Emergence of Carbapenem-Resistant blaPOM-1 Harboring Pseudomonas otitidis Isolated from River Water in Ghana
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Profiles
2.2. Genomic Characterization, Resistome, and Virulome Analysis of P. otitidis Tg_9B and BC12 Strains
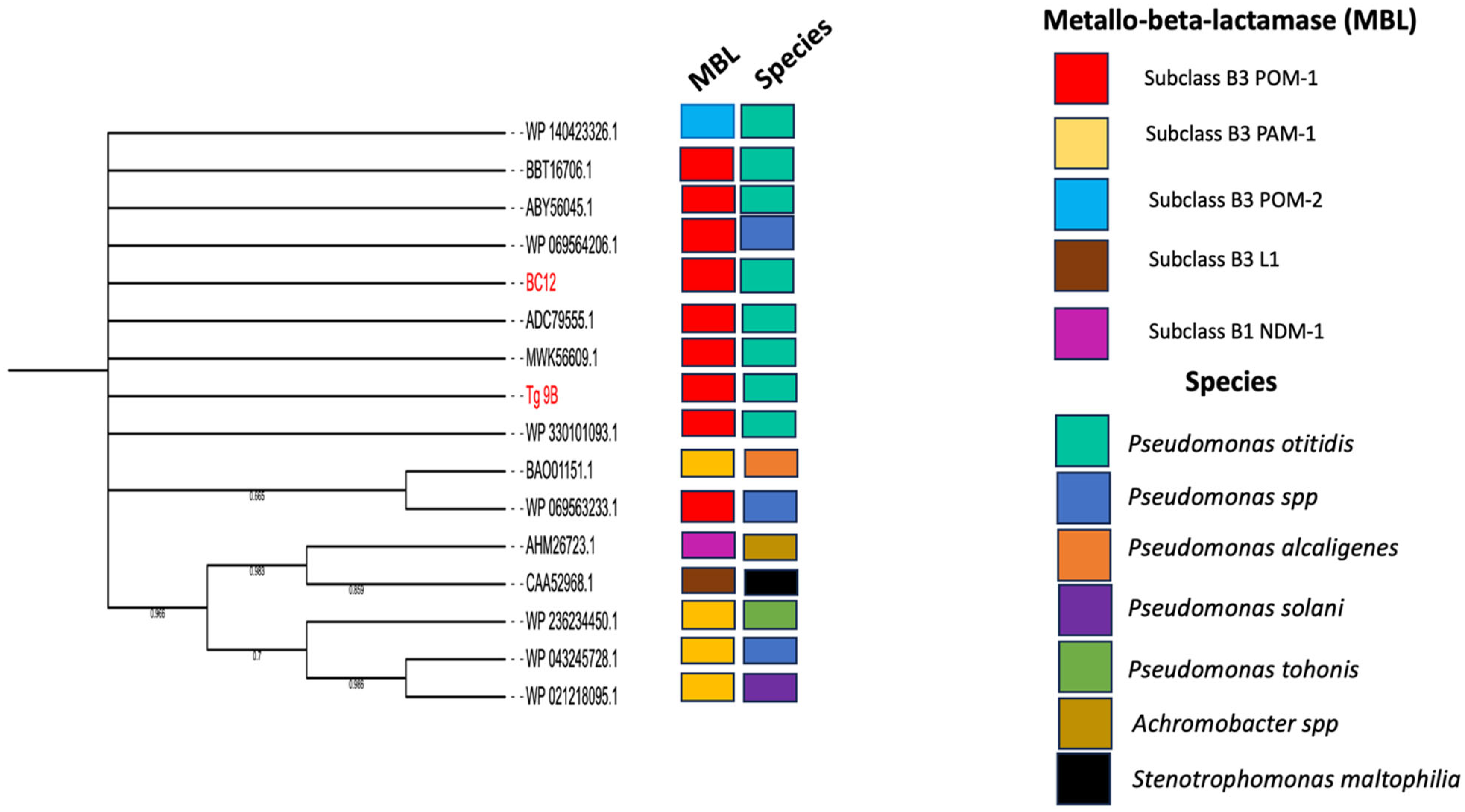
2.3. Phylogenetic Relationship of POM Amino Acid Sequences and P. otitidis Core Genomes
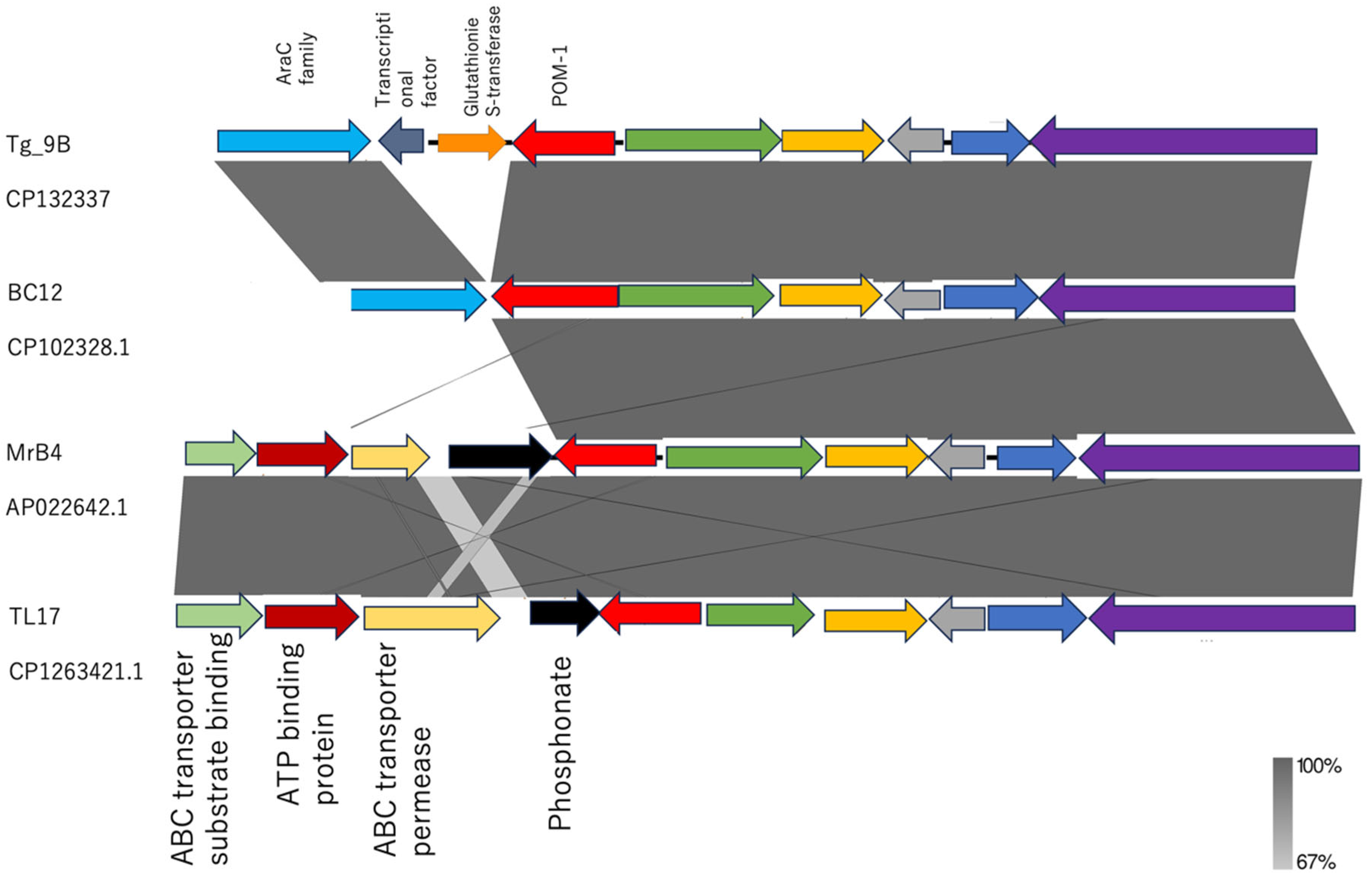
2.4. Genetic Environment of Tg_9B and BC12 and Their Associated Virulence Factors
3. Discussion
4. Materials and Methods
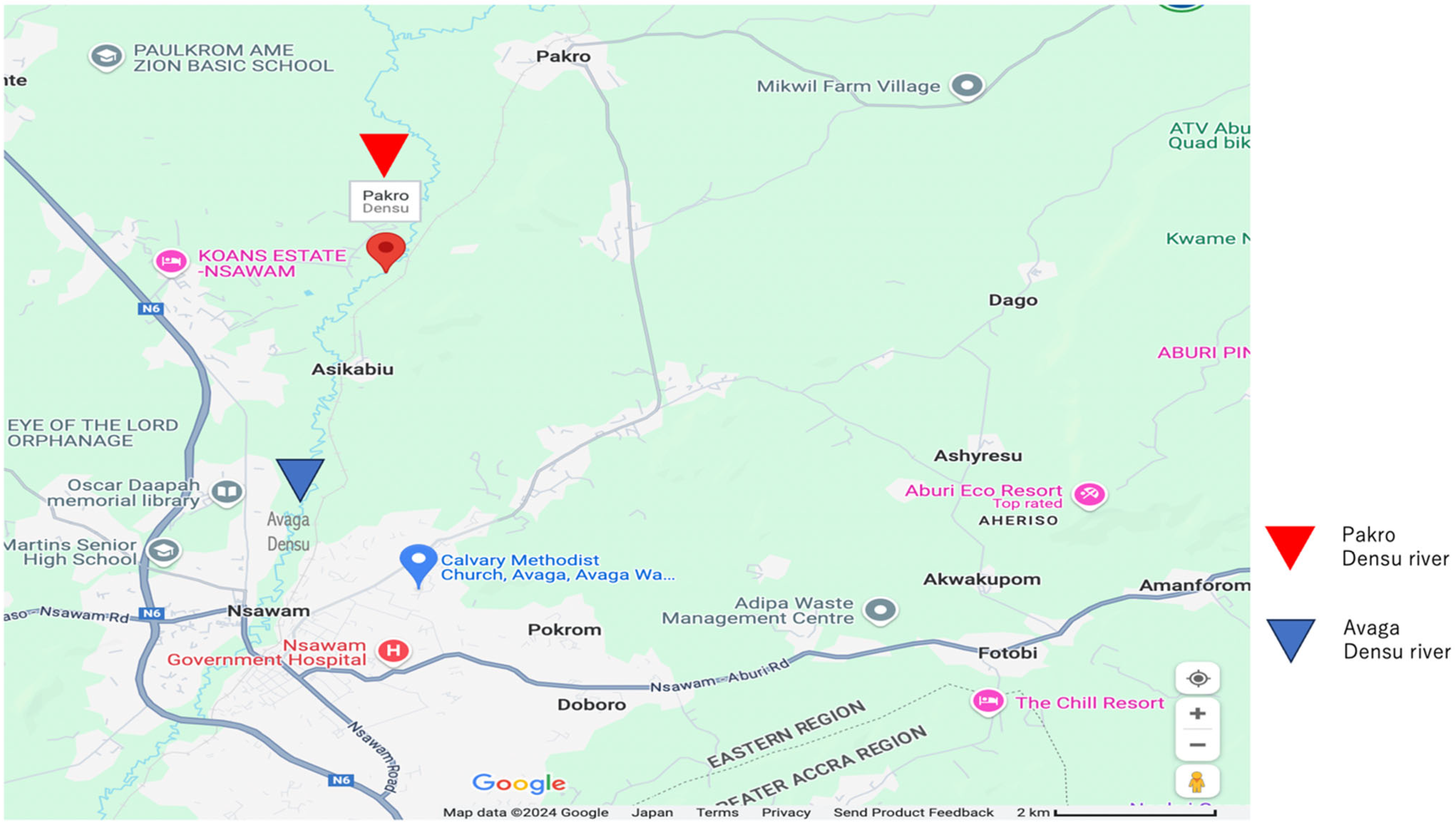
4.1. Sample Collection Site and Bacterial Isolation
4.2. 16 S rRNA Species Identification
4.3. Antimicrobial Susceptibility Testing and BlaPOM-1 Genes Screening
4.4. Genome Sequencing and Analysis
4.5. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornaglia, G.; Giamarellou, H.; Rossolini, G.M. Metallo-β-lactamases: A last frontier for β-lactams? Lancet Infect. Dis. 2011, 11, 381–393. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-β-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef]
- Thaller, M.C.; Borgianni, L.; Di Lallo, G.; Chong, Y.; Lee, K.; Dajcs, J.; Stroman, D.; Rossolini, G.M. Metallo-β-lactamase production by Pseudomonas otitidis: A species-related trait. Antimicrob. Agents Chemother. 2011, 55, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2016, 3, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.L.; Dajcs, J.J.; McLean, C.H.; Bartell, J.G.; Stroman, D.W. Pseudomonas otitidis sp. nov., isolated from patients with otic infections. Int. J. Syst. Evol. Microbiol. 2006, 56, 709–714. [Google Scholar] [CrossRef]
- Borgianni, L.; De Luca, F.; Thaller, M.C.; Chong, Y.; Rossolini, G.M.; Docquier, J.-D. Biochemical characterization of the POM-1 metallo-β-lactamase from Pseudomonas otitidis. Antimicrob. Agents Chemother. 2015, 59, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X. Prevalence of metallo-β-lactamase genes among Pseudomonas aeruginosa isolated from various clinical samples in China. J. Lab. Med. 2020, 44, 197–203. [Google Scholar] [CrossRef]
- Jianfeng, G.; Mohamad, R.; Halim, M.; Mohamed, M.S. Pseudomonas otitidis: Discovery, Mechanisms and Potential Biotechnological Applications. Eur. J. Biol. 2023, 82, 224–238. [Google Scholar] [CrossRef]
- Kim, D.; Hong, S.K.; Seo, Y.H.; Kim, M.S.; Kim, H.S.; Yong, D.; Chong, Y. Two non-otic cases of POM-1 metallo-β-lactamase-producing Pseudomonas otitidis infection: Necrotizing fasciitis and pan-peritonitis. J. Glob. Antimicrob. Resist. 2016, 7, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Logtong, E.G.; Zakpaa, H.D. The The Assessment of Antibiotic-Resistant Bacteria in Pharmaceutical Effluents from Major Pharmaceutical Companies in Accra, Ghana. Ghana J. Sci. Technol. Dev. 2024, 10, 50–68. [Google Scholar]
- Vieira, T.R.; Sambrano, G.E.; Da Silva, N.M.V.; Vasconcelos, P.C.; De Oliveira, E.F.C.; De Oliveira, C.J.B.; Cibulski, S.P.; Cardoso, M. In-depth genomic characterization of a meropenem-non-susceptible Pseudomonas otitidis strain contaminating chicken carcass. Acta Sci. Vet. 2020, 48, 1743. [Google Scholar]
- Rodriguez-Verdugo, A.; Souza, V.; Eguiarte, L.E.; Escalante, A.E. Diversity across seasons of culturable Pseudomonas from a desiccation lagoon in Cuatro Cienegas, Mexico. Int. J. Microbiol. 2012, 2012, 201389. [Google Scholar] [CrossRef]
- Wong, M.H.Y.; chi Chan, E.W.; Chen, S. Isolation of carbapenem-resistant Pseudomonas spp. from food. J. Glob. Antimicrob. Resist. 2015, 3, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jung, B.G.; Kim, K.S.; Lee, Y.C.; Sung, N.C. Isolation and characterization of Pseudomonas otitidis WL-13 and its capacity to decolorize triphenylmethane dyes. J. Environ. Sci. 2009, 21, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Khairalla, A.S.; El-Gendy, A.O.; Elkhatib, W.F. Isolation and characterization of mercury-resistant bacteria from wastewater sources in Egypt. Can. J. Microbiol. 2019, 65, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Tacao, M.; Correia, A.; Henriques, I.S. Low prevalence of carbapenem-resistant bacteria in river water: Resistance is mostly related to intrinsic mechanisms. Microb. Drug Resist. 2015, 21, 497–506. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hase, E.; Maruya, T. Complete genome sequence of Pseudomonas otitidis strain MrB4, isolated from Lake Biwa in Japan. Microbiol. Resour. Announc. 2020, 9, e00148-20. [Google Scholar] [CrossRef]
- Ali, S.; Mahmood, R.; Muneer, A.; Khalil, M.; Sheikh, N.; Tahir, H.M.; Andleeb, S.; Liaqat, I.; Ashfaq Kiani, K.; Ali, N.M.; et al. Assessment of spring water microbiology and role of Typha angustata as biosorbent. Water Environ. Res. 2019, 91, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Davies, P. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zinger, L.; Gobet, A.; Pommier, T. Two decades of describing the unseen majority of aquatic microbial diversity. Mol. Ecol. 2012, 21, 1878–1896. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Diversity of microbial communities. In Advances in Microbial Ecology; Springer: Boston, MA, USA, 1984; pp. 1–47. [Google Scholar]
- Ibekwe, A.M.; Ma, J.; Murinda, S.E. Bacterial community composition and structure in an Urban River impacted by different pollutant sources. Sci. Total Environ. 2016, 566, 1176–1185. [Google Scholar] [CrossRef]
- Abraham, W.R. Megacities as sources for pathogenic bacteria in rivers and their fate downstream. Int. J. Microbiol. 2011, 2011, 798292. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Document M100-S30; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Suzuki, M.; Suzuki, S.; Matsui, M.; Hiraki, Y.; Kawano, F.; Shibayama, K. A subclass B3 metallo-β-lactamase found in Pseudomonas alcaligenes. J. Antimicrob. Chemother. 2014, 69, 1430–1432. [Google Scholar] [CrossRef][Green Version]
- Morris, D.O.; Davis, M.F.; Palmeiro, B.S.; O’Shea, K.; Rankin, S.C. Molecular and epidemiological characterization of canine Pseudomonas otitis using a prospective case-control study design. Vet. Dermatol. 2017, 28, 118-e25. [Google Scholar] [CrossRef]
- Prah, I.; Nukui, Y.; Yamaoka, S.; Saito, R. Emergence of a high-risk Klebsiella michiganensis clone disseminating carbapenemase genes. Front. Microbiol. 2022, 13, 880248. [Google Scholar] [CrossRef] [PubMed]
- Shibu, P.; McCuaig, F.; McCartney, A.L.; Kujawska, M.; Hall, L.J.; Hoyles, L. Improved molecular characterization of the Klebsiella oxytoca complex reveals the prevalence of the kleboxymycin biosynthetic gene cluster. Microb. Genom. 2021, 7, 000592. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, G.; Induja, K.; Aiswarya, D.; Deepak, P.; Arul, D.; Kavitha, M.; Perumal, P. Isolation and characterization of human foot crack–associated bacterium, Pseudomonas Otitidis, and its biological propensity. Smart Sci. 2019, 7, 79–90. [Google Scholar] [CrossRef]
- Yamada, K.; Aoki, K.; Nagasawa, T.; Sasaki, M.; Murakami, H.; Ishii, T.; Tateda, K. Complete whole-genome sequence of the novel Pseudomonas species strain TUM18999, isolated from a patient with a burn wound in Japan. J. Glob. Antimicrob. Resist. 2021, 24, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Caixinha, A.L.; Valsamidis, A.N.; Chen, M.; Lindberg, M. Pseudomonas otitidis bacteraemia in a patient with COPD and recurrent pneumonia: Case report and literature review. BMC Infect. Dis. 2021, 21, 868. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Singh, D.; Kesavan, A.K.; Kaur, R. Molecular characterization and antimicrobial susceptibility of bacterial isolates present in tap water of public toilets. Int. Health 2020, 12, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Mathys, D.A.; Mollenkopf, D.F.; Feicht, S.M.; Adams, R.J.; Albers, A.L.; Stuever, D.M.; Wittum, T.E. Carbapenemase-producing Enterobacteriaceae and Aeromonas spp. present in wastewater treatment plant effluent and nearby surface waters in the US. PLoS ONE 2019, 14, e0218650. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.J.; Barsottini, M.R.D.O.; Rocha, R.R.; Laurindo, M.V.; Moraes, F.L.L.D.; Rocha, S.L.D. Pseudomonas aeruginosa: Virulence factors and antibiotic resistance genes. Braz. Arch. Biol. Technol. 2019, 62, e19180503. [Google Scholar] [CrossRef]
- Skariyachan, S.; Sridhar, V.S.; Packirisamy, S.; Kumargowda, S.T.; Challapilli, S.B. Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal. Folia Microbiol. 2018, 63, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Pier, G.B.; Ramphal, R. Pseudomonas aeruginosa. In Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases, 6th ed.; Mandell, G.L., Bennet, J.E., Dolin, R., Eds.; Churchill Livingstone: New York, NY, USA, 2005. [Google Scholar]
- Mandell, G.L.; Bennett, J.E.; Dolin, R.; Schwartz, D.A. Principles and practice of infectious disease. Arch. Pathol. Lab. Med. 1997, 121, 908. [Google Scholar]
- Bielecki, P.; Glik, J.; Kawecki, M.; Martins dos Santos, V.A. Towards understanding Pseudomonas aeruginosa burn wound infections by profiling gene expression. Biotechnol. Lett. 2008, 30, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Diggle, S.P.; Cornelis, P.; Williams, P.; Cámara, M. 4-quinolone signalling in Pseudomonas aeruginosa: Old molecules, new perspectives. Int. J. Med. Microbiol. 2006, 296, 83–91. [Google Scholar] [CrossRef]
- Le Terrier, C.; Masseron, A.; Uwaezuoke, N.S.; Edwin, C.P.; Ekuma, A.E.; Olugbeminiyi, F.; Nordmann, P. Wide spread of carbapenemase-producing bacterial isolates in a Nigerian environment. J. Glob. Antimicrob. Resist. 2020, 21, 321–323. [Google Scholar] [CrossRef]
- Fernando, D.M.; Kumar, A. Resistance-nodulation-division multidrug efflux pumps in gram-negative bacteria: Role in virulence. Antibiotics 2013, 2, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Dhanarani, S.; Shankar, C.; Prakash, P.; Ranganathan, K.; Saratale, R.G.; Thamaraiselvi, K. Electrophoretic pattern of glutathione S-transferase (GST) in antibiotic resistance Gram-positive bacteria from poultry litter. Microb. Pathog. 2017, 110, 285–290. [Google Scholar] [CrossRef]
- Mahazu, S.; Prah, I.; Ayibieke, A.; Sato, W.; Hayashi, T.; Suzuki, T.; Saito, R. Possible Dissemination of Escherichia coli Sequence Type 410 Closely Related to B4/H24RxC in Ghana. Front. Microbiol. 2021, 12, 770130. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Parkhill, J. Roary: Rapid large-scale prokaryote pangenome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]

| Antimicrobial Agents | Breakpoint for Resistance (µg/mL) | Tg_9B (µg/mL) | BC12 (µg/mL) |
|---|---|---|---|
| Piperacillin | ≥128 | 4 | 4 |
| Piperacilli–tazobactem | ≥128/4 | 4/4 | 4/4 |
| Cefipime | ≥32 | 1 | ≤0.5 |
| Ceftazidime | ≥32 | 2 | 2 |
| Gentamicin | ≥16 | ≤1 | ≤1 |
| Amikacin | ≥64 | ≤4 | ≤4 |
| Levofloxacin | ≥4 | 2 | ≤0.5 |
| Aztreonam | ≥32 | 4 | 8 |
| Imipenem | ≥8 | 2 | 2 |
| Meropenem | ≥8 | 4 | ≤0.5 |
| Ciprofloxacin | ≥2 | ≤0.5 | ≤0.25 |
| Tobramycin | ≥16 | ≤1 | ≤1 |
| Colistin | ≥4 | ≤1 | <1 |
| Virulence Factors | Number of Related Genes | |
|---|---|---|
| Tg_9B | BC12 | |
| Adherence | 77 | 79 |
| Anti-phagocytosis | 23 | 24 |
| Enzyme | 2 | 2 |
| Iron uptake | 16 | 16 |
| Protease | 1 | 1 |
| Quorum sensing | 1 | 1 |
| Regulation | 4 | 3 |
| Secretion system | 23 | 24 |
| Toxin | 1 | 2 |
| Amino acid and purine metabolism | 1 | 1 |
| Efflux pump | 1 | 1 |
| Endotoxin | 1 | 1 |
| Glycosylation system | 1 | 1 |
| Immune evasion | 2 | 3 |
| Invasion | 2 | 2 |
| Magnesium uptake | 1 | 1 |
| Serum resistance | 1 | 1 |
| Stress adaption | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ofosu Appiah, F.; Mahazu, S.; Prah, I.; Kawamura, T.; Ota, Y.; Nishikawa, Y.; Yoshida, M.; Suzuki, M.; Hoshino, Y.; Suzuki, T.; et al. Emergence of Carbapenem-Resistant blaPOM-1 Harboring Pseudomonas otitidis Isolated from River Water in Ghana. Antibiotics 2025, 14, 50. https://doi.org/10.3390/antibiotics14010050
Ofosu Appiah F, Mahazu S, Prah I, Kawamura T, Ota Y, Nishikawa Y, Yoshida M, Suzuki M, Hoshino Y, Suzuki T, et al. Emergence of Carbapenem-Resistant blaPOM-1 Harboring Pseudomonas otitidis Isolated from River Water in Ghana. Antibiotics. 2025; 14(1):50. https://doi.org/10.3390/antibiotics14010050
Chicago/Turabian StyleOfosu Appiah, Frederick, Samiratu Mahazu, Isaac Prah, Taira Kawamura, Yusuke Ota, Yohei Nishikawa, Mitsunori Yoshida, Masato Suzuki, Yoshihiko Hoshino, Toshihiko Suzuki, and et al. 2025. "Emergence of Carbapenem-Resistant blaPOM-1 Harboring Pseudomonas otitidis Isolated from River Water in Ghana" Antibiotics 14, no. 1: 50. https://doi.org/10.3390/antibiotics14010050
APA StyleOfosu Appiah, F., Mahazu, S., Prah, I., Kawamura, T., Ota, Y., Nishikawa, Y., Yoshida, M., Suzuki, M., Hoshino, Y., Suzuki, T., Ishino, T., Ablordey, A., & Saito, R. (2025). Emergence of Carbapenem-Resistant blaPOM-1 Harboring Pseudomonas otitidis Isolated from River Water in Ghana. Antibiotics, 14(1), 50. https://doi.org/10.3390/antibiotics14010050






