Monitoring the Spread of Multidrug-Resistant Escherichia coli Throughout the Broiler Production Cycle
Abstract
1. Introduction
2. Results
2.1. Characteristics of Farms and E. coli Isolates
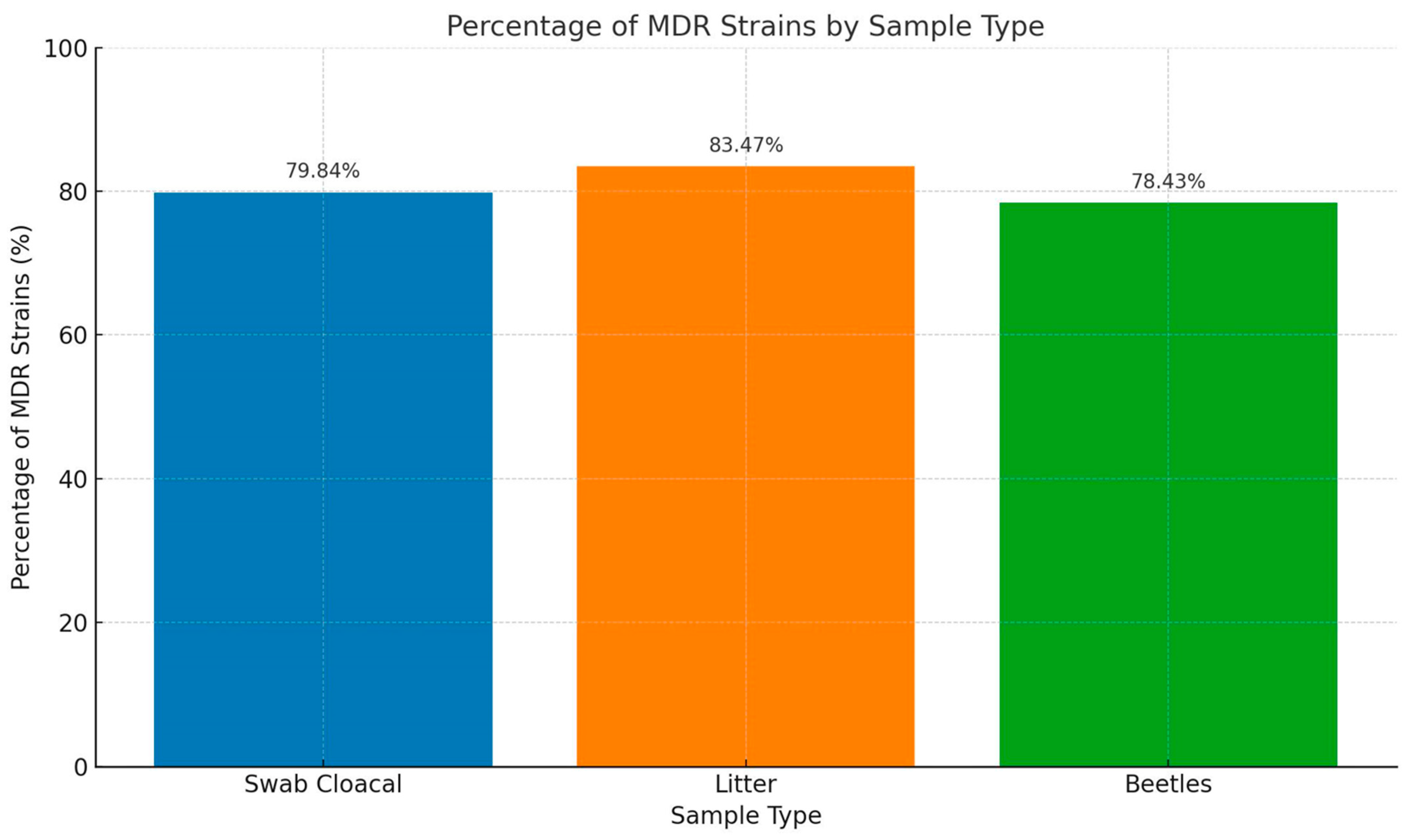
2.2. Phenotypic Antimicrobial Resistance
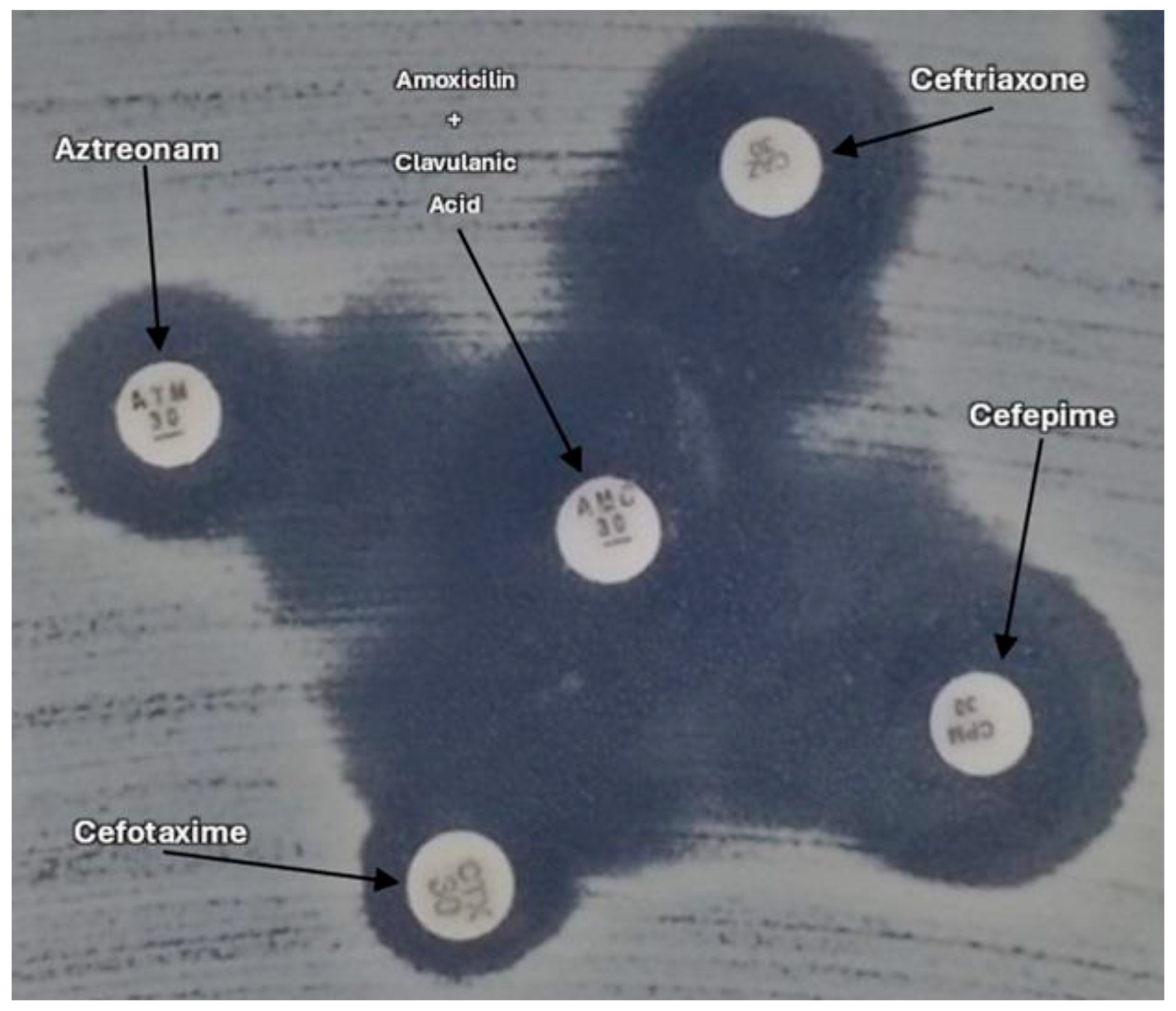
2.3. Detection of ESBL Genes (blaCTX-M; blaTEM; blaSHV) and Virulence Genes
2.4. Multivariate Logistic Regression
3. Discussion
4. Materials and Methods
4.1. Study Period and Location
4.2. Characteristics of the Farms
4.3. Sample Collection Procedure
4.4. Isolation of E. coli
4.5. Antimicrobial Susceptibility Testing
4.6. DNA Extraction
4.7. Detection of ESBL Genes and Virulence Genes for Potential Avian Pathogenic Escherichia coli (APEC)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brazilian Association of Animal Protein. Annual Poultry Report. ABPA. 2024. Available online: https://abpa-br.org/wp-content/uploads/2024/04/ABPA-Relatorio-Anual-2024_capa_frango.pdf (accessed on 5 November 2024).
- Erian, I.; Philips, C.J. Public understanding and attitudes towards meat chicken production and relations to consumption. Animals 2017, 7, 20. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Gou, M.; Wang, J.T.; Chen, D.; He, J.Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle, and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Alvez, H.C.; Cruz, F.P.N.; Assis, P.C.P.; Pessoa, J.D.C.; Trevelin, L.C.; Leal, A.M.O.; Sousa, C.P. Antibiotic resistance among Escherichia coli: Isolates and novel approaches to the control of E. coli infections. In Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; Samie, A., Ed.; IntechOpen: London, UK, 2017; pp. 99–122. [Google Scholar]
- Penido, C. Antibióticos carbapenêmicos. In Curso Básico de Antimicrobianos; Divisão de MI, Faculdade de Medicina de Ribeirão Preto, USP: Ribeirão Preto, Brazil, 2018. [Google Scholar]
- Bajaj, P.; Singh, N.S.; Virdi, J.S. Escherichia coli β-lactamases: What really matters. Front. Microbiol. 2016, 7, 417. [Google Scholar] [CrossRef] [PubMed]
- Apata, D.F. The emergence of antibiotic resistance and utilization of probiotics for poultry production. Sci. J. Microbiol. 2012, 2, 8–13. [Google Scholar]
- Labro, M.; Bryskier, J. Antibacterial resistance: An emerging ‘zoonosis’? Expert. Rev. Anti Infect. Ther. 2014, 12, 1441–1461. [Google Scholar] [CrossRef]
- Menck-Costa, M.F.; Baptista, A.A.S.; Gazal, L.E.S.; Justino, L.; Sanches, M.S.; de Souza, M.; Nishio, E.K.; Queiroz dos Santos, B.; Cruz, V.D.; Berbert, J.V.M.; et al. High-frequency detection of fosA3 and blaCTX-M-55 genes in Escherichia coli from longitudinal monitoring in broiler chicken farms. Front. Microbiol. 2022, 13, 846116. [Google Scholar] [CrossRef]
- Gazal, L.E.S.; Medeiros, L.P.; Dibo, M.; Nishio, E.K.; Koga, V.L.; Gonçalves, B.C. Detection of ESBL/AmpC-producing and fosfomycin-resistant Escherichia coli from different sources in poultry production in southern Brazil. Front. Microbiol. 2021, 11, 604544. [Google Scholar] [CrossRef]
- Koga, V.L.; Maluta, R.P.; Silveira, W.D.; Ribeiro, R.A.; Hungria, M.; Vespero, E.C.; Nakazato, G.; Kobayashi, R.K.T. Characterization of CMY-2-type β-lactamase-producing Escherichia coli isolated from chicken carcasses and human infection in a city of South Brazil. BMC Microbiol. 2019, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Johnston, B.; Clabots, C.R.; Kuskowski, M.A.; Roberts, E.; Debrouy, C. Virulence genotypes and phylogenetic background of Escherichia coli serogroup O6 isolates from humans, dogs and cats. J. Clin. Microbiol. 2008, 46, 417–422. [Google Scholar] [CrossRef]
- Rodriguez-Siek, K.E.; Nolan, L.; Giddings, C.; Doetkott, C.; Johnson, T. Characterizing the APEC pathotype. Vet. Res. 2005, 36, 241–256. [Google Scholar] [CrossRef]
- Ferreira, A.J.P.; Knobl, T. Colibacilose. In Doença das Aves, 2nd ed.; Junior, A.B., Silva, E.N., Fábio, J.D., Sesti, L., Zuanaze, M.A., Eds.; Fundação APINCO: Campinas, Brazil, 2009; pp. 457–471. [Google Scholar]
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J. Clin. Microbiol. 2008, 46, 3987–3996. [Google Scholar] [CrossRef]
- Brasil. Instrução Normativa MAPA n° 41, de 23 de outubro de 2017—Institui o Programa Nacional de Prevenção e Controle da Resistência aos Antimicrobianos na Agropecuária—Agroprevine, no âmbito do Ministério da Agricultura, Pecuária e Abastecimento. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-pecuarios/resistencia-aos-antimicrobianos/legislacao/INSTRUONORMATIVAN41DE23DEOUTUBRODE2017.pdf (accessed on 22 October 2024).
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Saliu, E.M.; Vahjen, W.; Zentek, J. Types and prevalence of extended-spectrum beta-lactamase producing Enterobacteriaceae in poultry. Anim. Health Res. Rev. 2017, 18, 46–57. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment: A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Han, T.; Zhang, Q.; Liu, N.; Wang, J.; Li, Y.; Huang, X.; Liu, J.; Wang, J.; Qu, Z.; Qi, K. Changes in antibiotic resistance of Escherichia coli during the broiler feeding cycle. Poult. Sci. 2020, 99, 6983–6989. [Google Scholar] [CrossRef] [PubMed]
- Gazal, L.E.S.; Puño-Sarmiento, J.J.; Medeiros, L.P.; Cyoia, P.S.; da Silveira, W.D.; Kobayashi, R.K.; Nakazato, G. Presence of pathogenicity islands and virulence genes of extraintestinal pathogenic Escherichia coli (ExPEC) in isolates from avian organic fertilizer. Poult. Sci. 2015, 94, 3025–3033. [Google Scholar] [CrossRef]
- Cyoia, P.S.; Koga, V.L.; Nishio, E.K.; Houle, S.; Dozois, C.M.; Brito, K.C.T.; De Brito, B.G.E.; Nakazato, G.; Kobayashi, R.K.T. Distribution of ExPEC virulence factors, blaCTX-M, fosA3, and mcr-1 in Escherichia coli isolated from commercialized chicken carcasses. Front. Microbiol. 2019, 9, 3254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Nhung, N.I.; Juan, J.C. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar]
- Hachesoo, B.A.; Asasi, K.; Sharifiyazdi, H. Farm-level evaluation of enrofloxacin resistance in Escherichia coli isolated from broiler chickens during a rearing period. Com. Clin. Pathol. 2017, 26, 471–476. [Google Scholar] [CrossRef]
- Azizpour, A.; Saeidi, N.V. Investigation of antibiotic resistance patterns in Escherichia coli isolated from broiler chickens with colibacillosis to ten antibacterial agents commonly used in the Iranian poultry industry. J. Comp. Pathobiol. Iran. 2018, 14, 59. [Google Scholar]
- Coque, T.M.; Baquero, F.; Canton, R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eurosurveillance 2008, 13, 1–11. [Google Scholar] [CrossRef]
- Li, S.; Zhao, M.; Liu, J.; Zhou, Y.; Miao, Z. Prevalence and antibiotic resistance profiles of extended-spectrum β-lactamase-producing Escherichia coli isolated from healthy broilers in Shandong Province, China. J. Food Prot. 2016, 79, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Partridge, S.R.; Shen, J.; Zeng, Z.; Liu, L.; Rao, L. CTX-M-123, a novel hybrid of the CTX-M-1 and CTX-M-9 group β-lactamases recovered from Escherichia coli isolates in China. Antimicrob. Agents Chemother. 2013, 57, 4068–4071. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galán, J.C.; González-Candelas, F.; Rolain, J.M.; Cantón, R. Antibiotics as selectors and accelerators of diversity in the mechanisms of resistance: From the resistome to genetic plasticity in the β-lactamases world. Front. Microbiol. 2013, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Segabinazi, S.D.; Flôres, M.L.; da Silva Barcelos, A.; Jacobsen, G.; Eltz, R.D. Bactérias da família Enterobacteriaceae em Alphitobius diaperinus oriundos de granjas avícolas dos Estados do Rio Grande do Sul e Santa Catarina, Brasil. Acta Sci. Veterinariae. 2005, 33, 51–55. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, 30th ed. In CLSI Supplement Document M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J. Antimicrob. Chemother. 2005, 57, 154–155. [Google Scholar] [CrossRef]
- Arlet, G.; Philippon, A. Construction by polymerase chain reaction and use of intragenic DNA probes for three main types of transferable β-lactamases (TEM, SHV, CARB). FEMS Microbiol. Lett. 1991, 66, 19–25. [Google Scholar] [PubMed]
- Menard, S. Applied Logistic Regression Analysis; Sage: New York, NY, USA, 2002; Volume 2, p. 07-106. [Google Scholar]


| Farms | (%) MDR | (%) ESBL | Poultry Age (Days) | Antimicrobials Used | Litter Treatment |
|---|---|---|---|---|---|
| G1 | 93.83 | 14 | 3 | SUT | Fermentation |
| G2 | 91.67 | 58 | 3 | SUT | Fermentation + calcium |
| G3 | 87.50 | 18.75 | 5 | - | - |
| G4 | 66.67 | 27 | 4 | - | - |
| G5 | 100 | 42 | 5 | SUT | Calcium |
| G6 | 100 | 20 | 5 | FLO | Fermentation |
| G7 | 100 | 10 | 7 | SUT | Calcium + disinfectant |
| G8 | 100 | 25 | 5 | SUT | Calcium |
| G9 | 100 | 36.60 | 10 | EN | Calcium |
| G10 | 83.33 | 66.66 | 3 | SUT | Fermentation |
| G11 | 100 | 36.66 | 10 | - | Calcium |
| G12 | 100 | 34.37 | 13 | - | - |
| G13 | 100 | 40 | 5 | - | Fermentation |
| G14 | 100 | 77.27 | 33 | AMO/VIR/ENR/EN/CIP | - |
| G15 | 100 | 40 | 20 | AMO/VIR/ENR/EN/CIP | - |
| G16 | 100 | 60.71 | 20 | AMO/VIR/ENR/EN/CIP | - |
| G17 | 100 | 100 | 25 | AMO/VIR/ENR/EN/CIP | Calcium |
| G18 | 86.66 | 53.33 | 40 | VIR | Chicken litter replacement |
| G19 | 100 | 44,82 | 15 | EN/CIP | Calcium |
| G20 | 100 | 63.33 | 14 | EN | Calcium + disinfectant |
| G21 | 100 | 75 | 8 | OR | Calcium + disinfectant |
| G22 | 100 | 70 | 6 | - | Chicken litter replacement |
| G23 | 100 | 66.66 | 12 | SUT | Formaldehyde |
| G24 | 100 | 50 | 43 | - | Chicken litter replacement |
| G25 | 100 | 21.21 | 40 | FLO | Chicken litter replacement |
| G26 | 71.43 | 57.14 | 21 | - | Chicken litter replacement |
| G27 | 100 | 55 | 43 | TYL | Chicken litter replacement |
| G28 | 95.83 | 41,38 | 40 | EN/NEO | - |
| Virulence Genes | Strain Number (%) |
|---|---|
| 0 | 130 (56.3) |
| 1–2 | 25 (10.8) |
| 3–4 | 43 (18.6) |
| 5 | 33 (14.3) |
| Total | 231 (100) |
| Sample Origin | Crude OR (95% CI) | Adjusted OR (95% CI) | p (LR Test) |
|---|---|---|---|
| Litter | 3.24 (1.67, 6.3) | 1.91 (1.35, 2.71) | <0.001 |
| Cloacal Swab | 0.72 (0.44, 1.19) | 1.15 (0.86, 1.54) | 0.359 |
| Beetle | 0.51 (0.31, 0.86) | 0.87 (0.65, 1.17) | 0.359 |
| Antimicrobial | Crude OR (95% CI) | p (LR Test) |
|---|---|---|
| Fosfomycin | 1.66 (1.28, 2.17) | <0.001 |
| Chicken Litter | Crude OR (95% CI) | Adj. OR (95% CI) | p (LR Test) |
|---|---|---|---|
| Aztreonam | 1.09 (0.8, 1.49) | 1.38 (1, 1.9) | 0.05 |
| Cefotaxime | 0.93 (0.69, 1.26) | 0.7 (0.51, 0.95) | 0.025 |
| Ciprofloxacin | 1.69 (1.17, 2.43) | 1.93 (1.42, 2.64) | <0.001 |
| Tetracycline | 1.43 (1.06, 1.92) | 1.26 (1.04, 1.52) | 0.016 |
| Nalidixic acid | 0.93 (0.62, 1.38) | 0.56 (0.4, 0.8) | <0.001 |
| Cloacal Swab | Crude OR (95% CI) | Adj. OR (95% CI) | p (LR Test) |
|---|---|---|---|
| Cefepime | 0.85 (0.63, 1.15) | 0.78 (0.62, 0.98) | 0.035 |
| Cefazolin | 1.29 (0.94, 1.78) | 1.46 (1.15, 1.86) | 0.002 |
| Gentamycin | 1.13 (0.84, 1.53) | 1.19 (0.98, 1.44) | 0.082 |
| Ciprofloxacin | 1.68 (1.17, 2.42) | 1.66 (1.31, 2.11) | <0.001 |
| Ampicillin | 0.63 (0.43, 0.91) | 0.76 (0.58, 0.99) | 0.043 |
| Factor | Crude OR (95% CI) | Adjusted OR (95% CI) | p (LR Test) |
|---|---|---|---|
| Previous treatment | 0.58 (0.31, 1.09) | 0.66 (0.48, 0.91) | 0.009 |
| Antimicrobial use | 1.86 (1.12, 3.07) | 1.5 (1.15, 1.97) | 0.003 |
| Use | Crude OR (95% CI) | Adj. OR (95% CI) | p (LR Test) |
|---|---|---|---|
| Amoxicillin + C. acid | 0.86 (0.59, 1.25) | 0.6 (0.44, 0.83) | 0.001 |
| Aztreonam | 2.63 (1.9, 3.65) | 1.8 (1.16, 2.81) | 0.007 |
| Cefotaxime | 1.99 (1.45, 2.75) | 0.34 (0.2, 0.57) | <0.001 |
| Gentamycin | 0.54 (0.39, 0.75) | 0.78 (0.61, 1) | 0.053 |
| Fosfomycin | 3.61 (2.35, 5.54) | 1.49 (1.01, 2.22) | 0.044 |
| Enrofloxacin | 31.47 (9.92, 99.79) | 3.58 (1.96, 6.56) | <0.001 |
| Antibiotic | Crude OR (95% CI) | Adjusted OR (95% CI) | p (LR Test) |
|---|---|---|---|
| Amoxicillin/clavulanate | 0.71 (0.44, 1.14) | 0.61 (0.43, 0.87) | 0.006 |
| Cefazolin | 1.81 (1.15, 2.85) | 1.69 (1.07, 2.65) | 0.021 |
| Cefotaxime | 1.58 (1.08, 2.33) | 0.51 (0.33, 0.8) | 0.004 |
| Ciprofloxacin | 24.95 (6.1, 101.96) | 6.36 (2.94, 13.78) | <0.001 |
| Nalidixic acid | 28509842.59 (0, Inf) | 187.35 (5.26 × 10126) | <0.001 |
| Genes | Sequence (5′→3′) | PCR Product Size (bp) | Reference |
|---|---|---|---|
| bla-CTX-M-1 | AAAAATCACTGCGCCAGTTC AGCTTATTCATCGCCACGTT | 415 | (Woodford et al., 2005) [32] |
| bla-CTX-M-2 | CGACGCTACCCCTGCTATT CCAGCGTCAGATTTTTCAGG | 552 | |
| bla-CTX-M-8 | TCGCGTTAAGCGGATGATGC AACCCACGATGTGGGTAGC | 666 | |
| bla-CTX-M-9 | CAAAGAGAGTGCAACGGATG ATTGGAAAGCGTTCATCACC | 205 | |
| bla-CTX-M-25 | GCACGATGACATTCGGG AACCCACGATGTGGGTAGC | 327 | |
| bla-TEM | TTGGGTGCACGAGTGGGTTA TAATTGTTGCCGGGAAGCTA | 504 | (Arlet; Philippon, 1991) [33] |
| bla-SHV | TCGGGCCGCGTAGGCATGAT AGCAGGGCGACAATCCCGCG | 626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, V.D.; Alves, D.H.R.; de Souza, J.K.; Menck-Costa, M.F.; de Oliva, B.H.D.; Baptista, A.A.S.; Oba, A.; Matté, F.; Baierle, K.E.B.; da Rocha, S.P.D.; et al. Monitoring the Spread of Multidrug-Resistant Escherichia coli Throughout the Broiler Production Cycle. Antibiotics 2025, 14, 69. https://doi.org/10.3390/antibiotics14010069
Cruz VD, Alves DHR, de Souza JK, Menck-Costa MF, de Oliva BHD, Baptista AAS, Oba A, Matté F, Baierle KEB, da Rocha SPD, et al. Monitoring the Spread of Multidrug-Resistant Escherichia coli Throughout the Broiler Production Cycle. Antibiotics. 2025; 14(1):69. https://doi.org/10.3390/antibiotics14010069
Chicago/Turabian StyleCruz, Victor Dellevedove, Danilo Henrique Rabaçal Alves, Jamile Kellen de Souza, Maísa Fabiana Menck-Costa, Bruno Henrique Dias de Oliva, Ana Angelita Sampaio Baptista, Alexandre Oba, Fabrizio Matté, Kácio Emílio Borges Baierle, Sérgio Paulo Dejato da Rocha, and et al. 2025. "Monitoring the Spread of Multidrug-Resistant Escherichia coli Throughout the Broiler Production Cycle" Antibiotics 14, no. 1: 69. https://doi.org/10.3390/antibiotics14010069
APA StyleCruz, V. D., Alves, D. H. R., de Souza, J. K., Menck-Costa, M. F., de Oliva, B. H. D., Baptista, A. A. S., Oba, A., Matté, F., Baierle, K. E. B., da Rocha, S. P. D., de Brito, K. C. T., de Brito, B. G., Nakazato, G., Costa, M., & Kobayashi, R. K. T. (2025). Monitoring the Spread of Multidrug-Resistant Escherichia coli Throughout the Broiler Production Cycle. Antibiotics, 14(1), 69. https://doi.org/10.3390/antibiotics14010069








