Does the Silver Nanoparticles Production Route Affect the Proliferation of Antibiotic Resistance in Soil Ecosystem?
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Used Silver Nanoparticles
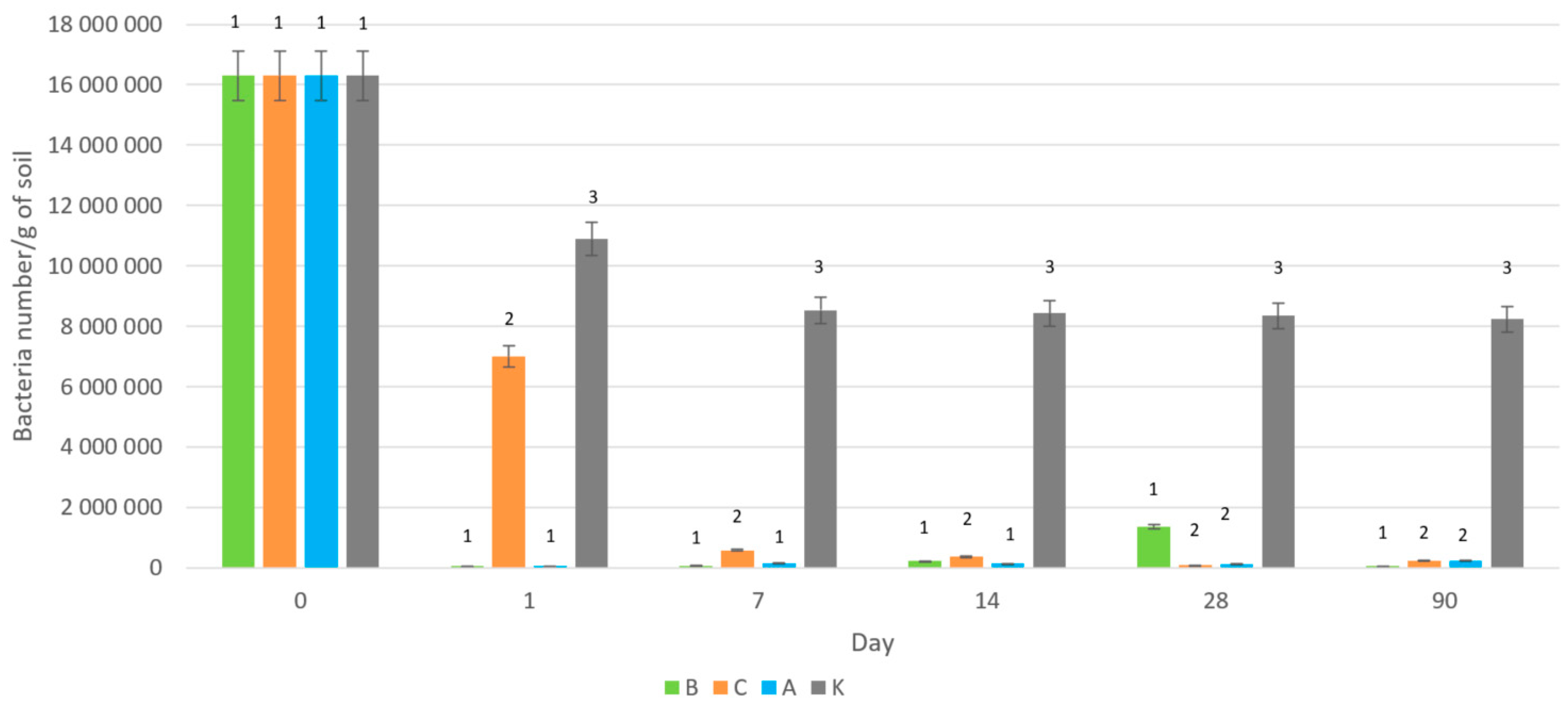
2.2. Effect of Silver Forms on Total Soil Bacteria
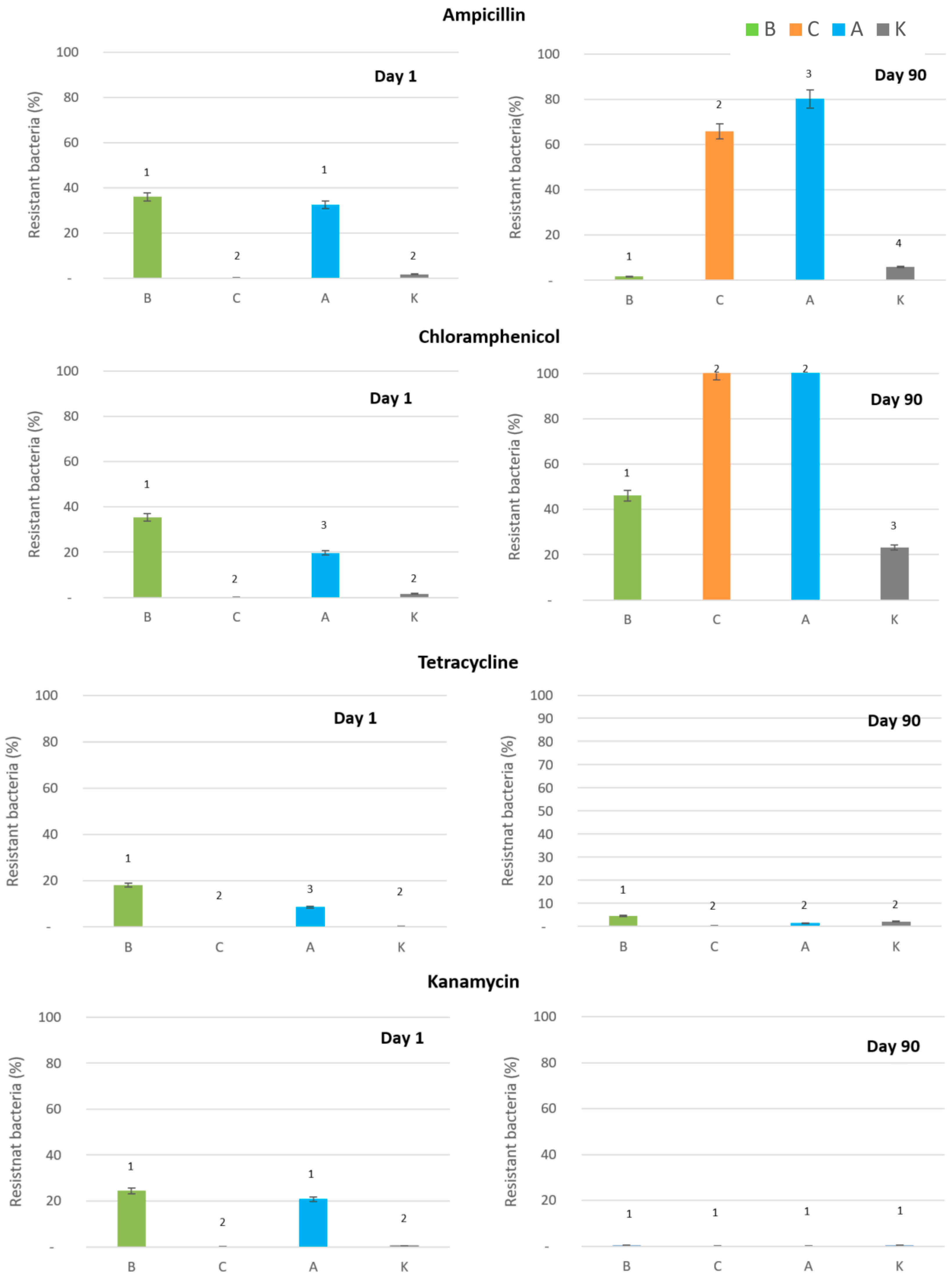
2.3. Impact of Silver Forms on Antibiotic Resistance
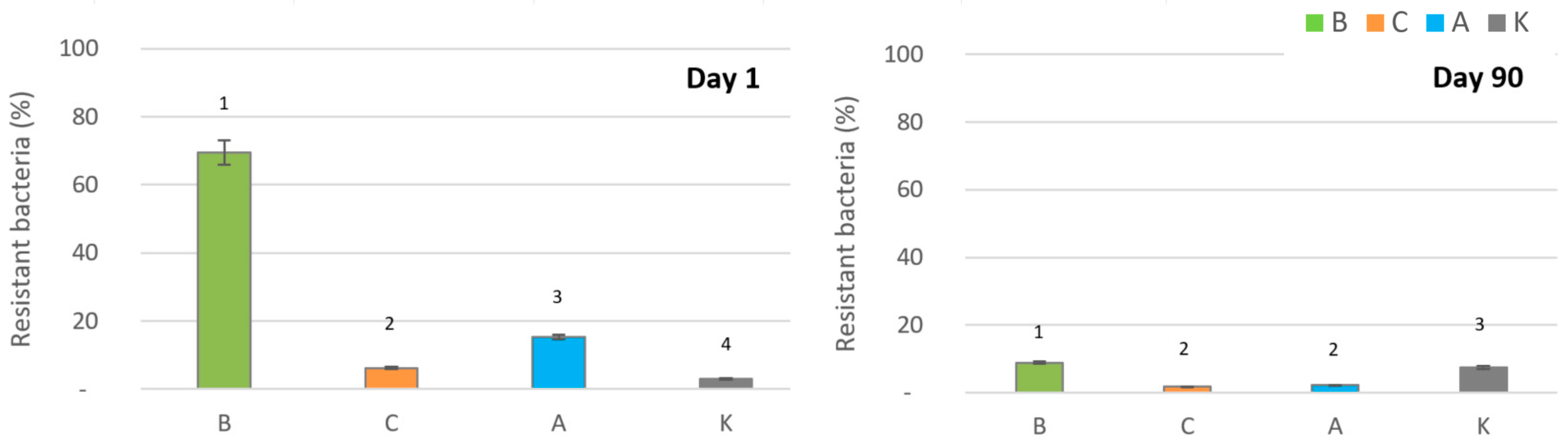
2.4. Bacterial Resistance to Silver Ions
3. Materials and Methods
3.1. Soil Sampling
3.2. Preparation of Chemically and Biologically Synthesized Silver Nanoparticles
3.3. Soil Ecosystem Effect Study
3.4. Isolation, Enumeration and Resistance Assay of Soil Bacteria
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Courtois, P.; Rorat, A.; Lemiere, S.; Guyoneaud, R.; Attard, E.; Levard, C.; Vandenbulcke, F. Ecotoxicology of silver nanoparticles and their derivatives introduced in soil with or without sewage sludge: A review of effects on microorganisms, plants and animals. Environ. Pollut. 2019, 253, 578–598. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, H.B. Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: Exploring their scope and potential in agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Agarwal, S.; Nair, K.K.; Harris, R.A.; Swart, H. Biomolecular assisted synthesis and mechanism of silver and gold nanoparticles. Mater. Res. Express 2019, 6, 082009. [Google Scholar] [CrossRef]
- Grün, A.L.; Emmerling, C. Long-term effects of environmentally relevant concentrations of silver nanoparticles on major soil bacterial phyla of a loamy soil. Environ. Sci. Eur. 2018, 30, 1–13. [Google Scholar] [CrossRef]
- Grün, A.L.; Manz, W.; Kohl, Y.L.; Meier, F.; Straskraba, S.; Jost, C.; Drexel, R.; Emmerling, C. Impact of silver nanoparticles (AgNP) on soil microbial community depending on functionalization, concentration, exposure time, and soil texture. Environ. Sci. Eur. 2019, 31, 1–22. [Google Scholar] [CrossRef]
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver nanoparticles (AgNP) in the environment: A review of potential risks on human and environmental health. Water Air Soil Pollut. 2016, 227, 306. [Google Scholar] [CrossRef]
- Chavan, S.; Nadanathangam, V. Effects of nanoparticles on plant growth-promoting bacteria in Indian agricultural soil. Agronomy 2019, 9, 140. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef]
- Stamplecoskie, K. Silver Nanoparticles: From Bulk Material to Colloidal Nanoparticles. In Silver Nanoparticle Applications: In the Fabrication and Design of Medical and Biosensing Devices; Springer Nature: Berlin/Heidelberg, Germany, 2015; pp. 1–12. [Google Scholar] [CrossRef]
- Nartop, P.; Çetin, B.N.; Zaidan, G. Dose-dependent effects of Bio-AgNPs on Rubia tinctorum callus and root biomass. Iran J. Sci. 2023, 47, 337–345. [Google Scholar] [CrossRef]
- Kaweeteerawat, C.; Na Ubol, P.; Sangmuang, S.; Aueviriyavit, S.; Maniratanachote, R. Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J. Toxicol. Environ. Health Part A 2017, 80, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Zhu, D.; An, X.L.; Ding, J.; Zhu, Y.G.; Cui, L. Does nano silver promote the selection of antibiotic resistance genes in soil and plant? Environ. Int. 2019, 128, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kadukova, J. Surface sorption and nanoparticle production as a silver detoxification mechanism of the freshwater alga Parachlorella kessleri. Bioresour. Technol. 2016, 216, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Chahar, V.; Sharma, B.; Shukla, G.; Srivastava, A.; Bhatnagar, A. Study of antimicrobial activity of silver nanoparticles synthesized using green and chemical approaches. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 149–155. [Google Scholar] [CrossRef]
- Antony, J.J.; Sivalingam, P.; Siva, D.; Kamalakkannan, S.; Anbarasu, K.; Sukirtha, R.; Krishnan, M.; Achiraman, S. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf. B Biointerfaces 2011, 88, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Y.; Jin, M.; Yuan, Z.; Bond, P.; Guo, J. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020, 169, 115229. [Google Scholar] [CrossRef] [PubMed]
- Mousavi-Khattat, M.; Keyhanfar, M.; Razmjou, A. A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1022–1031. [Google Scholar] [CrossRef]
- Guo, X.P.; Chen, X.J.; Sidikjan, N.; Sha, R.R. Silver nanoparticles regulate antibiotic resistance genes by shifting bacterial community and generating anti-silver genes in estuarine biofilms. Aquat. Toxicol. 2024, 276, 107131. [Google Scholar] [CrossRef] [PubMed]
- Campo-Beleño, C.; Villamizar-Gallardo, R.A.; López-Jácome, L.E.; González, E.E.; Muñoz-Carranza, S.; Franco, B.; Morales-Espinosa, R.; Coria-Jimenez, R.; Franco-Cendejas, R.; Hernández-Durán, M.; et al. Biologically synthesized silver nanoparticles as potent antibacterial effective against multidrug-resistant Pseudomonas aeruginosa. Lett. Appl. Microbiol. 2022, 75, 680–688. [Google Scholar] [CrossRef]
- Ali, A.R.; Anani, H.A.; Selim, F.M. Biologically formed silver nanoparticles and in vitro study of their antimicrobial activities on resistant pathogens. Iran. J. Microbiol. 2021, 13, 848. [Google Scholar] [CrossRef] [PubMed]
- Sampath, G.; Chen, Y.Y.; Rameshkumar, N.; Krishnan, M.; Nagarajan, K.; Shyu, D.J. Biologically synthesized silver nanoparticles and their diverse applications. Nanomaterials 2022, 12, 3126. [Google Scholar] [CrossRef] [PubMed]
- Sedlakova-Kadukova, J.; Demcakova, V. Stability of Biologically Produced Silver Nanoparticles in Various Environments. In Proceedings of the 6th International Scientific Conference Biotechnology and Metals, Stará Lesná, High Tatras, Slovakia, 10–11 October 2024; pp. 208–211, ISBN 978-80-89883-15-8. [Google Scholar]
- Slovak Technical Standard ISO 10381-6. Soil Quality—Sampling—Part 6: Guidance on the Collection, Handling and Storage of Soil Under Aerobic Conditions for the Assessment of Microbiological Processes, Biomass and Diversity in the Laboratory. Available online: https://www.iso.org/standard/43691.html (accessed on 28 November 2024).
- Mikac, L.; Ivanda, M.; Gotić, M.; Mihelj, T.; Horvat, L. Synthesis and characterization of silver colloidal nanoparticles with different coatings for SERS application. J. Nanoparticle Res. 2014, 16, 2748. [Google Scholar] [CrossRef]
- Schumacher, A.; Vranken, T.; Malhotra, A.; Arts, J.J.C.; Habibovic, P. In vitro antimicrobial susceptibility testing methods: Agar dilution to 3D tissue-engineered models. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 187–208. [Google Scholar] [CrossRef] [PubMed]
| B-AgNP | C-AgNP | |
|---|---|---|
| Size | 9 ± 2 nm | 20–90 nm |
| Shape | Spherical | Spherical |
| Surface | Protein biocorona | Citrate anions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlakova-Kadukova, J.; Sincak, M.; Demčakova, V. Does the Silver Nanoparticles Production Route Affect the Proliferation of Antibiotic Resistance in Soil Ecosystem? Antibiotics 2025, 14, 15. https://doi.org/10.3390/antibiotics14010015
Sedlakova-Kadukova J, Sincak M, Demčakova V. Does the Silver Nanoparticles Production Route Affect the Proliferation of Antibiotic Resistance in Soil Ecosystem? Antibiotics. 2025; 14(1):15. https://doi.org/10.3390/antibiotics14010015
Chicago/Turabian StyleSedlakova-Kadukova, Jana, Miroslava Sincak, and Veronika Demčakova. 2025. "Does the Silver Nanoparticles Production Route Affect the Proliferation of Antibiotic Resistance in Soil Ecosystem?" Antibiotics 14, no. 1: 15. https://doi.org/10.3390/antibiotics14010015
APA StyleSedlakova-Kadukova, J., Sincak, M., & Demčakova, V. (2025). Does the Silver Nanoparticles Production Route Affect the Proliferation of Antibiotic Resistance in Soil Ecosystem? Antibiotics, 14(1), 15. https://doi.org/10.3390/antibiotics14010015






