Comparative Structural and Biophysical Investigation of Lycosa erythrognatha Toxin I (LyeTx I) and Its Analog LyeTx I-b
Abstract
:1. Introduction
2. Results
2.1. Structure and Orientation of the Peptides
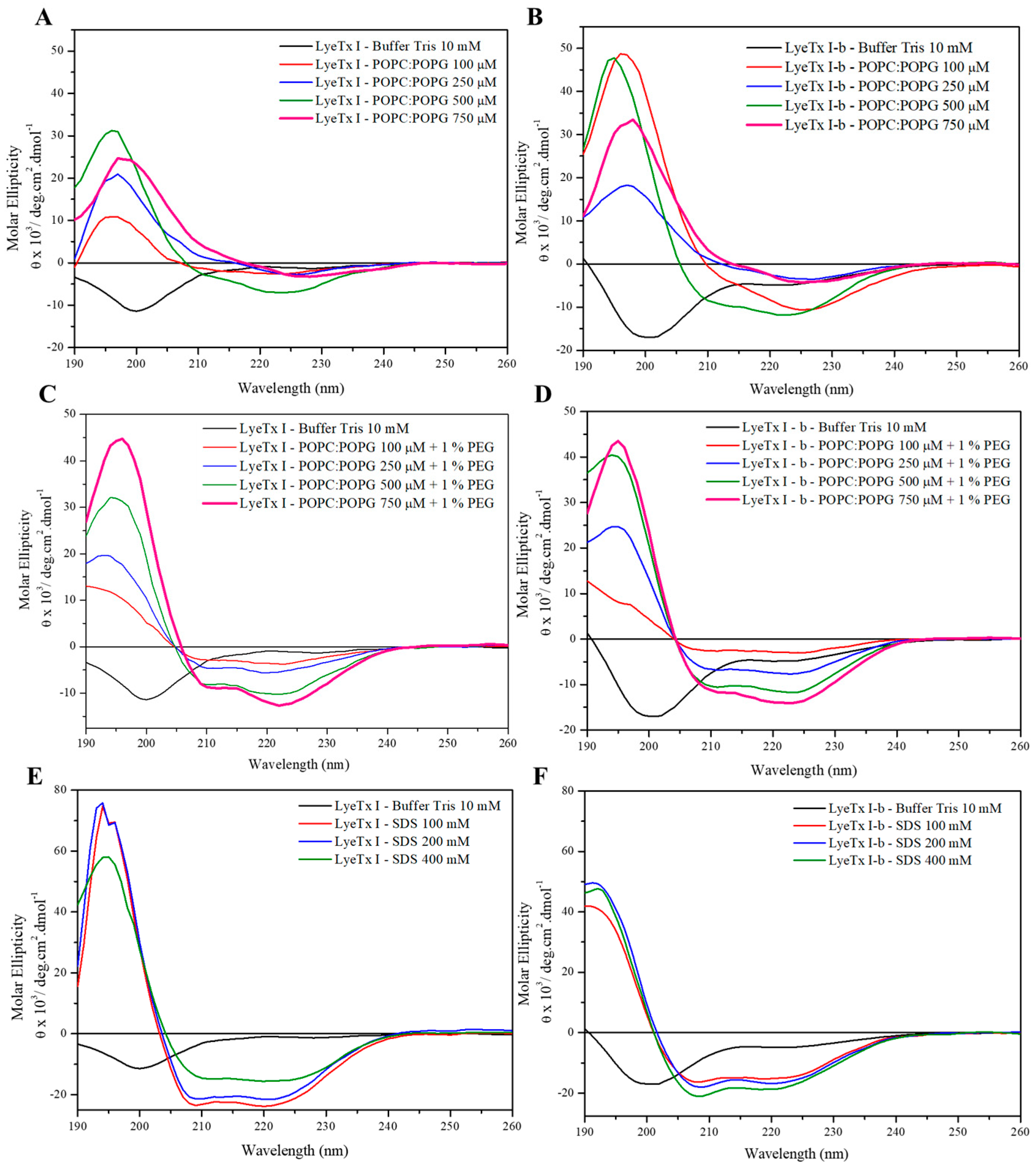
2.1.1. Conformational Preferences
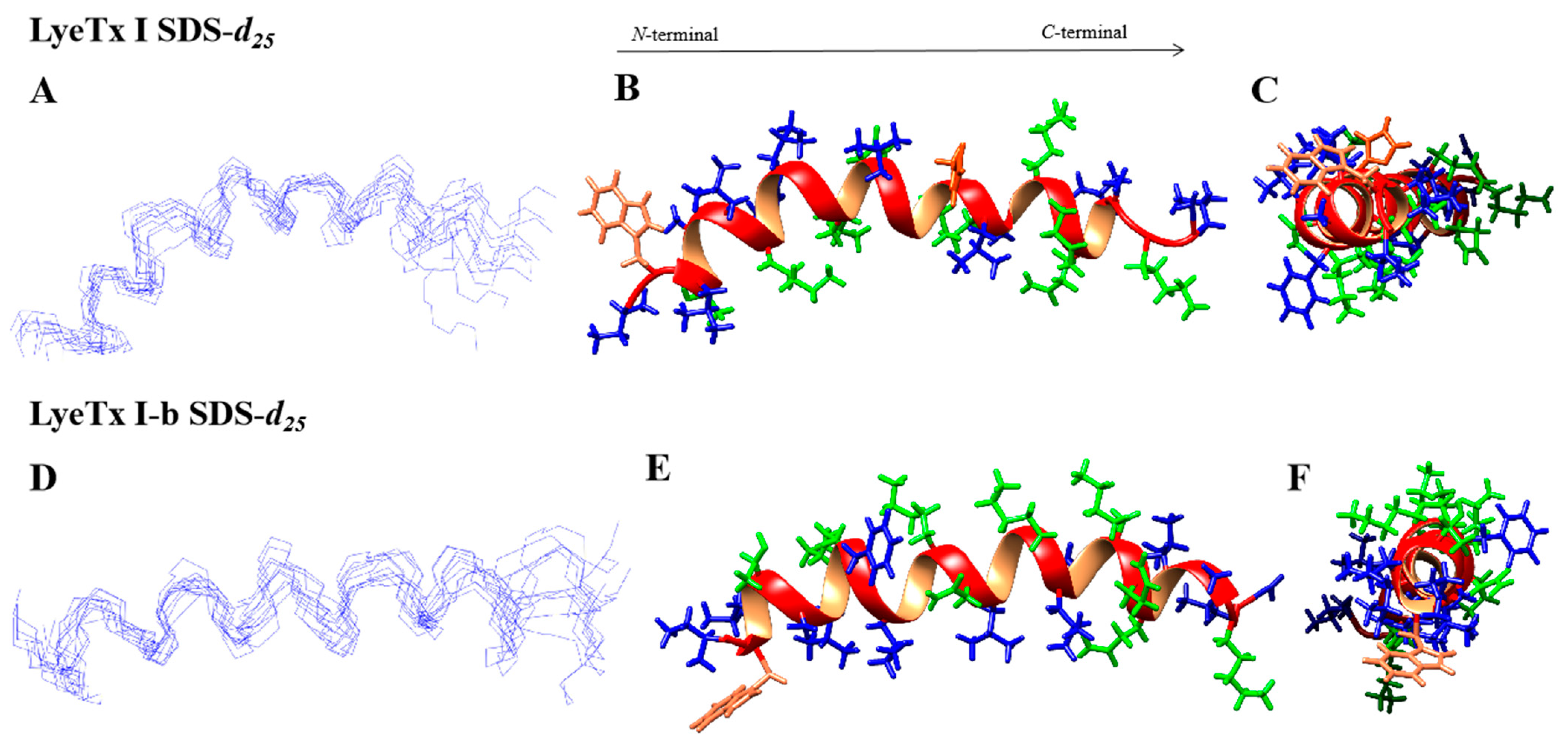
2.1.2. Tree-Dimensional NMR Structures
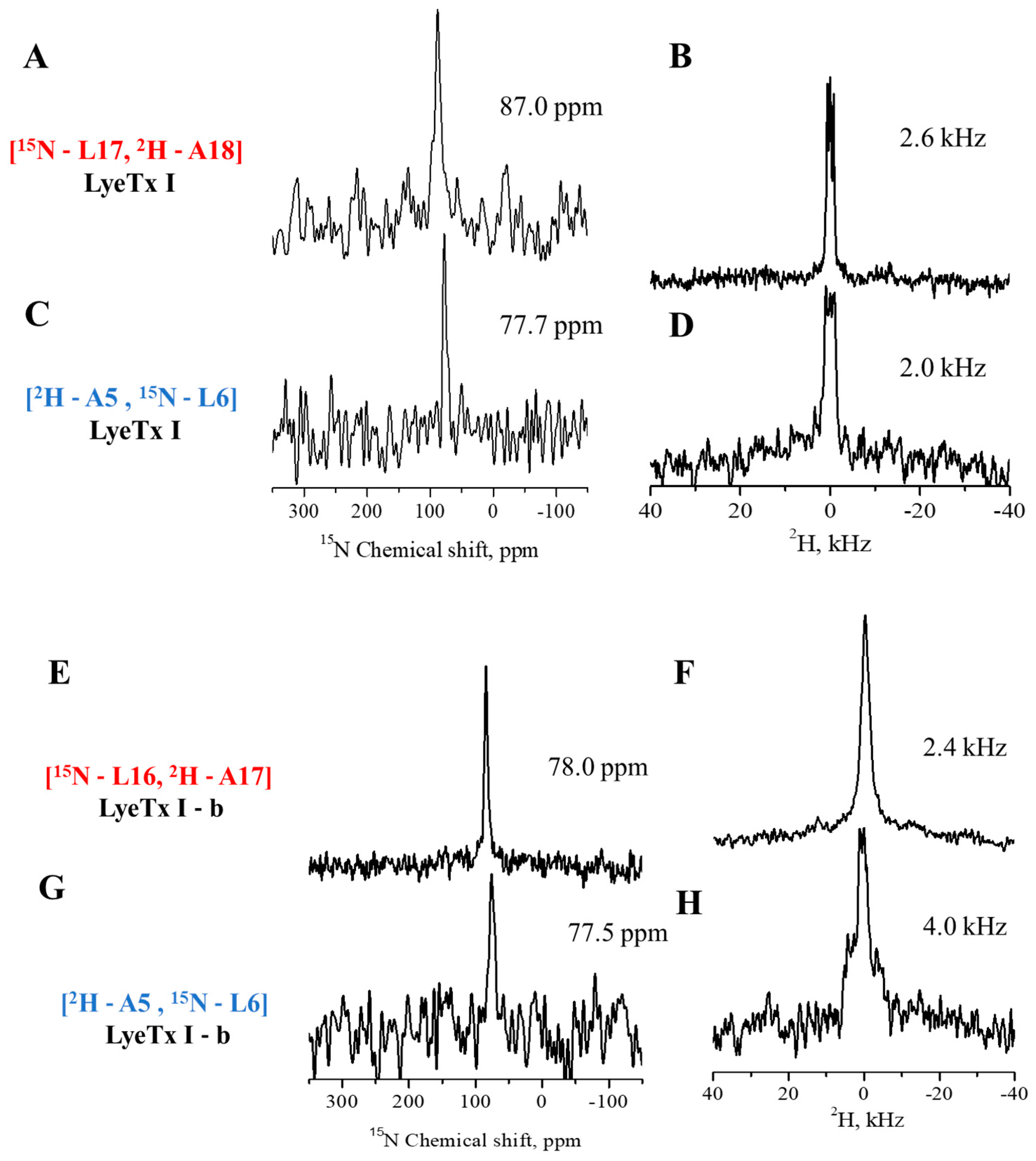
2.1.3. Topology of the Peptides in Oriented Membranes
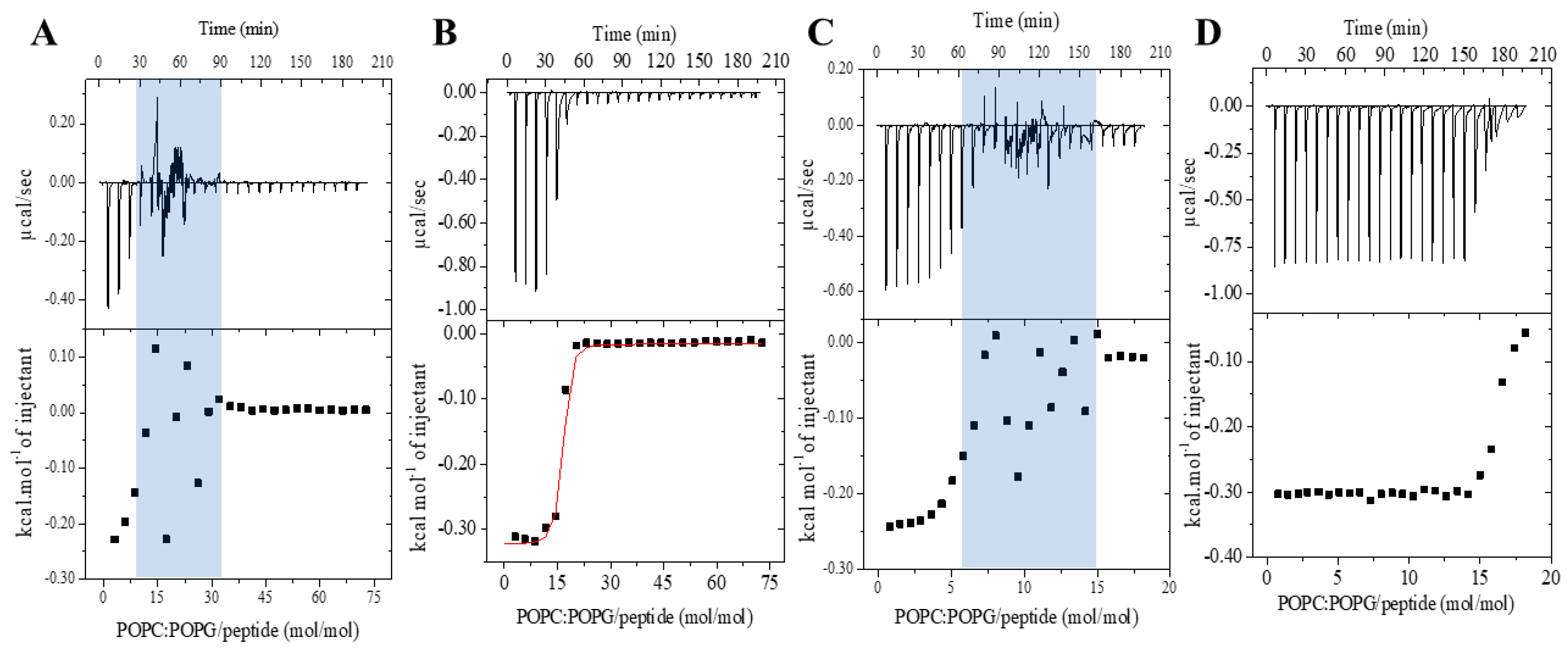
2.2. Membrane Affinity of the Peptide with Anionic Vesicles
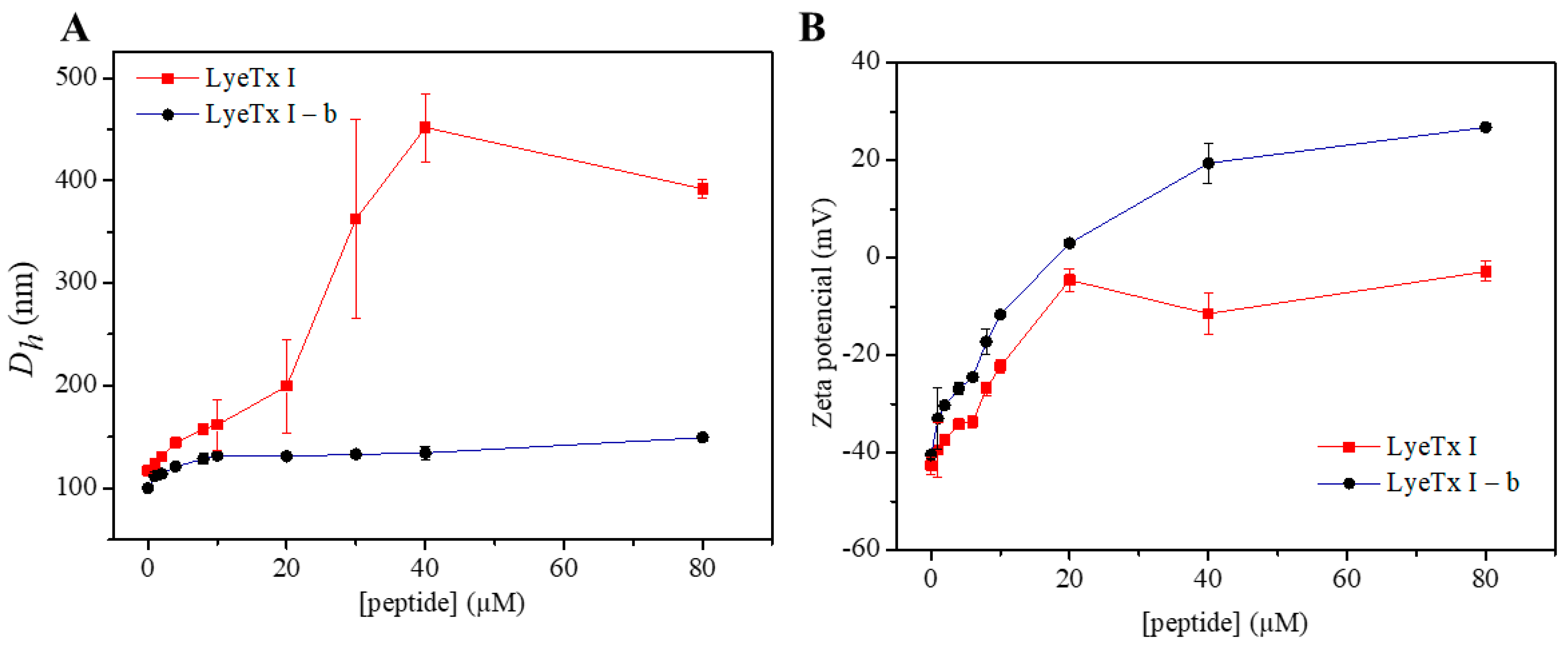
2.3. Effect of the Peptides on the Charge and Size Distribution of Anionic LUVs
2.4. Comparison of the Effect of Peptides on the Stability of Anionic and Zwitterionic Membranes
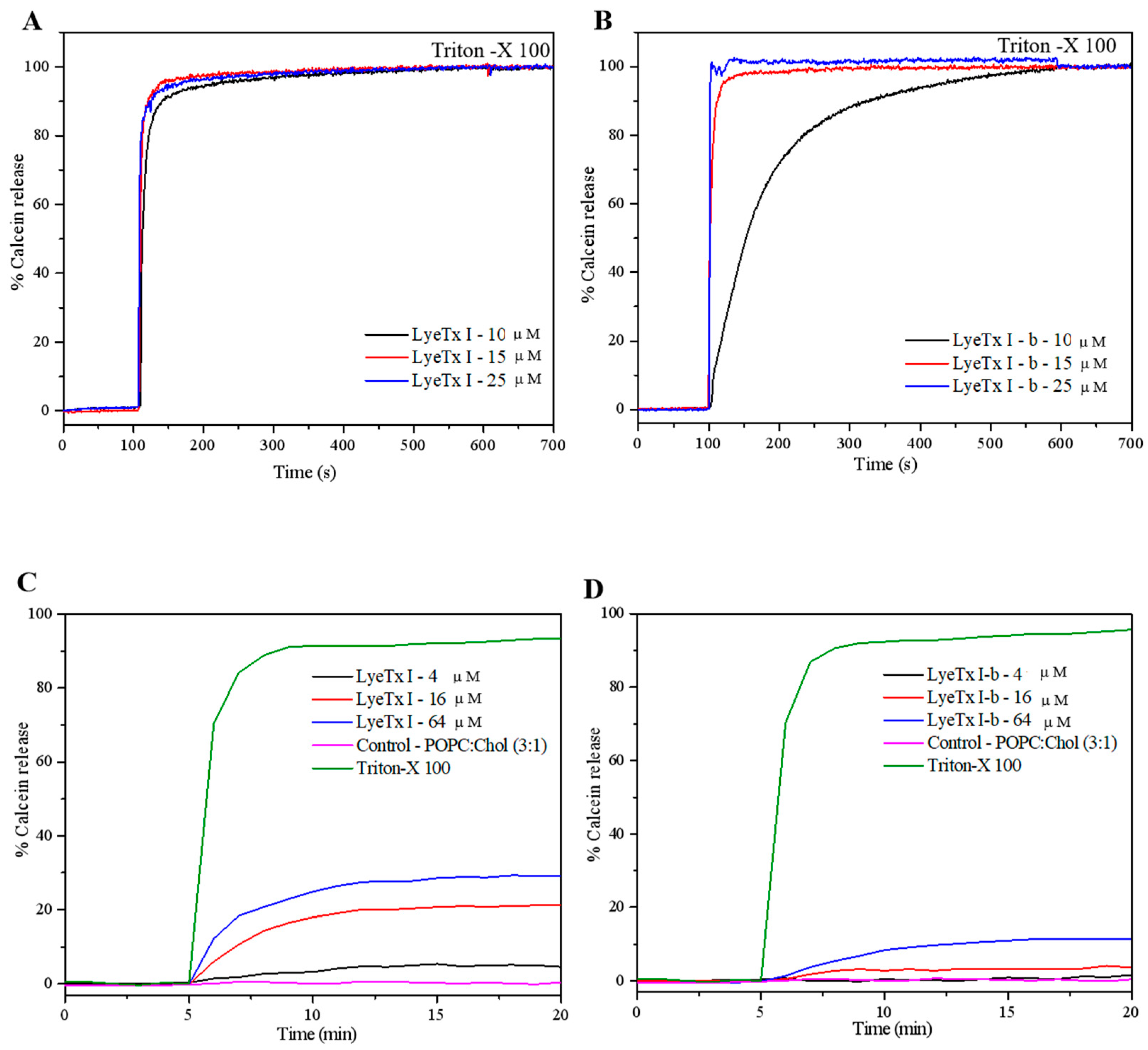
2.4.1. Evaluation of Lytic Activity
2.4.2. Effect on the the LUVs Thermotropic Behavior
3. Discussion
4. Material and Methods
4.1. Solid-Phase Peptide Synthesis
4.2. Characterizations and Purification
High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry
4.3. Preparation of Large Unilamellar Vesicles (LUVs)
4.4. Conformational and Topological Studies
4.4.1. Circular Dichroism Spectroscopy
4.4.2. Solution NMR Spectroscopy
4.4.3. NMR Data Analysis and Structure Calculations
4.4.4. Solid-State NMR Spectroscopy
4.5. Interaction with Membrane
4.5.1. Isothermal Titration Calorimetry (ITC)
4.5.2. Dynamic Light Scattering and Zeta Potential
4.5.3. Differential Scanning Calorimetry (DSC)
4.5.4. Fluorescence Spectroscopy
Calcein Release Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.; Verly, R.M.; Piló-Veloso, D.; De Maria, M.; De Carvalho, M.A.R.; Cisalpino, P.S.; Soares, B.M.; Diniz, C.G.; Farias, L.M.; Moreira, D.F.F.; et al. LyeTx I, a potent antimicrobial peptide from the venom of the spider Lycosa erythrognatha. Amino Acids 2010, 39, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Weiss, R.M.; Terwilliger, T.C. The helical hydrophobic moment: A measure of the amphiphilicity of a helix. Nature 1982, 299, 371–374. [Google Scholar] [CrossRef]
- Reis, P.V.M.; Boff, D.; Verly, R.M.; Melo-Braga, M.N.; Cortés, M.E.; Santos, D.M.; Pimenta, A.M.D.C.; Amaral, F.A.; Resende, J.M.; De Lima, M.E. LyeTxI-b, a Synthetic Peptide Derived from Lycosa erythrognatha Spider Venom, Shows Potent Antibiotic Activity In Vitro and In Vivo. Front. Microbiol. 2018, 9, 667. [Google Scholar] [CrossRef]
- Abdel-Salam, M.A.L.; Carvalho-Tavares, J.; Gomes, K.S.; Teixeira-Carvalho, A.; Kitten, G.T.; Nyffeler, J.; Dias, F.F.; dos Reis, P.V.M.; Pimenta, A.M.C.; Leist, M.; et al. The synthetic peptide LyeTxI-b derived from Lycosa erythrognatha spider venom is cytotoxic to U-87 MG glioblastoma cells. Amino Acids 2019, 51, 433–449. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.N.; da Silva, F.R.; Dourado, L.F.N.; dos Reis, P.V.M.; Silva, R.O.; da Costa, B.L.; Nunes, P.S.; Amaral, F.A.; dos Santos, V.L.; de Lima, M.E.; et al. A New Topical Eye Drop Containing LyeTxI-b, a Synthetic Peptide Designed from A Lycosa erithrognata Venom Toxin, Was Effective to Treat Resistant Bacterial Keratitis. Toxins 2019, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.W.; Hui, C.; Cullis, P.R.; Madden, T.D. Poly(ethylene glycol)−Lipid Conjugates Regulate the Calcium-Induced Fusion of Liposomes Composed of Phosphatidylethanolamine and Phosphatidylserine. Biochemistry 1996, 35, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.E.; Vanderlick, T.K. Aggregation and hemi-fusion of anionic vesicles induced by the antimicrobial peptide cryptdin-4. Biochim. Biophys. Acta (BBA)—Biomembr. 2007, 1768, 1796–1804. [Google Scholar] [CrossRef]
- Shi, S.; Fan, H.; Hoernke, M. Leaky membrane fusion: An ambivalent effect induced by antimicrobial polycations. Nanoscale Adv. 2022, 4, 5109–5122. [Google Scholar] [CrossRef]
- Khan, A.; Shah, S. Determination of critical micelle concentration (Cmc) of sodium dodecyl sulfate (SDS) and the effect of low concentration of pyrene on its Cmc using ORIGIN software. J. Chem. Soc. Pak. 2008, 30, 186–191. [Google Scholar]
- Wüthrich, K. NMR with Proteins and Nucleic Acids. Eur. News 1986, 17, 11–13. [Google Scholar] [CrossRef]
- Aisenbrey, C.; Bechinger, B. Tilt and Rotational Pitch Angle of Membrane-Inserted Polypeptides from Combined 15N and 2H Solid-State NMR Spectroscopy. Biochemistry 2004, 43, 10502–10512. [Google Scholar] [CrossRef]
- Stockton, G.W.; Polnaszek, C.F.; Leitch, L.; Tulloch, A.; Smith, I.C. A study of mobility and order in model membranes using 2H NMR relaxation rates and quadrupole splittings of specifically deuterated lipids. Biochem. Biophys. Res. Commun. 1974, 60, 844–850. [Google Scholar] [CrossRef]
- Bouchemal, K.; Mazzaferro, S. How to conduct and interpret ITC experiments accurately for cyclodextrin–guest interactions. Drug Discov. Today 2012, 17, 623–629. [Google Scholar] [CrossRef]
- Lewis, E.A.; Murphy, K.P. Isothermal Titration Calorimetry; Humana: Totowa, NJ, USA, 2005; pp. 1–15. [Google Scholar] [CrossRef]
- Malvern Instruments Ltd. Zetasizer Nano Series User Manual; Malvern Instruments Ltd.: Malvern, UK, 2013. [Google Scholar]
- Juszczyk, P.; Kołodziejczyk, A.S.; Grzonka, Z. Circular dichroism and aggregation studies of amyloid beta (11-8) fragment and its variants. Acta Biochim. Pol. 2005, 52, 425–431. [Google Scholar] [CrossRef]
- Danielsson, J.; Jarvet, J.; Damberg, P.; Gräslund, A. The Alzheimer β-peptide shows temperature-dependent transitions between left-handed 31-helix, β-strand and random coil secondary structures. FEBS J. 2005, 272, 3938–3949. [Google Scholar] [CrossRef] [PubMed]
- Jarvet, J.; Damberg, P.; Bodell, K.; Eriksson, L.E.G.; Gräslund, A. Reversible Random Coil to β-Sheet Transition and the Early Stage of Aggregation of the Aβ(12−28) Fragment from the Alzheimer Peptide. J. Am. Chem. Soc. 2000, 122, 4261–4268. [Google Scholar] [CrossRef]
- Holtzer, M.E.; Holtzer, A. α-helix to random coil transitions: Determination of peptide concentration from the CD at the isodichroic point. Biopolymers 1992, 32, 1675–1677. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Tiltak, D.; Ehni, S.; Wadhwani, P.; Ulrich, A.S. Lipid shape is a key factor for membrane interactions of amphipathic helical peptides. Biochim. Biophys. Acta (BBA)—Biomembr. 2012, 1818, 1764–1776. [Google Scholar] [CrossRef]
- Bischoff, N.; Wimberger, S.; Maresca, M.; Brakebusch, C. Improving Precise CRISPR Genome Editing by Small Molecules: Is there a Magic Potion? Cells 2020, 9, 1318. [Google Scholar] [CrossRef]
- Alvares, D.S.; Wilke, N.; Neto, J.R. Effect of N-terminal acetylation on lytic activity and lipid-packing perturbation induced in model membranes by a mastoparan-like peptide. Biochim. Biophys. Acta (BBA)—Biomembr. 2018, 1860, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Mischerikow, N.; Heck, A.J.R. Targeted large-scale analysis of protein acetylation. Proteomics 2011, 11, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Raja, Z.; André, S.; Piesse, C.; Sereno, D.; Nicolas, P.; Foulon, T.; Oury, B.; Ladram, A. Structure, Antimicrobial Activities and Mode of Interaction with Membranes of Bovel Phylloseptins from the Painted-Belly Leaf Frog, Phyllomedusa sauvagii. PLoS ONE 2013, 8, e70782. [Google Scholar] [CrossRef]
- Bastos, M.; Adão, R.; Nazmi, K.; Bolscher, J.G. C- and N-truncated antimicrobial peptides from LFampin 265–284: Biophysical versus microbiology results. J. Pharm. Bioallied Sci. 2011, 3, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-López, J.; Oliveira, J.C.L.; Michel, D.A.G.R.; Ferreira, C.S.; Neto, F.G.; Salnikov, E.S.; Verly, R.M.; Bechinger, B.; Resende, J.M. Membrane interactions of Ocellatins. Where do antimicrobial gaps stem from? Amino Acids 2021, 53, 1241–1256. [Google Scholar] [CrossRef] [PubMed]
- Bacalum, M.; Radu, M. Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept. Int. J. Pept. Res. Ther. 2015, 21, 47–55. [Google Scholar] [CrossRef]
- Geethanjali, H.; Nagaraja, D.; Melavanki, R. Exploring the mechanism of fluorescence quenching in two biologically active boronic acid derivatives using Stern-Volmer kinetics. J. Mol. Liq. 2015, 209, 669–675. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. On the analysis of membrane protein circular dichroism spectra. Protein Sci. 2004, 13, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Jimah, J.; Schlesinger, P.; Tolia, N. Liposome Disruption Assay to Examine Lytic Properties of Biomolecules. Bio-Protocol 2017, 7, e2433. [Google Scholar] [CrossRef]
- Determination of Total Phosphorus|Avanti Polar Lipids (EN-US). Available online: https://avantilipids.com/tech-support/analytical-procedures/determination-of-total-phosphorus (accessed on 31 May 2024).
- Braun, W.; Wider, G.; Lee, K.; Wüthrich, K. Conformation of glucagon in a lipid-water interphase by 1H nuclear magnetic resonance. J. Mol. Biol. 1983, 169, 921–948. [Google Scholar] [CrossRef] [PubMed]
- Rucker, S.P.; Shaka, A. Broadband homonuclear cross polarization in 2D N.M.R. using DIPSI-2. Mol. Phys. 1989, 68, 509–517. [Google Scholar] [CrossRef]
- Willker, W.; Leibfritz, D.; Kerssebaum, R.; Bermel, W. Gradient selection in inverse heteronuclear correlation spectroscopy. Magn. Reson. Chem. 1993, 31, 287–292. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef]
- Johnson, B.A.; Blevins, R.A. NMR View: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 1994, 4, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Hyberts, S.G.; Goldberg, M.S.; Havel, T.F.; Wagner, G. The solution structure of eglin c based on measurements of many NOEs and coupling constants and its comparison with X-ray structures. Protein Sci. 1992, 1, 736–751. [Google Scholar] [CrossRef]
- Shen, Y.; Delaglio, F.; Cornilescu, G.; Bax, A. TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 2009, 44, 213–223. [Google Scholar] [CrossRef]
- Rice, L.M.; Brünger, A.T. Torsion angle dynamics: Reduced variable conformational sampling enhances crystallographic structure refinement. Proteins Struct. Funct. Bioinform. 1994, 19, 277–290. [Google Scholar] [CrossRef]
- Schwieters, C.D.; Kuszewski, J.J.; Mariusclore, G. Using Xplor–NIH for NMR molecular structure determination. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 48, 47–62. [Google Scholar] [CrossRef]
- Koradi, R.; Billeter, M.; Wüthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Koumkoua, P.K.; Aisenbrey, C.; Salnikov, E.; Rifi, O.; Bechinger, B. On the design of supramolecular assemblies made of peptides and lipid bilayers. J. Pept. Sci. 2014, 20, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, C.; Bertani, P.; Bechinger, B. Solid-State NMR Investigations of Membrane-Associated Antimicrobial Peptides. Methods Mol. Biol. 2010, 618, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Rance, M.; Byrd, R. Obtaining high-fidelity powder spectra in anisotropic media: Phase-cycled Hahn echo spectroscopy. J. Magn. Reson. (1969) 1983, 52, 221–240. [Google Scholar] [CrossRef]
- Munhoz, V.H.; Ferreira, C.S.; Nunes, L.O.; Santos, T.L.; Aisenbrey, C.; Adão, R.; de Carvalho Alcântara, A.F.; de Magalhães, M.T.; Piló-Veloso, D.; Resende, J.M.; et al. Epimers l- and d-Phenylseptin: How the relative stereochemistry affects the peptide-membrane interactions. Biochim. Biophys. Acta (BBA)—Biomembr. 2021, 1863, 183708. [Google Scholar] [CrossRef]



| Property | LyeTx I | LyeTx I-b |
|---|---|---|
| Sequence | IWLTALKFLGKNLGKHLAKQQLAKL-NH2 | CH3CO-IWLTALKFLGKNLGKLAKQQLAKL-NH2 |
| Net charge (pH 7) | +5 | +4 |
| Length (residues) | 25 | 24 |
| Hydrophobic residues | I, W, L, A, L, F, L, L, L, A, L, A, L (13 total) | I, W, L, A, L, F, L, L, L, A, L, A, L (13 total) |
| Hydrophobicity (H) | 0.523 * | 0.539 * |
| Hydrophobic moment (μ) | 0.198 * | 0.426 * |
| Molecular weight (Da) | 2831.7 | 2735.6 |
| N-terminal modification | None | Acetylation (-CH3CO) |
| C-terminal modification | Amidation (-NH2) | Amidation (-NH2) |
| LyeTx I | LyeTx I-b | |
|---|---|---|
| NMR distance and dihedral constraints | 310 | 238 |
| Distance constraints | ||
| Total NOE | ||
| Intra-residue | 195 | 137 |
| Inter-residue | ||
| Sequential (|i − j| = 1) | 63 | 28 |
| Medium-range (|i − j| < 4) | 52 | 33 |
| Total dihedral angle restraints | 25 | 40 |
| Structure statistics | ||
| Ramachandran analysis | ||
| Residues in most favored regions | 100% | 99.0% |
| Residues in additional allowed regions | 0.0% | 1.0% |
| Residues in generously allowed regions | 0.0% | 0.0% |
| Residues in disallowed regions | 0.0% | 0.0% |
| Average pairwise r.m.s. deviation ** (Å) | ||
| Residue 1 to 12—Backbone | 0.93 | 0.56 Å |
| Residue 12 to 25—Backbone | 0.98 | 0.88 Å |
| Peptide | Kapp (L·mol−1) | ∆G° (cal·mol−1) | ∆H° (cal·mol−1) | T∆S° (cal·mol−1) |
|---|---|---|---|---|
| LyeTx I a | 1.8 × 104 ± 1.6 × 103 | −5902 | −410 ± 20 | 18.1 ± 0.9 |
| LyeTx I-b a | 1.0 × 106 ± 1.7 × 105 | −8308 | −1150 ± 50 | 23.6 ± 1.2 |
| LyeTx I-b b | 1.2 × 106 ± 1.5 × 105 | −6881 | −721 ± 250 | 25.5 ± 7.5 |
| Peptides | Characteristics | Sequences |
| LyeTx I | Unlabeled | IWLTALKFLGKNLGKHLAKQQLAKL-NH2 |
| Labeled [15N—L17, 2H—A18] | IWLTALKFLGKNLGKHLAKQQLAKL-NH2 | |
| Labeled [2H—A5, 15N—L6] | IWLTALKFLGKNLGKHLAKQQLAKL-NH2 | |
| LyeTx I-b | Unlabeled | CH3CO-IWLTALKFLGKNLGKLAKQQLAKL-NH2 |
| Labeled [15N—L16, 2H—A17] | CH3CO-IWLTALKFLGKNLGKLAKQQLAKL-NH2 | |
| Labeled [2H—A5, 15N—L6] | CH3CO-IWLTALKFLGKNLGKLAKQQLAKL-NH2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.N.; Cardoso, G.d.A.; Nunes, L.O.; Aisenbrey, C.; Salnikov, E.; de Souza, K.R.; Saad, A.; de Lima, M.E.; Resende, J.M.; Bechinger, B.; et al. Comparative Structural and Biophysical Investigation of Lycosa erythrognatha Toxin I (LyeTx I) and Its Analog LyeTx I-b. Antibiotics 2025, 14, 66. https://doi.org/10.3390/antibiotics14010066
de Souza AN, Cardoso GdA, Nunes LO, Aisenbrey C, Salnikov E, de Souza KR, Saad A, de Lima ME, Resende JM, Bechinger B, et al. Comparative Structural and Biophysical Investigation of Lycosa erythrognatha Toxin I (LyeTx I) and Its Analog LyeTx I-b. Antibiotics. 2025; 14(1):66. https://doi.org/10.3390/antibiotics14010066
Chicago/Turabian Stylede Souza, Amanda Neves, Gabriele de Azevedo Cardoso, Lúcio Otávio Nunes, Christopher Aisenbrey, Evgeniy Salnikov, Kelton Rodrigues de Souza, Ahmad Saad, Maria Elena de Lima, Jarbas Magalhães Resende, Burkhard Bechinger, and et al. 2025. "Comparative Structural and Biophysical Investigation of Lycosa erythrognatha Toxin I (LyeTx I) and Its Analog LyeTx I-b" Antibiotics 14, no. 1: 66. https://doi.org/10.3390/antibiotics14010066
APA Stylede Souza, A. N., Cardoso, G. d. A., Nunes, L. O., Aisenbrey, C., Salnikov, E., de Souza, K. R., Saad, A., de Lima, M. E., Resende, J. M., Bechinger, B., & Verly, R. M. (2025). Comparative Structural and Biophysical Investigation of Lycosa erythrognatha Toxin I (LyeTx I) and Its Analog LyeTx I-b. Antibiotics, 14(1), 66. https://doi.org/10.3390/antibiotics14010066






