Selective Targeting and Enhanced Photodynamic Inactivation of Methicillin-Resistant Staphylococcus aureus (MRSA) by a Decacationic Vancomycin–Mesochlorin Conjugate
Abstract
1. Introduction
2. Results
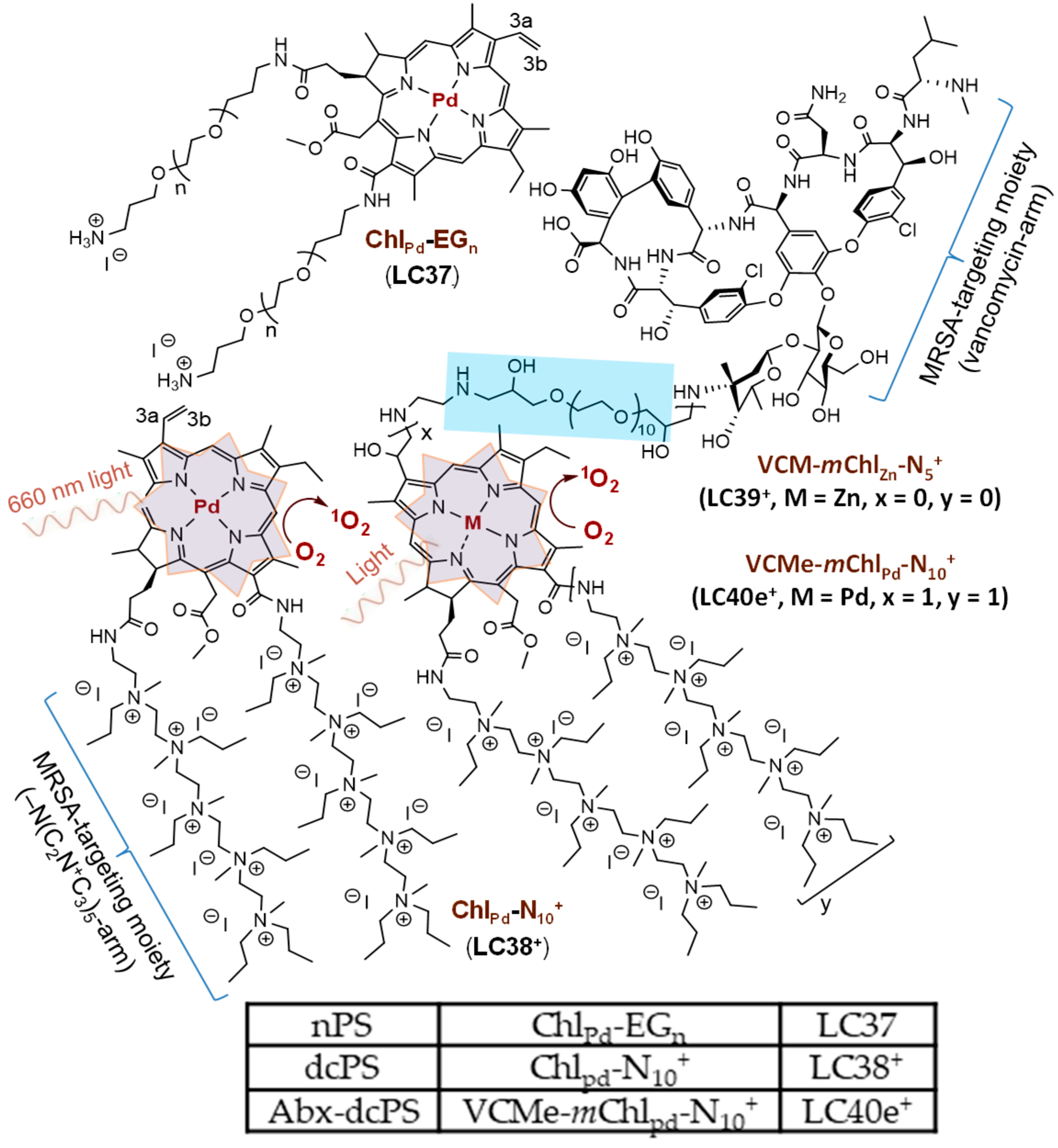
2.1. Material Preparation
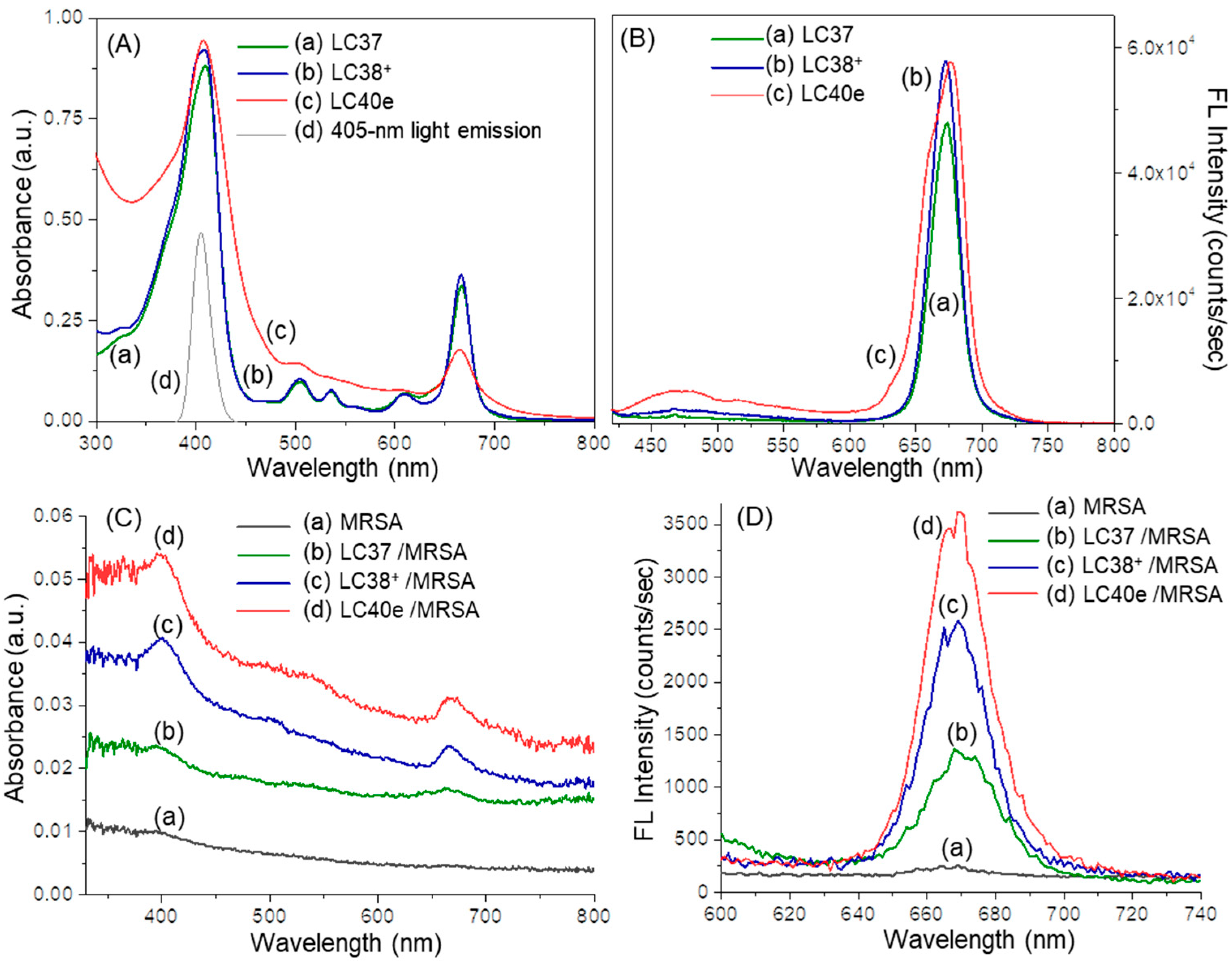
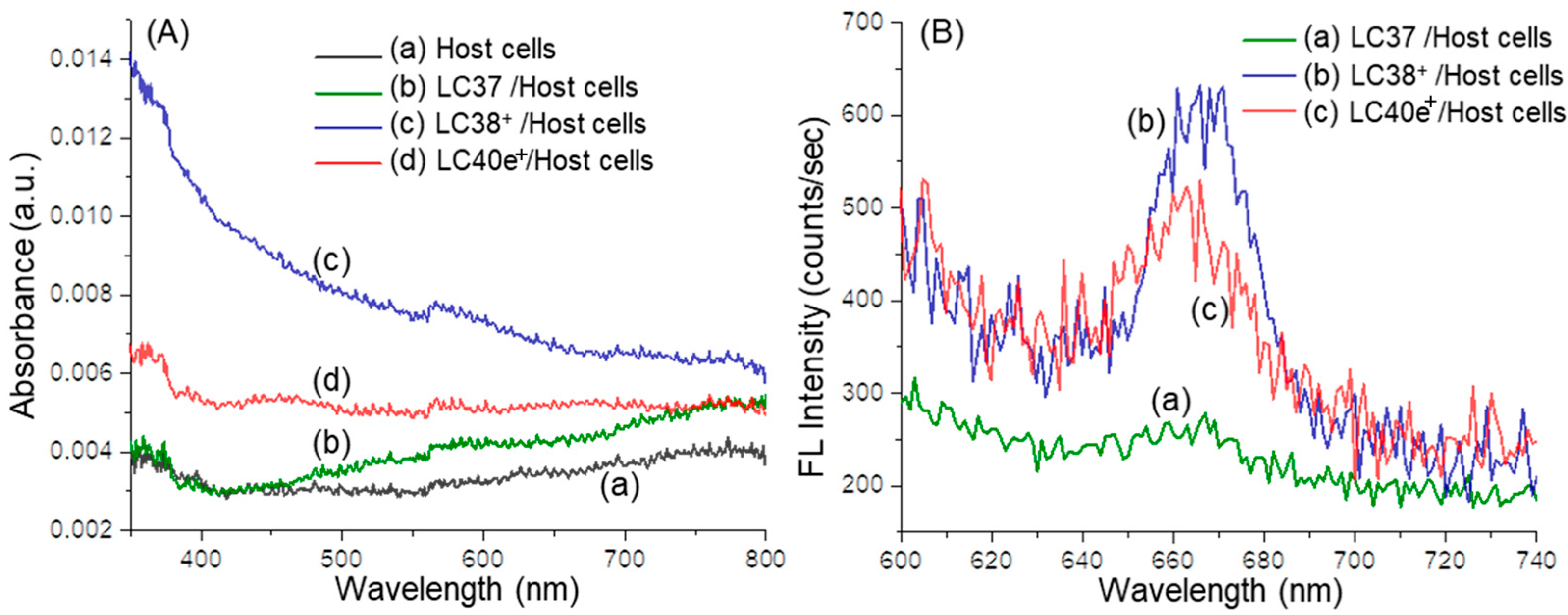
2.2. Photophysical Properties of dcPS ChlPd-N10+ (LC38+) and Abx-dcPS VCMe-mChlPd-N10+ (LC40e+)
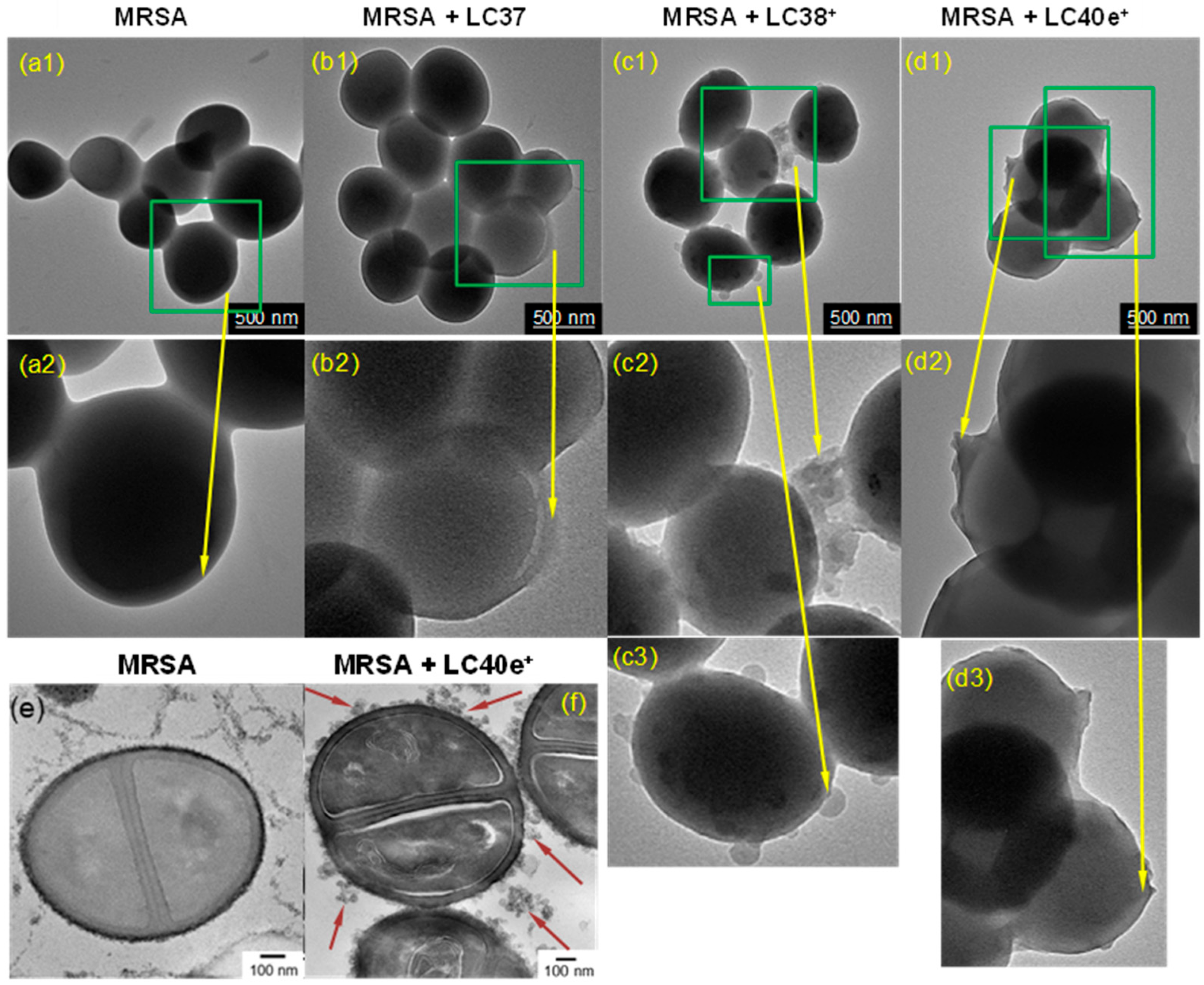
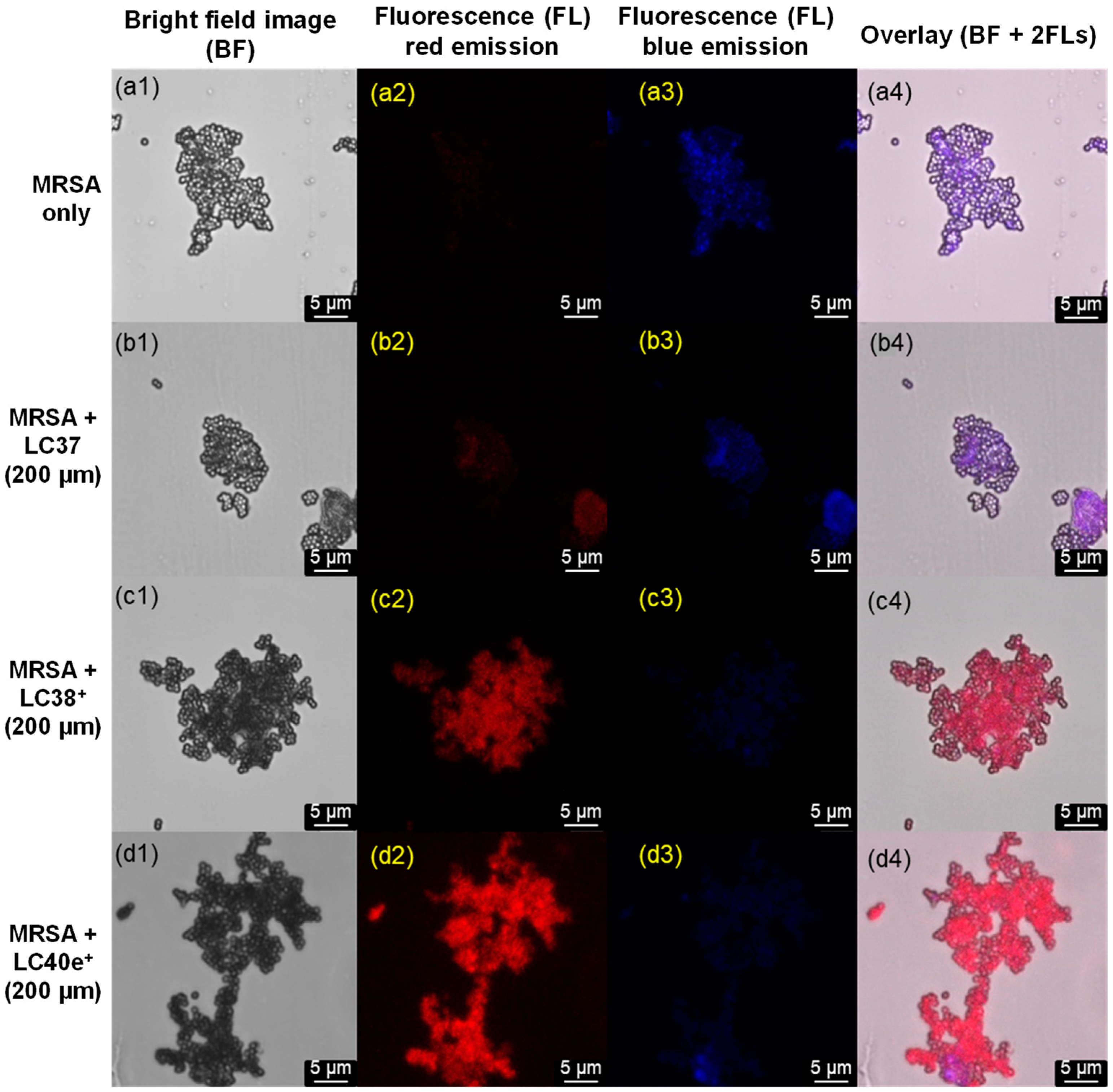
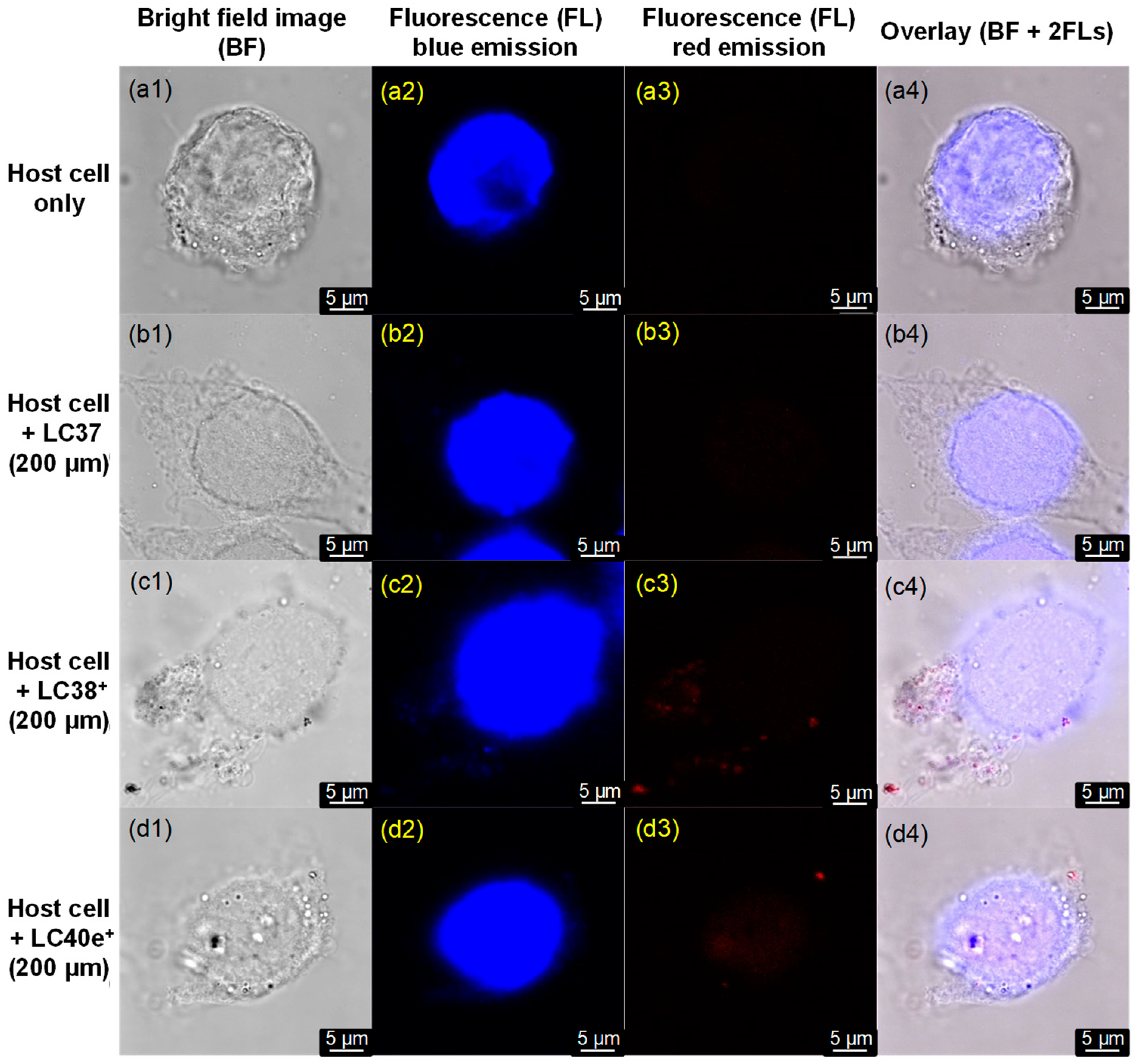
2.3. MRSA Cell Targeting Evaluation of dcPS ChlPd-N10+ (LC38+) and Abx-dcPS VCMe-mChlPd-N10+ (LC40e+)
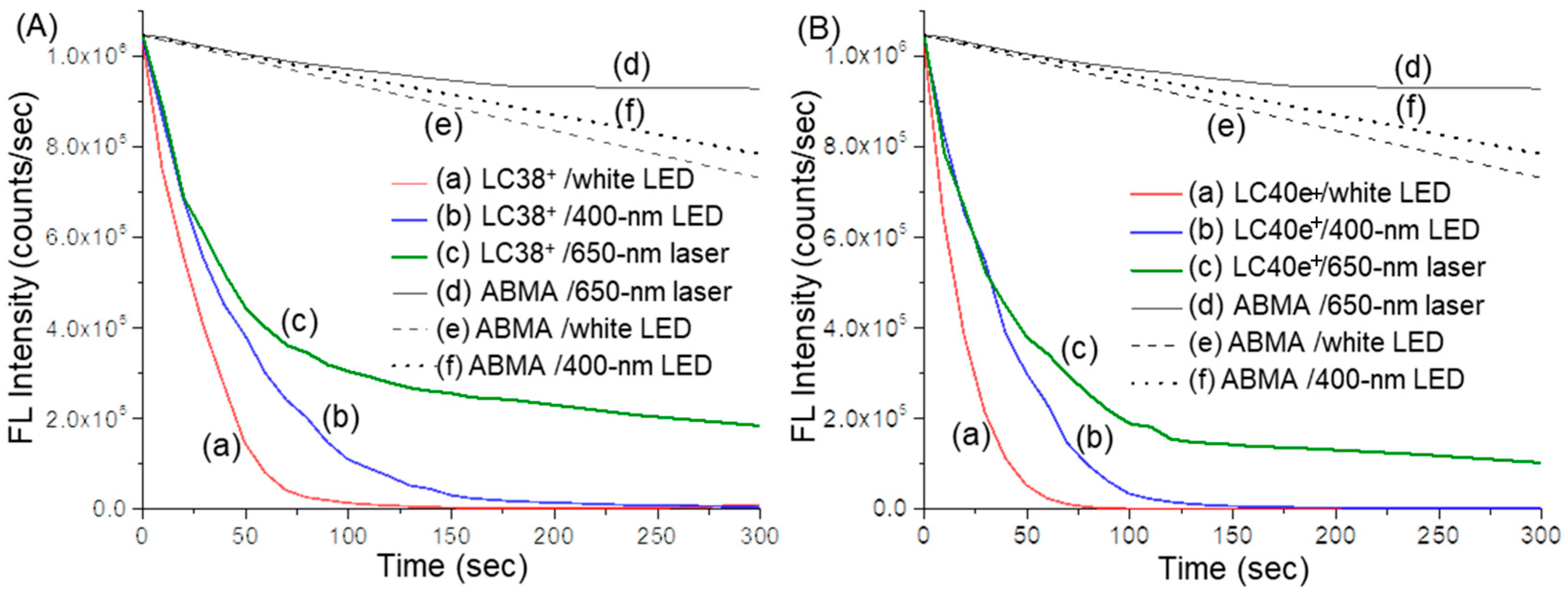
2.4. Reactive Oxygen Species (ROS) Generation Efficiency of dcPS ChlPd-N10+ (LC38+) and Abx-dcPS VCMe-mChlPd-N10+ (LC40e+)
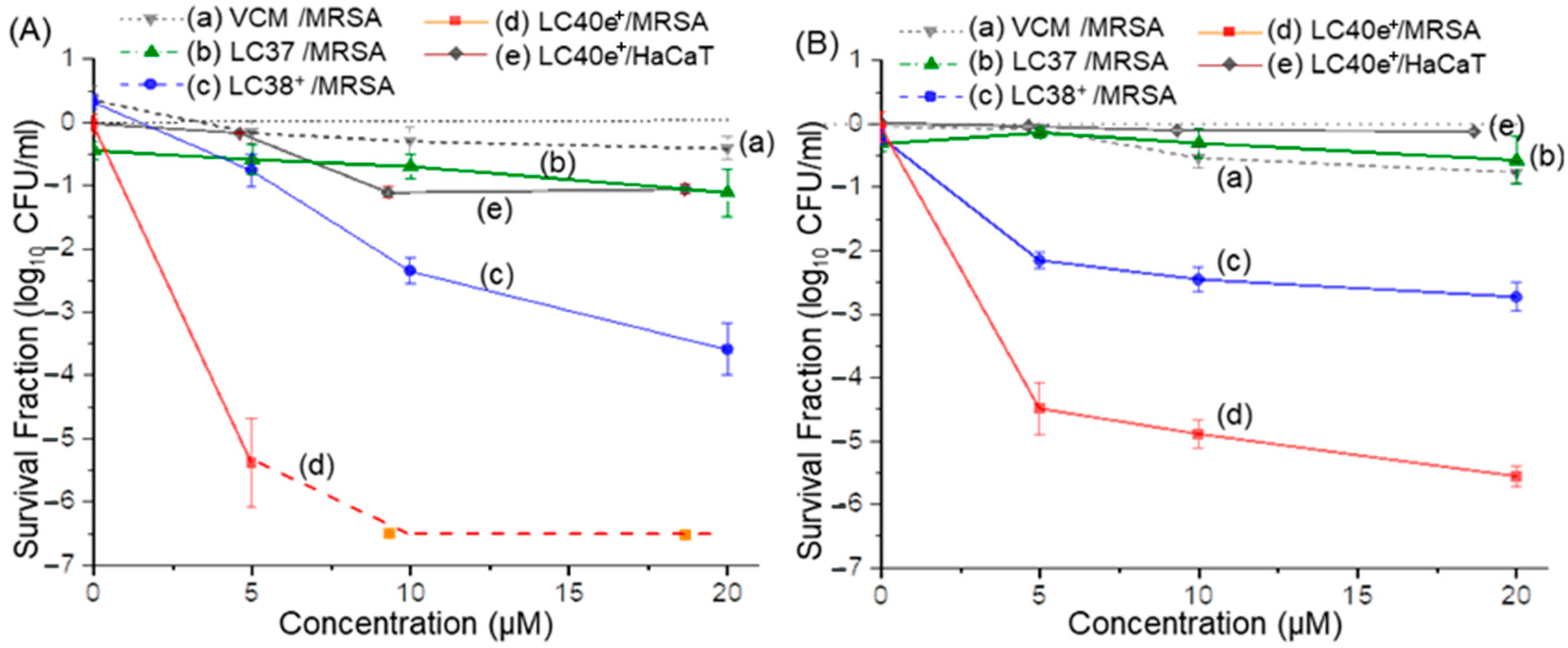
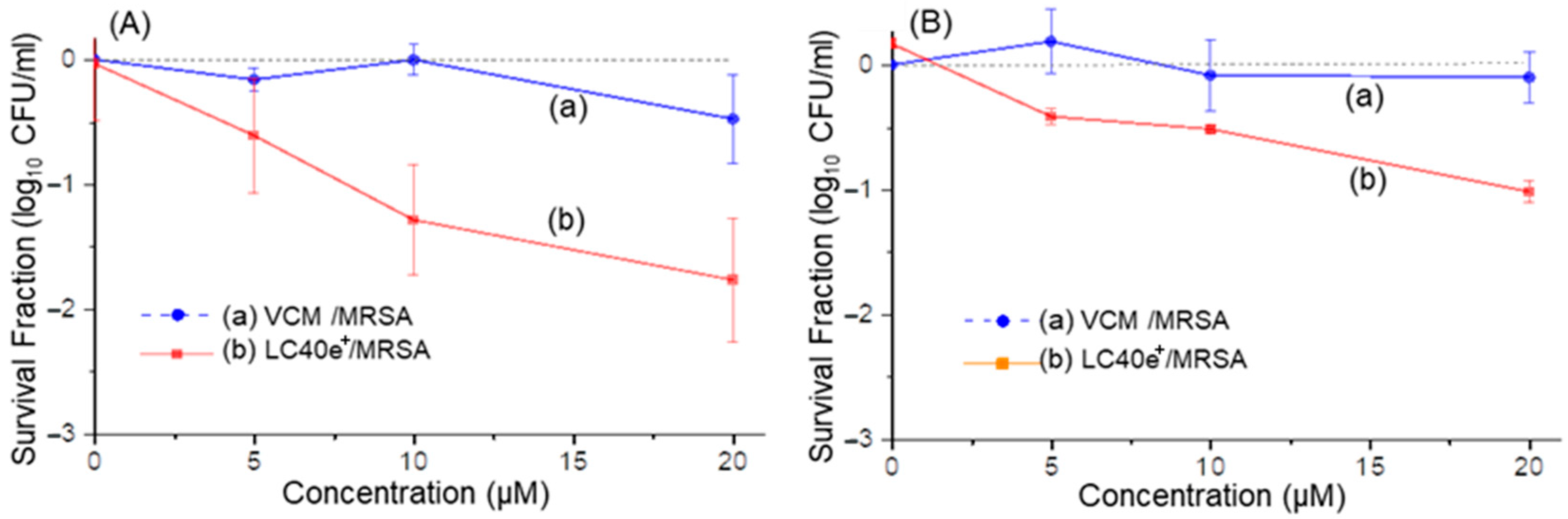
2.5. aPDI Efficiency of ChlPd-N10+ (LC38+) and VCMe-mChlPd-N10+ (LC40e+) Against MRSA Cells
2.6. aPDI Efficiency of VCMe-mChlPd-N10+ (LC40e+) Against MRSA Biofilms
2.7. Cytotoxicity of LC40e+ Mediated aPDI to Human Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Synthesis of 15b-Methyl-13a,17c-di[aminopropylpolyoxyethylene]-chlorin e6 (Chlpd-EGn, LC37, nPS); 15b-Methyl-13a,17c-di[N,N’,N,N,N,N-hexapropyl-panta(aminoethylene)] Amide-[Pd+2]chlorin e6-deca(quaternary methyl-ammonium iodide) (ChlPd-N10+, LC38+, dcPS) and 3a-Hydroxy-3b-aminoethylene-aminoPEGlycolated vancomycin-15b-methyl-13a,17c-di[N,N’,N,N,N,N-hexapropyl-panta(aminoethylene)]amide-mesochlorin-deca(quaternary methyl-ammonium iodide) (VCMe-mChlPd-N10+, LC40e+, Abx-dcPS)
4.3. Spectroscopic and Photophysical Characterization Instruments
4.4. Light Sources Applied in aPDI Experiments
4.5. Reactive Oxygen Species (ROS) Detection Using Singlet Oxygen (1O2)-Sensitive Fluorescent Probe
4.6. Bacterial Stain and Culture Conditions
4.7. Human Keratinocytes and Culture Conditions
4.8. Planktonic MRSA Cell Preparation and Conditions for aPDI Evaluations
4.9. MRSA Biofilm Preparation and Conditions for aPDI Evaluations
4.10. Examination of Photocytotoxicity to Human Cells
4.11. Statistical Analysis
4.12. Fixation of Bacteria and Host Cells for Targeting Imaging Studies
4.13. Transmission Electron Microscopic (TEM) Image Evaluation
4.14. Confocal Microscopic Fluorescent Imaging Measurements
4.15. MIC Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, M.W.; Griffith, M.E.; Jorgensen, J.H.; Hospenthal, D.R.; Mende, K.; Patterson, J.E. Presence and molecular epidemiology of virulence factors in methicillin-resistant Staphylococcus aureus strains colonizing and infecting soldiers. J. Clin. Microbiol. 2009, 47, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; David, M.Z.; Salata, R.A. Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2012, 61 Pt 9, 1179–1193. [Google Scholar] [CrossRef]
- Lacey, K.A.; Geoghegan, J.A.; McLoughlin, R.M. The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens. Pathogens 2016, 5, 22. [Google Scholar] [CrossRef]
- Copin, R.; Sause, W.E.; Fulmer, Y.; Balasubramanian, D.; Dyzenhaus, S.; Ahmed, J.M.; Kumar, K.; Lees, J.; Stachel, A.; Fisher, J.C.; et al. Sequential evolution of virulence and resistance during clonal spread of community-acquired methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2019, 116, 1745–1754. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Harkins, C.P.; Pichon, B.; Doumith, M.; Parkhill, J.; Westh, H.; Tomasz, A.; de Lencastre, H.; Bentley, S.D.; Kearns, A.M.; Holden, M.T.G. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017, 18, 130. [Google Scholar] [CrossRef]
- Okwu, M.U.; Olley, M.; Akpoka, A.O.; Izevbuwa, O.E. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol. 2019, 5, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Abbanat, D.; Morrow, B.; Bush, K. New agents in development for the treatment of bacterial infections. Curr. Opin. Pharmacol. 2008, 8, 582–592. [Google Scholar] [CrossRef]
- Devasahayam, G.; Scheld, W.M.; Hoffman, P.S. Newer antibacterial drugs for a new century. Expert. Opin. Investig. Drugs 2010, 19, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Rai, J.; Randhawa, G.K.; Kaur, M. Recent advances in antibacterial drugs. Int. J. Appl. Basic. Med. Res. 2013, 3, 3–10. [Google Scholar]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Rosa, L.P.; da Silva, F.C. Antimicrobial Photodynamic Therapy: A New Therapeutic Option to Combat Infections. J. Med. Microb. Diagn. 2014, 03, 158. [Google Scholar]
- Zhou, W.; Jiang, X.; Zhen, X. Development of organic photosensitizers for antimicrobial photodynamic therapy. Biomater. Sci. 2023, 11, 5108–5128. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.; Faustino, M.A.; Neves, M.G.; Tome, J.P.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Gomes, N.C.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Lauro, F.M.; Pretto, P.; Covolo, L.; Jori, G.; Bertoloni, G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem. Photobiol. Sci. 2002, 1, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Heal. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Leger, D.Y.; Liagre, B.; Therrien, B. Photosensitizers Used in the Photodynamic Therapy of Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 3339. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, M.; Huang, Y.Y.; Huang, H.C.; Avci, P.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with decacationic (60)fullerene monoadducts: Effect of a light absorbing electron-donor antenna and micellar formulation. Nanomedicine 2014, 10, 795–808. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Sharma, S.K.; Wang, M.; Jeon, S.; Huang, Y.-Y.; Dai, T.; Nayka, S.; de Sousa, S.C.O.M.; Chiang, L.Y.; Hamblin, M.R. Photoinduced electron-transfer mechanisms for radical-enhanced photodynamic therapy mediated by water-soluble decacationic C70 and C84O2 Fullerene Derivatives. Nanomed. Nanotech. Biol. Med. 2013, 9, 570–579. [Google Scholar] [CrossRef]
- Yin, R.; Wang, M.; Huang, Y.Y.; Landi, G.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: Oxygen-independent photokilling in presence of azide and new mechanistic insights. Free Radic. Biol. Med. 2015, 79, 14–27. [Google Scholar] [CrossRef]
- Wang, M.; Maragani, S.; Huang, L.; Jeon, S.; Canteenwala, T.; Hamblin, M.R.; Chiang, L.Y. Synthesis of decacationic (60)fullerene decaiodides giving photoinduced production of superoxide radicals and effective PDT-mediation on antimicrobial photoinactivation. Eur. J. Med. Chem. 2013, 63, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Rajda, P.J.; Szewczyk, G.; Bhayana, B.; Chiang, L.Y.; Sarna, T.; Hamblin, M.R. Sodium nitrite potentiates antimicrobial photodynamic inactivation: Possible involvement of peroxynitrate. Photochem. Photobiol. Sci. 2019, 18, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Shimizu, M.; Katafuchi, A.; Grúz, P.; Fujii, S.; Usui, Y.; Fuchs, R.P.; Nohmi, T. Escherichia coli DNA polymerase III is responsible for the high level of spontaneous mutations in mutT strains. Mol. Microbiol. 2012, 86, 1364–1375. [Google Scholar] [CrossRef]
- Xing, B.; Jiang, T.; Wu, X.; Liew, R.; Zhou, J.; Zhang, D.; Yeow, E.K.L. Molecular interactions between glycopeptide vancomycin and bacterial cell wall peptide analogues. Chemistry 2011, 17, 14170–14177. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, H.; Olademehin, O.P.; Kim, S.J.; Tao, P. Insights into Key Interactions between Vancomycin and Bacterial Cell Wall Structures. ACS Omega 2018, 3, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Bellnier, D.A.; Smith, K.M.; Dougherty, T.J. Chlorin and porphyrin derivatives as potential photosensitizers in photodynamic therapy. Photochem. Photobiol. 1991, 53, 65–72. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Beauregard, D.A.; Williams, D.H.; Gwynn, M.N.; Knowles, D.J. Dimerization and membrane anchors in extracellular targeting of vancomycin group antibiotics. Antimicrob. Agents Chemother. 1995, 39, 781–785. [Google Scholar] [CrossRef]
- Mackay, J.P.; Gerhard, U.; Beauregard, D.A.; Williams, D.H.; Westwell, M.S.; Searle, M.S. Glycopeptide antibiotic activity and the possible role of dimerization: A model for biological signaling. J. Am. Chem. Soc. 1994, 116, 4581–4590. [Google Scholar] [CrossRef]
- Huang, L.; Wang, M.; Huang, Y.Y.; El-Hussein, A.; Wolf, L.M.; Chiang, L.Y.; Hamblin, M.R. Progressive cationic functionalization of chlorin derivatives for antimicrobial photodynamic inactivation and related vancomycin conjugates. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef]
- Wang, M.; Huang, L.; Sharma, S.K.; Jeon, S.; Thota, S.; Sperandio, F.F.; Nayka, S.; Chang, J.; Hamblin, M.R.; Chiang, L.Y. Synthesis and photodynamic effect of new highly photostable decacationically armed (60)- and (70)fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells. J. Med. Chem. 2012, 55, 4274–4285. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rohatgi-Mukherjee, K.K. Solvent Effect on Photosensitized Oxidation of Iodide Ion by Anthracene Sulphonates. Photochem. Photobiol. 1978, 27, 539–543. [Google Scholar] [CrossRef]
- Maeda, H.; Yamamoto, K.; Nomura, Y.; Kohno, I.; Hafsi, L.; Ueda, N.; Yoshida, S.; Fukuda, M.; Fukuyasu, Y.; Yamauchi, Y.; et al. A design of fluorescent probes for superoxide based on a nonredox mechanism. J. Am. Chem. Soc. 2005, 127, 68–69. [Google Scholar] [CrossRef]
- de Melo, W.C.; Avci, P.; de Oliveira, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huang, Y.Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert. Rev. Anti Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.; Figueiro Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Codling, C.E.; Maillard, J.Y.; Russell, A.D. Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine. J. Antimicrob. Chemother. 2003, 51, 1153–1158. [Google Scholar] [CrossRef]
- Kügler, R.; Bouloussa, O.; Rondelez, F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 2005, 151, 1341–1348. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Demidova, T.N.; Arcila-Lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 2005, 12, 1127–1135. [Google Scholar] [CrossRef]
- Hamblin, M.R. Fullerenes as photosensitizers in photodynamic therapy: Pros and cons. Photochem. Photobiol. Sci. 2018, 17, 1515–1533. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, M.; Dai, T.; Sperandio, F.F.; Huang, Y.Y.; Xuan, Y.; Chiang, L.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy with decacationic monoadducts and bisadducts of (70)fullerene: In vitro and in vivo studies. Nanomedicine 2014, 9, 253–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, T.; Wang, M.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: In vitro and in vivo studies. Nanomedicine 2015, 10, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Bruniera, F.R.; Ferreira, F.M.; Saviolli, L.R.M.; Bacci, M.R.; Feder, D.; da Luz Gonçalves Pedreira, M.; Sorgini Peterlini, M.A.; Azzalis, L.A.; Campos Junqueira, V.B.; Fonseca, F.L.A. The use of vancomycin with its therapeutic and adverse effects: A review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 694–700. [Google Scholar]
- Nyenje, M.E.; Tanih, N.F.; Ndip, R.N. A comparative study of M.I.C evaluator test with the broth microdilution method for antimicrobial susceptibility testing of Enterobacter cloacae isolated from cooked food. Pak. J. Pharm. Sci. 2014, 27, 63–66. [Google Scholar] [PubMed]
- Hamblin, M.R.; O’Donnell, D.A.; Murthy, N.; Contag, C.H.; Hasan, T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem. Photobiol. 2002, 75, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.S.; Gomes, M.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Cavaleiro, J.A.S.; Almeida, A. Comparative photodynamic inactivation of antibiotic resistant bacteria by first and second generation cationic photosensitizers. Photochem. Photobiol. Sci. 2012, 11, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, D.D.; Reynoso, E.; Cordero, P.; Spesia, M.B.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Synthesis and properties of 5,10,15,20-tetrakis [4-(3-N,N-dimethylaminopropoxy)phenyl] chlorin as potential broad-spectrum antimicrobial photosensitizers. J. Photochem. Photobiol. B Biol. 2016, 158, 243–251. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Liu, X.; Wang, M.; Wang, Y.; Dai, T.; Chiang, L.Y. Selective Targeting and Enhanced Photodynamic Inactivation of Methicillin-Resistant Staphylococcus aureus (MRSA) by a Decacationic Vancomycin–Mesochlorin Conjugate. Antibiotics 2025, 14, 978. https://doi.org/10.3390/antibiotics14100978
Yin H, Liu X, Wang M, Wang Y, Dai T, Chiang LY. Selective Targeting and Enhanced Photodynamic Inactivation of Methicillin-Resistant Staphylococcus aureus (MRSA) by a Decacationic Vancomycin–Mesochlorin Conjugate. Antibiotics. 2025; 14(10):978. https://doi.org/10.3390/antibiotics14100978
Chicago/Turabian StyleYin, He, Xiaojing Liu, Min Wang, Ying Wang, Tianhong Dai, and Long Y. Chiang. 2025. "Selective Targeting and Enhanced Photodynamic Inactivation of Methicillin-Resistant Staphylococcus aureus (MRSA) by a Decacationic Vancomycin–Mesochlorin Conjugate" Antibiotics 14, no. 10: 978. https://doi.org/10.3390/antibiotics14100978
APA StyleYin, H., Liu, X., Wang, M., Wang, Y., Dai, T., & Chiang, L. Y. (2025). Selective Targeting and Enhanced Photodynamic Inactivation of Methicillin-Resistant Staphylococcus aureus (MRSA) by a Decacationic Vancomycin–Mesochlorin Conjugate. Antibiotics, 14(10), 978. https://doi.org/10.3390/antibiotics14100978







