1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by persistent inflammation in synovium, joint pain, as well as stiffness alongside the progressive erosion of cartilage and bone, leading to significant disability when not treated adequately. Systemic manifestations such as fatigue and extra-articular involvement are common, marking its complicated clinical presentation even more [
1,
2,
3]. The disease affects approximately 0.5–1.0% of the global population, with prevalence consistent across diverse populations, highlighting its widespread impact on public health [
4].
The pharmacological management of RA follows a stepwise approach, incorporating nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, disease-modifying antirheumatic drugs (DMARDs) [conventional synthetic (cs)DMARDs, biological (b)DMARDs, targeted synthetic (ts)DMARDs], and JAK inhibitors, aimed at controlling inflammation, relieving symptoms and halting disease advancement [
5]. Minocycline, as well as doxycycline, are members of the tetracycline class of semi-synthetic antibiotics that possess anti-inflammatory properties and are used to treat various inflammation-related diseases, including RA [
6,
7,
8].
Due to sulfasalazine’s combined anti-inflammatory and antibacterial qualities, antibiotics were first used to treat RA in the 1930s [
9,
10]. Tetracyclines gained attention in the 1960s and 1970s because of their immunomodulatory properties, as evidenced by research showing that they effectively reduced the symptoms of RA [
11,
12,
13,
14]. Recent clinical trials have examined macrolides such as clarithromycin and roxithromycin, which have demonstrated potential advantages mainly associated with their anti-inflammatory actions [
15,
16,
17,
18].
Significant anti-inflammatory and immunomodulatory effects are exhibited by tetracyclines, particularly doxycycline and minocycline, which reduce the generation of pro-inflammatory cytokines and T cell proliferation. These substances prevent the presentation of antigens, the activation of microglial cells, and the synthesis of important inflammatory chemicals like cyclooxygenase-2, phospholipase A2, and nitric oxide. Tetracyclines also efficiently block matrix metalloproteinases (MMPs), which may be used therapeutically to treat autoimmune disorders and diseases linked to inflammation, such as RA [
19,
20]. Concerns about scarce evidence and methodological inconsistency, followed by antimicrobial resistance, safety, and cost-effectiveness concerns, are the main reasons for the limited acceptance of tetracyclines in current clinical guidelines [
21,
22,
23].
It is projected that 17.6 million individuals of all ages worldwide had RA in 2020, a 121% increase from 1990 [
24]. The global prevalence rate increased by 14.1% from 1990. It is projected that 31.7 million people worldwide will have RA by 2050, representing an 80.2% rise in cases from 2020 to 2050, based on projected demographic trends, which highlights the significant burden of this disease and further emphasizes the significance of exploring alternative therapeutic options [
24].
This narrative review aims to give an all-inclusive look at the role of tetracyclines, notably minocycline and doxycycline in RA management, focusing on their historical evolution, mechanisms of action, and supporting clinical evidence. By critically evaluating the current body of evidence and umbrella guidelines, this review discusses patient selection criteria, safety profiles, and challenges in integrating these drugs into everyday clinical practice, including issues such as drug resistance and variations in patient responses. Furthermore, this review highlights emerging research and future directions to optimize the use of tetracyclines in RA care.
2. Mechanism of Action of Tetracyclines in RA
Tetracyclines are a group of antibiotics that inhibit protein synthesis by preventing the binding of aminoacyl-transfer RNA to the messenger RNA-ribosome complex [
10]. This is primarily achieved through binding to the 30S ribosomal subunit in the messenger RNA translation complex [
10]. Their anti-inflammatory effects are attributed to the inhibition of enzymes involved in the inflammatory cascade [
10].
Minocycline, one of the most extensively studied tetracyclines, has demonstrated anti-inflammatory effects in patients with RA [
25]. T cells play a critical role in RA pathogenesis, and studies have shown that minocycline suppresses T cell activity by acting on the T cell receptor (TCR)/CD3 complex [
25]. This leads to the reduced production of pro-inflammatory mediators, including interleukin-2 (IL-2), interferon-gamma (IFN-γ), and tumour necrosis factor-alpha (TNF-α) [
25]. Minocycline also inhibits T cell proliferation by decreasing IL-2 responsiveness [
25]. Further research has demonstrated that minocycline suppresses NFAT1-mediated transcriptional activation pathways in human CD4
+ T cells [
26,
27]. In rat models, minocycline significantly reduced the incidence of adjuvant-induced and collagen-induced arthritis—both considered T cell-dependent models of RA due to their pathophysiology [
28].
Minocycline has also been shown to suppress antigen-presenting capacity. Both minocycline and doxycycline inhibit the production of pro-inflammatory cytokines, such as TNF-α, interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), as well as matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9 [
19]. Additionally, tetracyclines inhibit inflammation-associated molecules, including inducible nitric oxide synthases (iNOSs), phospholipase A2 (PLA2), cyclooxygenase-2 (COX-2), and prostaglandins [
19].
RA and osteoarthritis (OA) progression often involve cartilage damage accompanied by the spontaneous release of nitric oxide (NO). A study evaluating the effects of tetracyclines on this process found that doxycycline and minocycline inhibited nitric oxide synthase enzymes, including OA-specific NOS and rodent inducible NOS (iNOS) [
29]. The mechanism of action was determined to involve the regulation of RNA expression and enzyme translation [
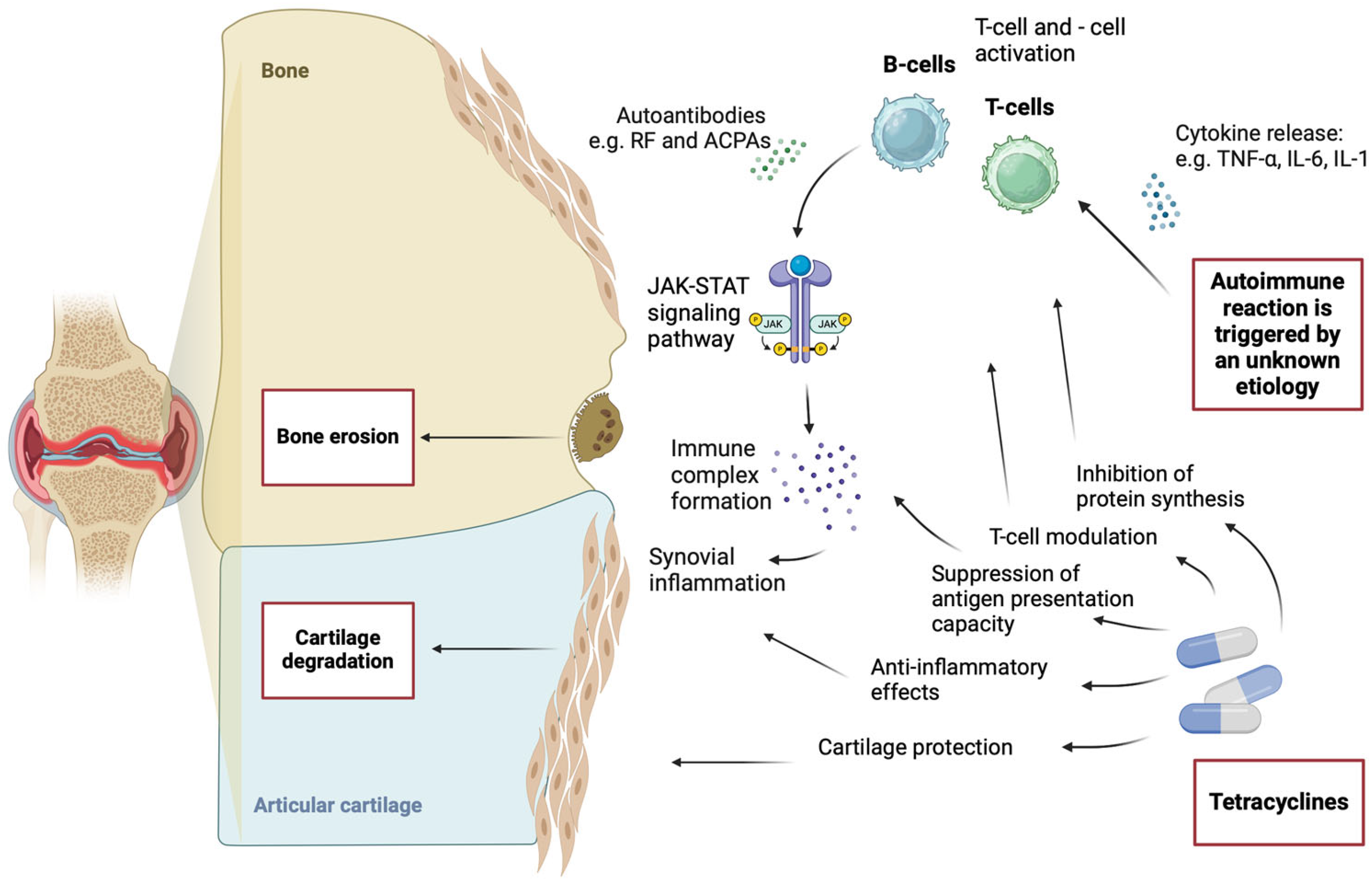
29]. A detailed visualization of the pathogenesis of RA and the mechanism of action of tetracyclines is presented in
Figure 1.
3. Tetracyclines in RA: A Historical Perspective
The exploration of tetracyclines in the treatment of RA has a rich history, evolving from their initial recognition as antibiotics to their current understanding as modulators of collagenase activity, which is critical in the pathology of RA. The earliest significant findings regarding tetracyclines’ therapeutic roles emerged in the 1970s, when researchers began to examine their effects beyond antimicrobial properties. In particular, a study conducted by Rokkanen et al. [
30] highlighted tetracyclines’ ability to suppress collagenase activity, implicating them in the management of conditions characterized by excessive tissue degradation, such as RA. This discovery laid the groundwork for further investigations into the biochemical mechanisms by which tetracyclines could exert protective effects on joint tissues.
Subsequent research, particularly noted in studies conducted by Skinner et al. [
31] and Tourtellotte [
32], explored the interaction of tetracyclines with human synovial tissues. In these studies, minocycline was administered to RA patients, showing a significant 67% reduction in collagenase activity post-treatment. This provided compelling evidence that tetracyclines could inhibit the destructive enzymatic processes associated with RA, suggesting a dual mechanism of action: antimicrobial effectiveness and the modulation of MMPs. The researchers proposed that this non-antimicrobial action could lead to the development of novel therapeutic strategies aimed at mitigating tissue damage caused by collagenases, a hallmark of RA pathology. The emergence of chemically modified tetracyclines (CMTs), such as the dedimethylaminotetracycline examined by Tourtellotte [
32], represented a significant advancement. These modifications aimed to retain anti-collagenase properties while eliminating antibiotic effects, thus reducing the risk of resistance. Preliminary animal studies demonstrated that CMTs could effectively reduce collagenase activity without affecting glucose metabolism, suggesting a favourable safety profile for long-term use in chronic conditions like RA.
By the 1990s, clinical trials began to substantiate the therapeutic potential of minocycline in RA treatment. Studies conducted by Kloppenburg et al. [
11] and Pruzanski et al. [
33] assessed minocycline’s safety and efficacy over extended periods. In a notable trial, 40 patients with active RA received minocycline, revealing statistically significant improvements in multiple clinical parameters, including joint tenderness and swelling. The absence of severe adverse effects further underscored minocycline’s promise as a safer alternative to traditional RA therapies. A larger multicenter trial [
34] confirmed these findings, demonstrating that minocycline administration led to substantial improvements in disease activity metrics after 48 weeks. Notably, patients treated with minocycline exhibited higher rates of improvement in joint swelling and tenderness compared to placebo, reinforcing the drug’s clinical relevance in managing RA symptoms.
In summary, the research trajectory on tetracyclines in RA has transitioned from their initial application as antibiotics to a nuanced understanding of their role in collagenase inhibition and tissue protection. These studies collectively underscore the potential of minocycline and its derivatives as effective therapeutic agents, warranting further exploration and validation through controlled clinical trials to establish their mechanisms of action and long-term safety in the treatment of RA. This evolving narrative highlights the importance of repurposing existing medications to address complex chronic conditions, ultimately enhancing patient care in rheumatology.
4. Clinical Evidence of Tetracyclines in RA Treatment
The first studies exploring the use of tetracyclines and their effectiveness in treating RA were conducted during the 1970s and 1980s. Minocycline was the most commonly studied antibiotic from the tetracycline group in these trials, which initially compared its efficacy to that of a placebo. Patients in these studies had previously been treated with DMARDs. The findings consistently demonstrated that minocycline was superior to placebo, generally safe, but with an unclear mechanism of action [
11,
12,
13].
Two notable studies, conducted by Kloppenburg and colleagues [
11] and the Rheumatoid Arthritis Investigational Network (RAIN) study group [
13], had relatively small sample sizes, which was a significant limitation. In the late 1990s, the RAIN group investigated minocycline, comparing its effectiveness to hydroxychloroquine in DMARD-naive RA patients [
13]. The results showed that the minocycline group achieved better outcomes, including a positive effect on glucocorticoid-sparing strategies. The drug’s efficacy was particularly pronounced in patients with more aggressive forms of RA, who made up the majority of the study population. At that time, minocycline was suggested as a DMARD with high potential for RA treatment [
14].
Doxycycline, another tetracycline-group antibiotic, was also tested as a potential treatment for RA. In one study, doxycycline was compared to azithromycin and placebo, but the results showed no significant reduction in disease activity across any of the three groups [
35].
Given methotrexate’s (MTX) established role as one of the most effective DMARDs for RA treatment, another study examined the combination of MTX and doxycycline versus MTX alone [
36]. This study included 66 seropositive RA patients with disease durations of less than a year, none of whom had received prior DMARD therapy [
36]. The primary endpoint, ACR50, was achieved more frequently in the group treated with doxycycline plus MTX, demonstrating superior outcomes [
36]. However, the authors emphasized the need for further studies to understand the mechanism of action [
36].
The largest observational study to date was conducted in the United States between 1998 and 2009, involving 15,716 patients with RA who were prescribed tetracyclines (minocycline or doxycycline) [
37]. The study concluded that tetracyclines were primarily used in refractory cases rather than as a first-line treatment [
37]. They were well tolerated, and the most common side effects were nausea, dizziness, and skin complications [
37].
Additionally, the combination of oral tetracyclines with clindamycin was tested as an RA treatment [
38]. However, this study was discontinued after the initial results from 20 patients failed to achieve the desired ACR50 positive response [
38].
Table 1 is a summary of all listed studies.
5. Current Guidelines and Clinical Use of Tetracyclines in RA
Current guidelines for managing RA do not explicitly include tetracyclines as a recommended therapeutic option. The European Alliance of Associations for Rheumatology (EULAR) guidelines employ the Oxford Centre for Evidence-Based Medicine Levels of Evidence to shape recommendations [
21]. The American College of Rheumatology (ACR) guidelines utilize the GRADE framework, combining systematic reviews with a voting panel of clinicians and patients to reach a consensus on the strength and direction of recommendations [
22]. The National Institute for Health and Care Excellence (NICE) integrates evidence interpretation with economic considerations in its methodology [
23]. Despite their differing approaches, all these guidelines focus on established DMARDs and biologics, without highlighting tetracyclines.
The limited inclusion of tetracyclines stems from inconsistent evidence, small-scale trials, and methodological heterogeneity, as well as concerns about antimicrobial resistance, safety risks, and economic factors. However, off-label use may be cautiously explored on a case-by-case basis when standard treatments fail to achieve an adequate clinical response. In particular, tetracyclines may be considered in refractory cases to leverage their immunomodulatory and anti-inflammatory properties, especially in patients with early-stage disease or coexisting conditions like infections, where other treatments pose higher risks. This approach requires careful monitoring to ensure that the benefits outweigh the potential risks.
6. Challenges and Limitations in Tetracycline Use for RA
Despite the benefits mentioned in this review, the use of tetracyclines in RA may carry some risks, and, in some cases, precautions should be taken with regard to antimicrobial resistance, drug interactions, or variations in response to treatment.
The global tetracycline resistance rate in European countries for methicillin-resistant Staphylococcus aureus (MRSA) and Streptococcus pneumoniae was 8.7% and 24.3%, respectively [
39], and, for Escherichia coli and Klebsiella species producing extended-spectrum β-lactamase (ESBL), the percentages were 66.9% and 44.9%, respectively [
40]. Tetracycline resistance occurs through active efflux, ribosomal protection, decreased permeability, target mutation, enzymatic degradation, and gene transfer via plasmids and transposons [
41].
When it comes to drug interactions, tetracyclines interact with several medications and must be carefully considered in clinical use. Doxycycline may prolong the prothrombin time in patients taking warfarin, and concomitant use with penicillin is not recommended due to the impairment of bactericidal activity. The absorption of doxycycline may be reduced by antacids, iron, zinc, and other cation-containing drugs, necessitating separate dosing. Enzyme inducers such as phenobarbital and rifampicin may accelerate the metabolism of doxycycline, resulting in subtherapeutic levels, while alcohol shortens the half-life of doxycycline. Caution should be exercised when administering doxycycline concomitantly with methotrexate, ciclosporin, retinoids, or methoxyflurane due to the increased risk of toxicity, and it may inactivate oral typhoid vaccines [
42]. Similar precautions should also be taken with minocycline [
43]. Furthermore, in terms of dietary interactions, milk and other dairy product consumption alongside tetracycline use can interfere with drug absorption by 50 to 90% or more [
44]. Considering this substantial interaction, dairy products, in general, should be avoided during tetracycline use, which can potentiate lower adherence to this therapy.
There is limited evidence regarding the differential response of patients to RA treatment with tetracyclines. They have been studied in naive RA, early RA, long-term RA, and RA unresponsive to DMARD treatment [
45], but there is a gap in knowledge about the differences between specific RA groups based on disease severity and other possible contributing factors.
7. Future Directions and Emerging Research
Although tetracyclines pose an interesting therapeutic option for RA, due to inconsistent evidence from small-scale clinical trials and concerns about antimicrobial resistance, the future of tetracyclines as an RA treatment remains far from common usage. In order to achieve this, the research community must first address all the current limitations and explore novel therapeutic applications, such as the development of safer compounds, in-depth in silico analyses, and integration with personalized medicine approaches.
A promising area of research is the synthesis of novel chemically modified tetracyclines that retain anti-collagenase properties while eliminating antibiotic effects, thus reducing the risk of antimicrobial resistance. Molecular modelling studies and docking can predict the interactions between these newly chemically modified or synthesized tetracyclines with their target protein(s) in order to identify compounds with high binding affinity and specificity. In addition, these novel compounds should be investigated for their synergistic effects with current therapeutic options for RA, which could enhance outcomes and reduce the doses of more toxic drugs, which could add to the long-term cost-effectiveness from the macroeconomic point of view. Recently, nanotechnology has also emerged as a promising new method for targeted drug delivery, which in turn can enhance bioavailability and reduce the systemic side effects of new drugs [
46,
47]. Nanotechnology also offers great potential in delivering a combination of multiple drugs, which would pose a great advantage for RA treatment as tetracyclines are proposed as an adjunct therapy option.
Emerging research in this field also lies in addressing the personalized medicine approach and the use of various omics potential. Advances in genomics and pharmacogenomics offer the opportunity to identify biomarkers that predict patient responses to tetracyclines. This would, in turn, help with patients’ stratification based on disease phenotypes and genetic profiles, to determine responders from non-responders, i.e., which individuals are most likely to benefit from these new treatment strategies. One systematic review investigated the potential of omics biomarkers in the field of RA, which identified 196 potential clinical biomarkers [
48]; however, to the best of our knowledge, there are currently no well-established biomarkers or omics studies specifically tailored to optimizing tetracycline use in RA. This remains an area of ongoing research and presents a significant opportunity for future studies to enhance the precision and effectiveness of tetracycline therapies. Apart from biomarker identification, future studies should incorporate the exploration of comorbidities, such as cardiovascular disease and osteoporosis, which are often associated with RA, and assess the effects of tetracyclines on these conditions in combination. The longitudinal effects of tetracycline use should also be investigated in future studies.
Finally, the vast majority of available in silico methods should be employed to maximize the identification of tetracyclines’ potential before clinical trials. One example is drug repurposing approaches based on transcriptomic profiling, which can help select the most potent novel chemically modified tetracyclines based on the transcriptomic signature that the drug induces.
8. Conclusions and Perspectives
This comprehensive review highlights the importance of exploring novel treatment options for RA, a chronic autoimmune disease featured by persistent inflammation, joint pain, and stiffness, which increases the overall healthcare burden and diminishes the quality of life. Current treatment strategies primarily focus on controlling inflammation, relieving symptoms, and halting disease progression. However, in recent years, tetracyclines have been proposed as a supplementary treatment option. With their dual mode of action, both antimicrobial and immunomodulatory, the future of tetracyclines in RA treatment is a very promising field. Notably, tetracyclines are of particular interest due to their ability to suppress collagenase activity, which is a critical process in RA pathology. Despite this potential, their integration into clinical practice remains limited due to small-scale clinical trials with inconsistent evidence and concerns about antimicrobial resistance.
The synthesis of novel chemically modified tetracyclines could further enhance their therapeutic applicability while minimizing antibacterial effects. Strategies to overcome these challenges could also be in use of natural products and nanoformulations as a collaborative method to improve antimicrobial effectiveness while addressing issues such as limited bioavailability and the development of resistance, as demonstrated by recent progress in flavonoid-based nanoparticle formulations [
49].
Nanomaterials, due to their high surface-to-volume ratio and adaptability, offer a cutting-edge platform for sustained antibacterial activity, and, when paired with natural compounds, they facilitate targeted delivery and enhanced therapeutic results [
50].
These strategies highlight the promise of utilizing natural substances like flavonoids alongside advanced nanoformulations to optimize the delivery of tetracycline, reduce side effects, and tackle significant limitations, thereby opening avenues for successful clinical applications. However, further research is needed to support these potentials in natural compound and nanoformulation use [
50,
51].
In conclusion, tetracyclines hold promise as a supplementary option in RA management, but their current role remains largely experimental. Addressing existing evidence gaps, coupled with leveraging technological advancements and personalized medicine approaches, could enable tetracyclines to transition from a supplementary treatment option to unlocking their full potential in improving outcomes for patients with RA. Finally, for tetracyclines to be more fully integrated into the treatment of RA, close collaboration between rheumatologists, pharmacologists, molecular biologists, and bioinformaticians is needed to address current challenges, encourage creativity in drug development, and ultimately translate untried approaches into medical practice.
Author Contributions
Conceptualization, M.R., A.B. and J.R.; methodology, M.R., A.B. and J.R.; writing—H.Đ., A.B., M.V., I.V., A.G. and A.F.; writing—review and editing, All authors; visualization, A.F.; supervision, M.R., A.B. and J.R.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038, Erratum in Lancet 2016, 388, 1984. [Google Scholar] [CrossRef] [PubMed]
- Radić, M.; Vlak, I.; Vučković, M.; Rendulić Slivar, S.; Kadojić, M.; Stamenković, D.; Bobek, D.; Radić, J.; Gelemanović, A.; Belančić, A.; et al. Disease Activity, Inflammation Markers, and Quality of Life Are Associated with Muscle Strength in Croatian Rheumatoid Arthritis Patients-A National-Based Study. Medicina 2024, 60, 1406. [Google Scholar] [CrossRef] [PubMed]
- Gibofsky, A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: A Synopsis. Am. J. Manag. Care 2014, 20, S128–S135. [Google Scholar]
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef]
- Voils, S.A.; Evans, M.E.; Lane, M.T.; Schosser, R.H.; Rapp, R.P. Use of macrolides and tetracyclines for chronic inflammatory diseases. Ann. Pharmacother. 2005, 39, 86–94. [Google Scholar] [CrossRef]
- Webster, G.; Del Rosso, J.Q. Anti-inflammatory activity of tetracyclines. Dermatol. Clin. 2007, 25, 133–135. [Google Scholar] [CrossRef]
- Svartz, N. The treatment of rheumatic polyarthritis with acid azo compounds. Rheumatism 1948, 4, 180–185. [Google Scholar]
- Ogrendik, M. Antibiotics for the treatment of rheumatoid arthritis. Int. J. Gen. Med. 2013, 7, 43–47. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Breedveld, F.C.; Terwiel, J.P.; Mallee, C.; Dijkmans, B.A. Minocycline in active rheumatoid arthritis. A double-blind, placebo-controlled trial. Arthritis Rheum. 1994, 37, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Tilley, B.C.; Alarcón, G.S.; Heyse, S.P.; Trentham, D.E.; Neuner, R.; Kaplan, D.A.; Clegg, D.O.; Leisen, J.C.; Buckley, L.; Cooper, S.M.; et al. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann. Intern. Med. 1995, 122, 81–89. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, J.R.; Haire, C.E.; Palmer, W.; Drymalski, W.; Wees, S.; Blakely, K.; Churchill, M.; Eckhoff, P.J.; Weaver, A.; Doud, D.; et al. Treatment of early rheumatoid arthritis with minocycline or placebo: Results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1997, 40, 842–848. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, J.R.; Blakely, K.W.; Mallek, J.A.; Eckhoff, P.J.; Leff, R.D.; Wees, S.J.; Sems, K.M.; Fernandez, A.M.; Palmer, W.R.; Klassen, L.W.; et al. Treatment of early seropositive rheumatoid arthritis: A two-year, double-blind comparison of minocycline and hydroxychloroquine. Arthritis Rheum. 2001, 44, 2235–2241. [Google Scholar] [CrossRef]
- Saviola, G.; Abdi Ali, L.; Rossini, P.; Campostrini, L.; Coppini, A.; Gori, M.; Ianaro, A.; Bucci, M.; de Nucci, G.; Cirino, G. Clarithromycin in rheumatoid arthritis patients not responsive to disease-modifying antirheumatic drugs: An open, uncontrolled pilot study. Clin. Exp. Rheumatol. 2002, 20, 373–378. [Google Scholar]
- Saviola, G.; Abdi-Ali, L.; Campostrini, L.; Sacco, S.; Baiardi, P.; Manfredi, M.; Benucci, M.; Bucci, M.; Cirino, G. Clarithromycin in rheumatoid arthritis: The addition to methotrexate and low-dose methylprednisolone induces a significant additive value-a 24-month single-blind pilot study. Rheumatol. Int. 2013, 33, 2833–2838. [Google Scholar] [CrossRef]
- Ogrendik, M. Efficacy of roxithromycin in adult patients with rheumatoid arthritis who had not received disease-modifying antirheumatic drugs: A 3-month, randomized, double-blind, placebo-controlled trial. Clin. Ther. 2009, 31, 1754–1764. [Google Scholar] [CrossRef]
- Ogrendik, M.; Karagoz, N. Treatment of rheumatoid arthritis with roxithromycin: A randomized trial. Postgrad. Med. 2011, 123, 220–227. [Google Scholar] [CrossRef]
- Park, C.S.; Kim, S.H.; Lee, C.K. Immunotherapy of Autoimmune Diseases with Nonantibiotic Properties of Tetracyclines. Immune Netw. 2020, 20, e47. [Google Scholar] [CrossRef]
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol.-Cell Physiol. 2010, 299, C539–C548. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. NICE Guideline [NG100]: Rheumatoid Arthritis in Adults: Management. Available online: https://www.nice.org.uk/guidance/ng100 (accessed on 2 December 2024).
- Black, R.J.; Cross, M.; Haile, L.M.; Culbreth, G.T.; Steinmetz, J.D.; Hagins, H.; Kopec, J.A.; Brooks, P.M.; Woolf, A.D.; Ong, K.L.; et al. Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e594–e610. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Verweij, C.L.; Miltenburg, A.M.; Verhoeven, A.J.; Daha, M.R.; Dijkmans, B.A.; Breedveld, F.C. The influence of tetracyclines on T cell activation. Clin. Exp. Immunol. 1995, 102, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Szeto, G.L.; Pomerantz, J.L.; Graham, D.R.; Clements, J.E. Minocycline suppresses activation of nuclear factor of activated T cells 1 (NFAT1) in human CD4+ T cells. J. Biol. Chem. 2011, 286, 11275–11282. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Sewell, K.L.; Breedveld, F.; Furrie, E.; O’Brien, J.; Brinckerhoff, C.; Dynesius-Trentham, R.; Nosaka, Y.; Trentham, D.E. The effect of minocycline in rat models of inflammatory arthritis: Correlation of arthritis suppression with enhanced T cell calcium flux. Cell. Immunol. 1996, 167, 195–204. [Google Scholar] [CrossRef]
- Amin, A.R.; Attur, M.G.; Thakker, G.D.; Patel, P.D.; Vyas, P.R.; Patel, R.N.; Abramson, S.B. A novel mechanism of action of tetracyclines: Effects on nitric oxide synthases. Proc. Natl. Acad. Sci. USA 1996, 93, 14014–14019. [Google Scholar] [CrossRef]
- Rokkanen, P.; Julkunen, H.; Laine, H. Changes of the metatarsal head in patients with rheumatoid arthritis. A histological, tetracycline-fluorescence and microradiographical study. Acta Rheumatol. Scand. 1971, 16 (Suppl. S16), 3–46. [Google Scholar] [CrossRef]
- Skinner, M.; Cathcart, E.S.; Mills, J.A.; Pinals, R.S. Tetracycline in the treatment of rheumatoid arthritis. A double blind controlled study. Arthritis Rheum. 1971, 14, 727–732. [Google Scholar] [CrossRef]
- Tourtellotte, C.D. Tetracycline in RA. Arthritis Rheum. 1971, 14, 788. [Google Scholar] [CrossRef] [PubMed]
- Pruzanski, W.; Greenwald, R.A.; Street, I.P.; Laliberte, F.; Stefanski, E.; Vadas, P. Inhibition of enzymatic activity of phospholipases A2 by minocycline and doxycycline. Biochem. Pharmacol. 1992, 44, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.A. Treatment of destructive arthritic disorders with MMP inhibitors. Potential role of tetracyclines. Ann. N. Y. Acad. Sci. 1994, 732, 181–198. [Google Scholar] [CrossRef] [PubMed]
- St Clair, E.W.; Wilkinson, W.E.; Pisetsky, D.S.; Sexton, D.J.; Drew, R.; Kraus, V.B.; Greenwald, R.A. The effects of intravenous doxycycline therapy for rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2001, 44, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, J.R.; Elliott, J.R.; Mallek, J.A.; Mikuls, T.R.; Weaver, C.A.; Glickstein, S.; Blakely, K.M.; Hausch, R.; Leff, R.D. Treatment of early seropositive rheumatoid arthritis: Doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum. 2006, 54, 621–627. [Google Scholar] [CrossRef]
- Smith, C.J.; Sayles, H.; Mikuls, T.R.; Michaud, K. Minocycline and doxycycline therapy in community patients with rheumatoid arthritis: Prescribing patterns, patient-level determinants of use, and patient-reported side effects. Arthritis Res. Ther. 2011, 13, R168. [Google Scholar] [CrossRef]
- Smith, A.; Doré, C.; Charles, P.; Vallance, A.; Potier, T.; Mackworth-Young, C. Randomised double-blind trial of combination antibiotic therapy in rheumatoid arthritis. Int. J. Rheumatol. 2011, 2011, 585497. [Google Scholar] [CrossRef][Green Version]
- Mendes, R.E.; Farrell, D.J.; Sader, H.S.; Streit, J.M.; Jones, R.N. Update of the telavancin activity in vitro tested against a worldwide collection of Gram-positive clinical isolates (2013), when applying the revised susceptibility testing method. Diagn. Microbiol. Infect. Dis. 2015, 81, 275–279. [Google Scholar] [CrossRef]
- Jones, R.N.; Flonta, M.; Gurler, N.; Cepparulo, M.; Mendes, R.E.; Castanheira, M. Resistance surveillance program report for selected European nations (2011). Diagn. Microbiol. Infect. Dis. 2014, 78, 429–436. [Google Scholar] [CrossRef]
- Thaker, M.; Spanogiannopoulos, P.; Wright, G.D. The tetracycline resistome. Cell. Mol. Life Sci. 2010, 67, 419–431. [Google Scholar] [CrossRef]
- National Agency for Food and Drug Administration and Control. Summary of Product Characteristics (SmPC). Available online: https://www.nafdac.gov.ng/wp-content/uploads/Files/SMPC/February_2023_Revised/DXTDOXY-CAPSULE.pdf (accessed on 1 December 2024).
- College ter Beoordeling Van Geneesmiddelen. Summary of Product Characteristics (Minocycline). Available online: https://db.cbg-meb.nl/smpc/h110440_smpc_en.pdf (accessed on 1 December 2024).
- Neuvonen, P.J. Interactions with the absorption of tetracyclines. Drugs 1976, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Heydari-Kamjani, M.; Demory Beckler, M.; Kesselman, M.M. Reconsidering the Use of Minocycline in the Preliminary Treatment Regime of Rheumatoid Arthritis. Cureus 2019, 11, e5351. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xu, Y.; Dong, X.; Mu, Q.; Chen, X.; Su, G. Nanotechnology-empowered combination therapy for rheumatoid arthritis: Principles, strategies, and challenges. J. Nanobiotechnol. 2024, 22, 431. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Tiwary, N.; Sharma, N.; Behl, T.; Antil, A.; Anwer, M.K.; Ramniwas, S.; Sachdeva, M.; Elossaily, G.M.; Gulati, M.; et al. Integrating Nanotechnological Advancements of Disease-Modifying Anti-Rheumatic Drugs into Rheumatoid Arthritis Management. Pharmaceuticals 2024, 17, 248. [Google Scholar] [CrossRef]
- Puentes-Osorio, Y.; Amariles, P.; Calleja, M.Á.; Merino, V.; Díaz-Coronado, J.C.; Taborda, D. Potential clinical biomarkers in rheumatoid arthritis with an omic approach. Autoimmun. Highlights 2021, 12, 9. [Google Scholar] [CrossRef]
- Wahnou, H.; Limami, Y.; Oudghiri, M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem 2024, 4, 38–61. [Google Scholar] [CrossRef]
- Modi, S.; Inwati, G.K.; Gacem, A.; Saquib Abullais, S.; Prajapati, R.; Yadav, V.K.; Syed, R.; Alqahtani, M.S.; Yadav, K.K.; Islam, S.; et al. Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics. Antibiotics 2022, 11, 708. [Google Scholar] [CrossRef]
- Wahnou, H.; Ndayambaje, M.; Ouadghiri, Z.; Benayad, S.; Elattar, H.; Chgari, O.; Naya, A.; Zaid, Y.; Oudghiri, M. Artemisia herba-alba: Antioxidant capacity and efficacy in preventing chronic arthritis in vivo. Inflammopharmacology 2024, 32, 1855–1870. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).