google Scholar] [CrossRef] [PubMed]
- Hodiamont, C.J.; Van Den Broek, A.K.; De Vroom, S.L.; Prins, J.M.; Mathôt, R.A.A.; Van Hest, R.M. Clinical Pharmacokinetics of Gentamicin in Various Patient Populations and Consequences for Optimal Dosing for Gram-Negative Infections: An Updated Review. Clin. Pharmacokinet. 2022, 61, 1075–1094. [Google Scholar] [CrossRef]
- Best, E.J.; Gazarian, M.; Cohn, R.; Wilkinson, M.; Palasanthiran, P. Once-Daily Gentamicin in Infants and Children: A Prospective Cohort Study Evaluating Safety and the Role of Therapeutic Drug Monitoring in Minimizing Toxicity. Pediatr. Infect. Dis. J. 2011, 30, 827–832. [Google Scholar] [CrossRef]
- Kalmanson, O.A.; McLoughlin, K.C.; Kiser, T.H.; Gubbels, S.P. Debilitating Gentamicin Ototoxicity: Case Report and Recommendations Against Routine Use in Surgical Prophylaxis. Ann. Otol. Rhinol. Laryngol. 2023, 132, 1686–1689. [Google Scholar] [CrossRef]
- Jodh, R.; Tawar, M.; Kachewar, A.; Ingole, Y.; Deshmukh, T.; Ijapure, V. Pharmacological Review on Tobramycin. Asian J. Res. Pharm. Sci. 2022, 12, 137–142. [Google Scholar] [CrossRef]
- Pacifici, G.M. Clinical Pharmacology of Tobramycin. Clin. Res. Stud. 2023, 2, 1–7. [Google Scholar] [CrossRef]
- Vazquez Espinosa, E.; Giron Moreno, R.M.; Gomez Punter, R.M.; Garcia Castillo, E.; Valenzuela, C.; Cisneros Serrano, C.; Zamora Garcia, E.; Garcia Perez, J.; Ancochea Bermudez, J. Long-Term Safety and Efficacy of Tobramycin in The Management of Cystic Fibrosis. Ther. Clin. Risk Manag. 2015, 11, 407–415. [Google Scholar] [CrossRef]
- Gao, Y.-H.; Lu, H.-W.; Zheng, H.-Z.; Cao, C.; Chu, D.-J.; Fan, H.; Fan, X.-Y.; Gu, H.-Y.; Guan, W.-J.; Jie, Z.-J.; et al. A Phase 4 Multicentre, 2 × 2 Factorial Randomised, Double-Blind, Placebo-Controlled Trial to Investigate the Efficacy and Safety of Tobramycin Inhalation Solution for Pseudomonas aeruginosa Eradication in Bronchiectasis: ERASE. ERJ Open Res. 2024, 10, 00938–02023. [Google Scholar] [CrossRef]
- Terpstra, L.C.; Altenburg, J.; Bronsveld, I.; De Kruif, M.D.; Berk, Y.; Snijders, D.; Rozemeijer, W.; Heijerman, H.G.M.; Boersma, W.G. Effects of Long-Term Tobramycin Inhalation Solution (TIS) Once Daily on Exacerbation Rate in Patients with Non-Cystic Fibrosis Bronchiectasis. Respir. Res. 2022, 23, 330. [Google Scholar] [CrossRef]
- Minichmayr, I.K.; Aranzana-Climent, V.; Friberg, L.E. Pharmacokinetic/Pharmacodynamic Models for Time Courses of Antibiotic Effects. Int. J. Antimicrob. Agents 2022, 60, 106616. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; Van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving Solubility and Chemical Stability of Natural Compounds for Medicinal Use by Incorporation into Liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Verma, T.; Aggarwal, A.; Singh, S.; Sharma, S.; Sarma, S.J. Current Challenges and Advancements towards Discovery and Resistance of Antibiotics. J. Mol. Struct. 2022, 1248, 131380. [Google Scholar] [CrossRef]
- Jager, N.G.L.; Van Hest, R.M.; Lipman, J.; Roberts, J.A.; Cotta, M.O. Antibiotic Exposure at the Site of Infection: Principles and Assessment of Tissue Penetration. Expert Rev. Clin. Pharmacol. 2019, 12, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Medeiros-Silva, J.; Parmar, A.; Vermeulen, B.J.A.; Das, S.; Paioni, A.L.; Jekhmane, S.; Lorent, J.; Bonvin, A.M.J.J.; Baldus, M.; et al. Mode of Action of Teixobactins in Cellular Membranes. Nat. Commun. 2020, 11, 2848. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Dawson, R.; Friedrich, S.O.; Venter, A.; Paige, D.; Zhu, T.; Silvia, A.; Gobey, J.; Ellery, C.; Zhang, Y.; et al. Mycobactericidal Activity of Sutezolid (PNU-100480) in Sputum (EBA) and Blood (WBA) of Patients with Pulmonary Tuberculosis. PLoS ONE 2014, 9, e94462. [Google Scholar] [CrossRef] [PubMed]
- Meibohm, B.; Derendorf, H. Pharmacokinetic/Pharmacodynamic Studies in Drug Product Development. J. Pharm. Sci. 2002, 91, 18–31. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Nation, R.L. Limitations of Antibiotic MIC-Based PK-PD Metrics: Looking Back to Move Forward. Front. Pharmacol. 2021, 12, 770518. [Google Scholar] [CrossRef]
- Rodríguez-Gascón, A.; Solinís, M.Á.; Isla, A. The Role of PK/PD Analysis in the Development and Evaluation of Antimicrobials. Pharmaceutics 2021, 13, 833. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Malhotra, Y.; Murante, B.; Laverty, S.; Cook, S.; Topa, D.; Hardy, D.; Wang, H.; Gigliotti, F. A Single-Blinded Randomized Clinical Trial Comparing Polymyxin B-Trimethoprim and Moxifloxacin for Treatment of Acute Conjunctivitis in Children. J. Pediatr. 2013, 162, 857–861. [Google Scholar] [CrossRef]
- Alaoui Mdarhri, H.; Benmessaoud, R.; Yacoubi, H.; Seffar, L.; Guennouni Assimi, H.; Hamam, M.; Boussettine, R.; Filali-Ansari, N.; Lahlou, F.A.; Diawara, I.; et al. Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics 2022, 11, 1826. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology Based Drug Delivery System: Current Strategies and Emerging Therapeutic Potential for Medical Science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Nazli, A.; He, D.L.; Liao, D.; Khan, M.Z.I.; Huang, C.; He, Y. Strategies and Progresses for Enhancing Targeted Antibiotic Delivery. Adv. Drug Deliv. Rev. 2022, 189, 114502. [Google Scholar] [CrossRef] [PubMed]
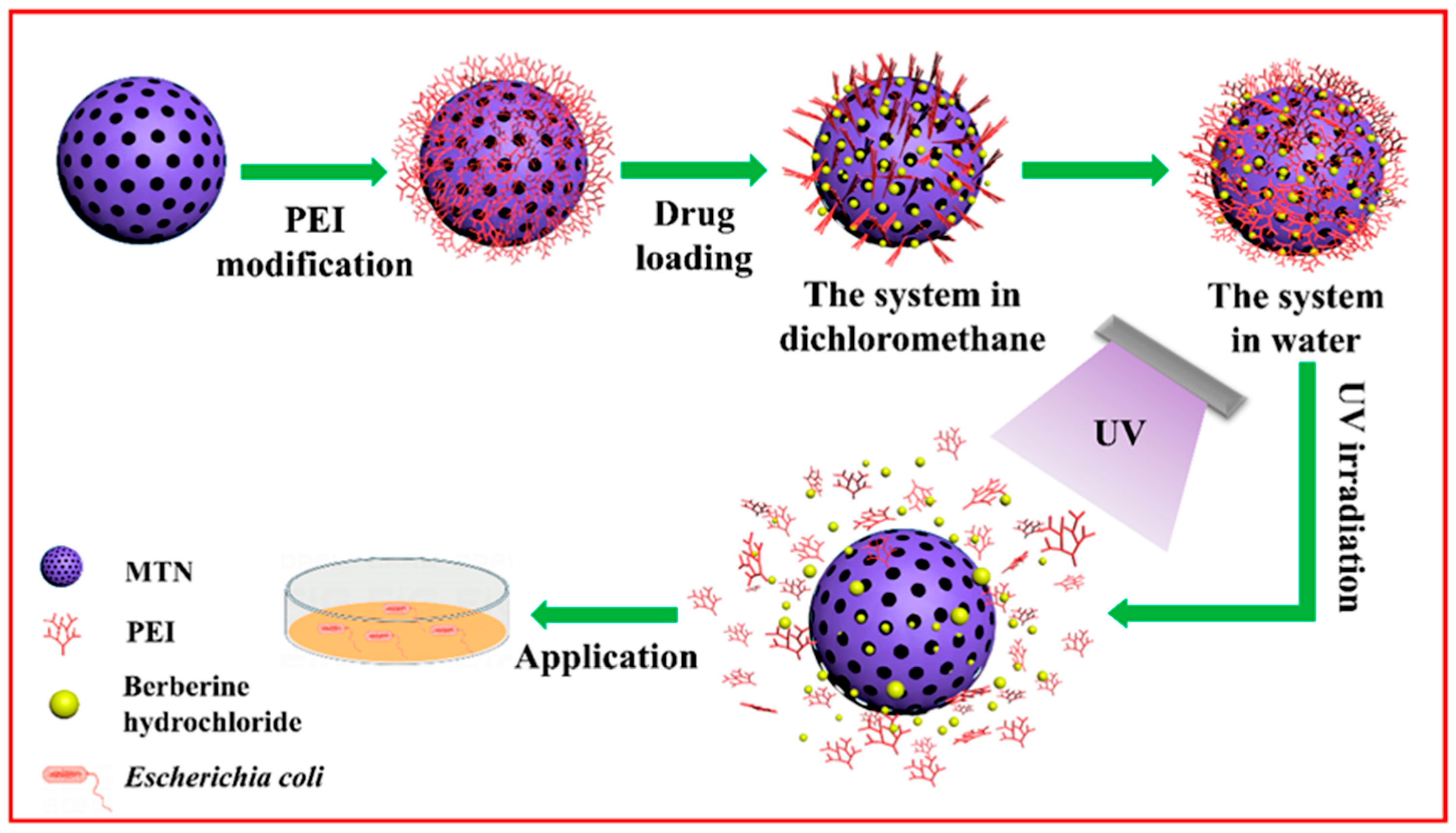

| Natural Product | Source Organism | Type | Target Bacteria | In Vivo Efficacy (Murine Model) | Synergistic Combination (FIC ≤ 0.5) | Ref. |
|---|---|---|---|---|---|---|
| Thymol | Plant (Thymus spp.) | Phenolic compound | S. typhimurium, S. aureus, E. coli | Effective in animal models [14] | Ampicillin, tetracycline, penicillin, erythromycin, novobiocin | [16] |
| Carvacrol | Plant (Oregano spp.) | Phenolic compound | S. typhimurium, S. aureus | Effective in animal models [17] | Ampicillin, penicillin, bacitracin | [22] |
| Cinnamaldehyde | Plant (Cinnamon spp.) | Aldehyde | E. coli, S. aureus | Effective in animal models [86] | Ampicillin, tetracycline, penicillin, erythromycin, novobiocin | [130] |
| Allyl isothiocyanate | Plant (Mustard spp.) | Isothiocyanate | S. pyogenes | Effective in animal models [87] | Erythromycin | [131] |
| Nisin | Bacteria (Lactococcus) | Bacteriocin peptide | Gram-positive bacteria | Effective in food models [88] | With other preservatives | [132] |
| Antimicrobial peptides | Animals, plants, fungi | Peptide | Broad-spectrum (bacteria, fungi, viruses) | Effective in animal models [89] | With antibiotics | [133] |
| Essential oils | Plants (various) | Oil mixture | Broad-spectrum | Effective in food/animal models [90] | With antibiotics | [134] |
| Nanoparticle Type | Key Features and Advantages | Example System and Application |
|---|---|---|
| Polymeric nanoparticles | Enhanced stability and solubility: protects drugs from degradation and improves their bioavailability. Controlled and targeted release: can be engineered to release antibiotics over an extended period or at specific sites of infection. Versatility: can be made from various biodegradable polymers like PLGA and chitosan [212]. | Chitosan–dextran sulfate nanocapsules: can be used to deliver ciprofloxacin, significantly prolonging its half-life and increasing concentration in tissues like the spleen and liver [213]. |
| Lipid-based nanoparticles | Biocompatibility: composed of lipids, making them highly compatible with biological systems. Encapsulation: can encapsulate both hydrophilic and hydrophobic drugs. Biofilm penetration: their structure allows them to penetrate the protective matrix of bacterial biofilms [214]. | Liposomes: encapsulating vancomycin to enhance its stability and bioavailability, particularly for treating infections in challenging environments. Solid lipid nanoparticles (SLNs): provide controlled release and enhance drug bioavailability [215]. |
| Inorganic and metal-based nanoparticles | Intrinsic antimicrobial activity: some, like silver and gold, can directly disrupt bacterial membranes or generate reactive oxygen species (ROS), reducing the likelihood of resistance. Synergistic effects: can be combined with conventional antibiotics to restore efficacy against resistant strains [216]. | Silver nanoparticles: can be used to combat biofilms by penetrating the matrix and targeting dormant bacteria. Gold nanoparticles: functionalized with antibiotics to increase activity against drug-resistant bacteria like MRSA [217]. |
| Drug-Delivery Systems | Advantages | Limitations | Ref. |
|---|---|---|---|
| Nanoparticles | Improve antibiotic activity; allow targeted release; multiple drugs can be loaded; enhance microbial activity; improve stability potential for more accurate evaluation. | Size- and shape-dependent toxicity; poor intracellular penetration; increased surface area of NPs increases chemical reactivity, which leads to critical instability. | [187,197,209,211,218] |
| Hydrogels | Biocompatible; supports cell adhesion; provides sustained release of antibiotics; increases patient compliance; enhanced biofilm activity when combined with enzymes. | Slow responsiveness of stimuli-sensitive hydrogels; risk of burst release or incomplete release; possibility of drug deactivation. | [205,223,241] |
| Liposomes | Excellent biocompatibility; biodegradable; encapsulate both hydrophilic and hydrophobic drugs; improved wound healing; possess flexibility to couple with specific ligands. | Short shelf-life of lipid vesicles, limiting drug stability; aggregation and fusion of liposomal vesicles influence the efficacy of the drug; high production costs. | [206,226,229] |
| Solid lipid nanoparticles (SLNs) | Biocompatibility and biodegradability; controlled drug release profile; low toxicity; enhanced biofilm inhibition. | Low expulsion of drug time; limited ability to encapsulate hydrophilic drugs; drug expulsion during storage. | [238,242,243] |
| Antibiotic | Class | Key Efficacy | Safety and Toxicity Concerns | Clinical Considerations | Ref. |
|---|---|---|---|---|---|
| Penicillin | β-lactam | Broad-spectrum activity against Gram-positive bacteria [201]. | - Allergic reactions (1–10% of population, often over-diagnosed). - IgE-mediated hypersensitivity, which is rare (1–2% of adverse reactions) [202,203]. | - Skin testing reliable for de-labeling allergies (especially in children). - Pharmacist-driven protocols improve accurate allergy assessment. | [247,248,249,250] |
| Vancomycin | Glycopeptide | Effective against MRSA and Gram-positive infections [205]. | - Nephrotoxicity risk (especially in kidney disease or obesity). - Altered PKs in critically ill patients [206]. | - Loading doses improve therapeutic levels without increasing toxicity. - Individualized dosing needed for obese/renal-impaired patients. | [251,252,253] |
| Gentamicin | Aminoglycoside | Synergistic with β-lactams; effective against Gram-negative bacilli [208]. | - Nephrotoxicity and irreversible ototoxicity (higher risk in children and elderly and critically ill people). - Narrow therapeutic window [209,210]. | - Once-daily dosing preferred (reduces toxicity). - Therapeutic drug monitoring (TDM) recommended for high-risk patients. | [254,255,256,257] |
| Tobramycin | Aminoglycoside | Superior anti-Pseudomonas activity (vs. gentamicin) [212]. | - Ototoxicity and nephrotoxicity (similar to gentamicin). - Weak rRNA binding may increase side effects [213,214]. | - Inhaled form reduces exacerbations in cystic fibrosis/bronchiectasis. - Long-term inhalation therapy improves quality of life. | [258,259,260,261,262] |
| Strategy | Description | Key Considerations | Ref. |
|---|---|---|---|
| Clinical protocols | Integrating natural antibiotics into standard treatment guidelines [201]. | Optimizing dosing (e.g., vancomycin loading doses), mitigating toxicity risks, and implementing allergy de-labeling (e.g., penicillin skin testing). | [247,253] |
| Industrial scalability | Overcoming challenges in large-scale production and standardization [18]. | Using biotechnological advancements like CRISPR-based strain engineering and “omics-driven” discovery to ensure stability and consistent quality. | [23,24] |
| Synergistic formulations | Developing combination therapies to enhance efficacy [79]. | Pairing natural antibiotics (e.g., polyphenols) with conventional drugs (e.g., β-lactams) to increase effectiveness and delay resistance. | [123,160] |
| Regulatory and economic rules | Advocating for policies that support the development of natural antibiotics [198]. | Highlighting their lower toxicity profiles and multi-target mechanisms to encourage investment and streamline approval processes. | [244,265] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, A.T.; Visan, A.I. Mechanism, Efficacy, and Safety of Natural Antibiotics. Antibiotics 2025, 14, 981. https://doi.org/10.3390/antibiotics14100981
Matei AT, Visan AI. Mechanism, Efficacy, and Safety of Natural Antibiotics. Antibiotics. 2025; 14(10):981. https://doi.org/10.3390/antibiotics14100981
Chicago/Turabian StyleMatei, Andrei Teodor, and Anita Ioana Visan. 2025. "Mechanism, Efficacy, and Safety of Natural Antibiotics" Antibiotics 14, no. 10: 981. https://doi.org/10.3390/antibiotics14100981
APA StyleMatei, A. T., & Visan, A. I. (2025). Mechanism, Efficacy, and Safety of Natural Antibiotics. Antibiotics, 14(10), 981. https://doi.org/10.3390/antibiotics14100981







