Evaluation of Cinnamon Essential Oil and Its Emulsion on Biofilm-Associated Components of Acinetobacter baumannii Clinical Strains
Abstract
1. Introduction
2. Results
2.1. Microdilution Assay
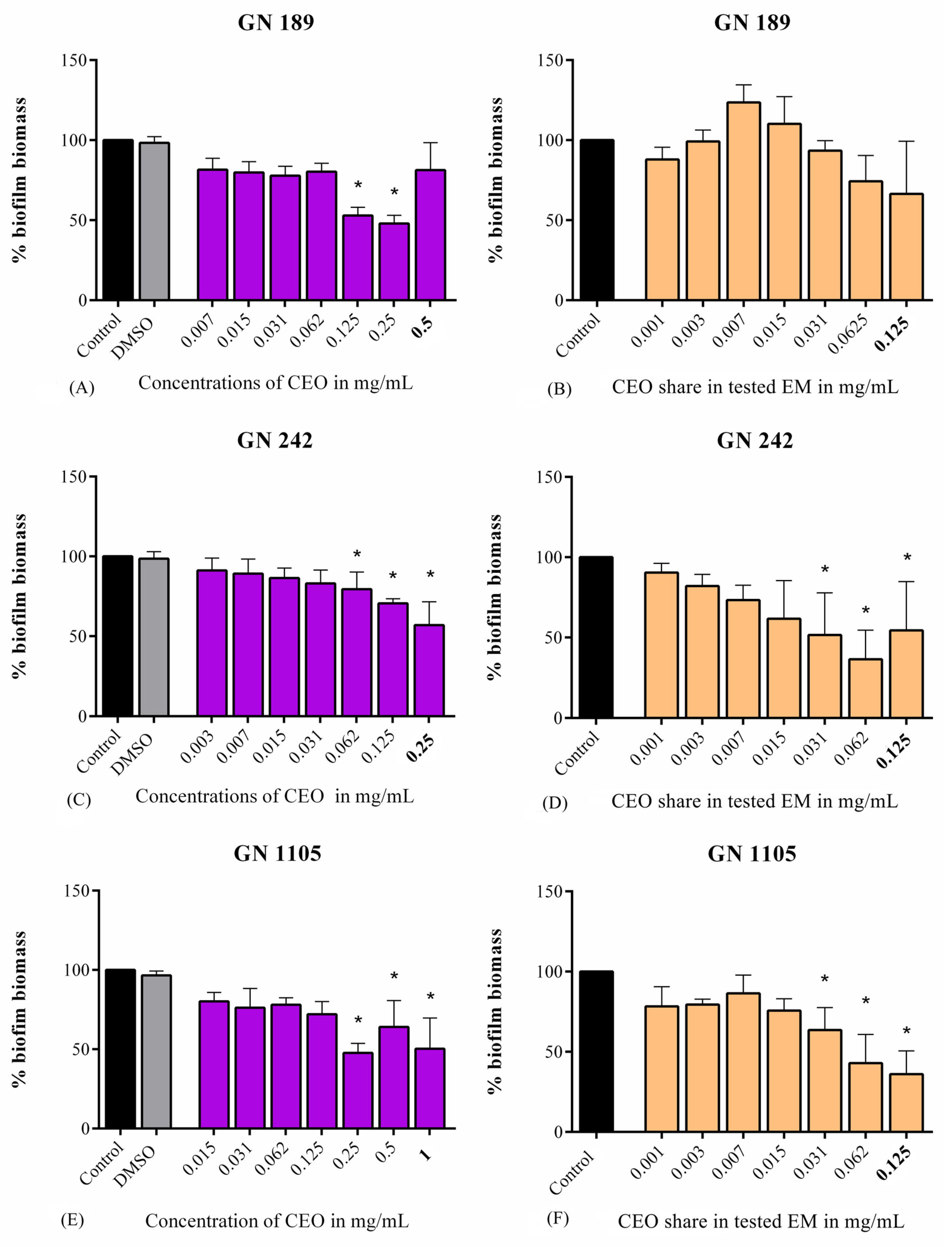
2.2. Antibiofilm Assays
2.3. Total Share of EPS
2.4. Total Share of Proteins
2.5. Concentrations of eDNA
2.6. Motility Assay
2.7. RT-qPCR
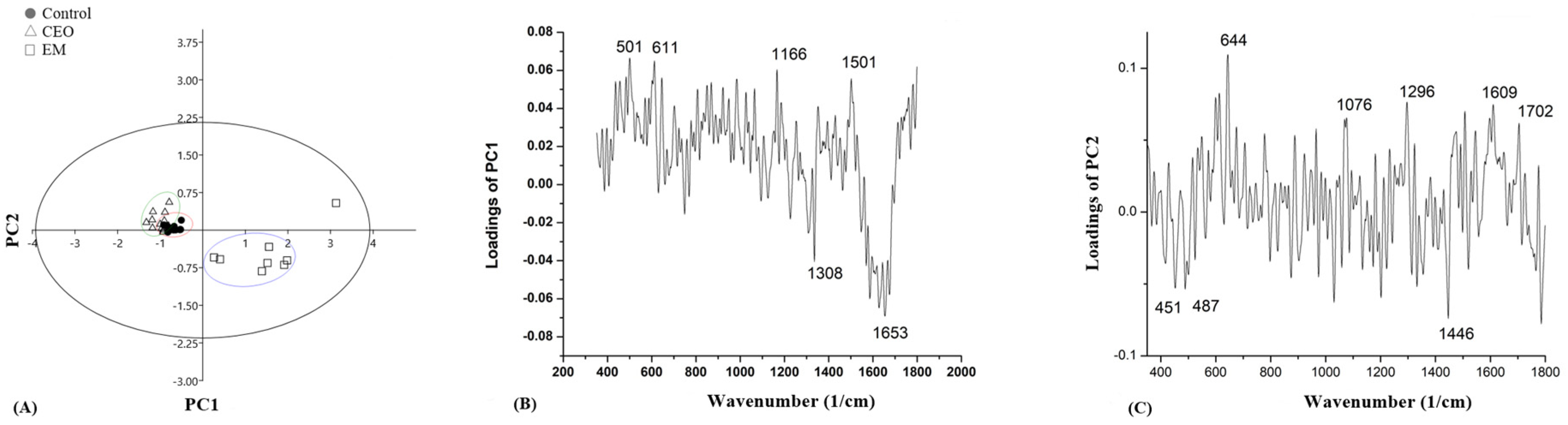
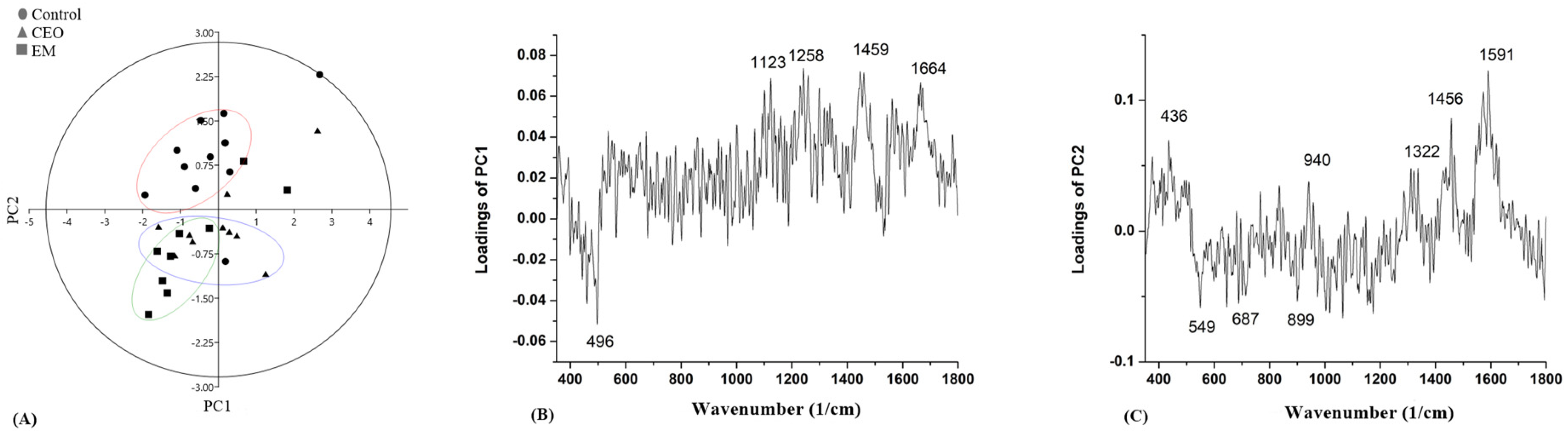
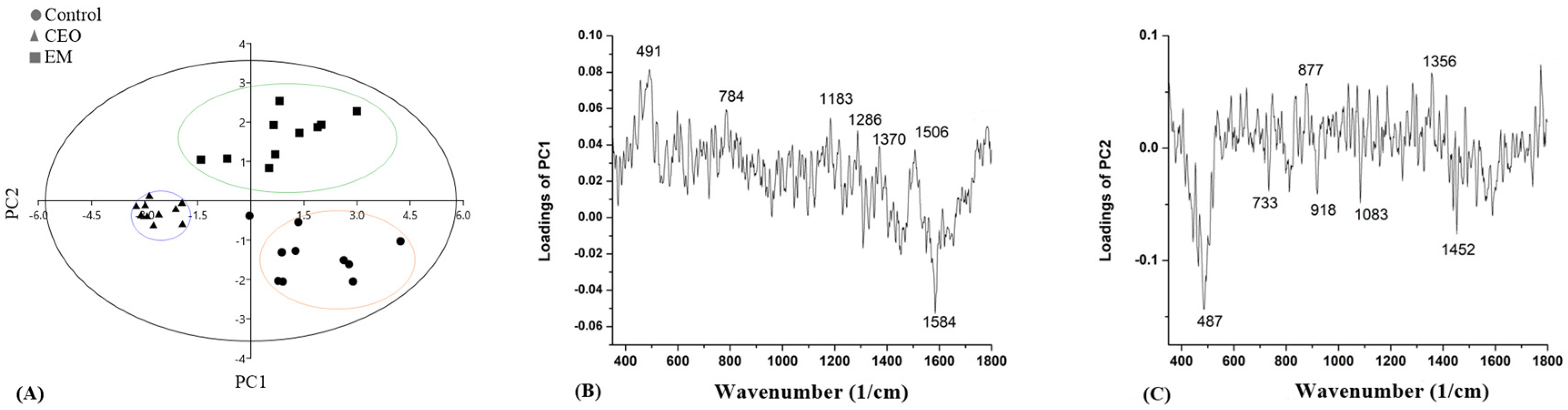
2.8. Raman Spectroscopy
3. Discussion
| Wave Number (cm−1) | Vibrational Mode | Chemical Moiety | References |
|---|---|---|---|
| 375–603 | CC bending in benzene ring; C–C–O bending; C–OH twisting | Carbohydrates | [63,65,68] |
| 549 | S-S stretch | Protein | [63] |
| 611 | Phenylalanine | Protein | [65] |
| 644–647 | C–S stretching and C–C twisting of proteins | Tyrosine, protein | [66] |
| 668–683 | Gunanine | DNA/RNA | [62] |
| 733 | Adenine | DNA/RNA | [62,63] |
| 744–828 | Timine, cytosine, uracil | DNA/RNA | [63] |
| 848 | Tyrosine | Protein | [65] |
| 877 | C–CH | Protein | [65] |
| 899 | Tryptophan | Protein | [66] |
| 915–918 | Side group (COH), (C–CH) stretching, (O–CH) | Carbohydrates | [64] |
| 940–948 | α 1,3 glucan; Deoxyribose | Carbohydrates | [62,65,69] |
| 1027–1037 | Phenylalanine/proline (C–H in plane deformation); CO and CC stretching | Protein | [63] |
| 1063 | C–C; C–N bands | Lipid, protein | [65] |
| 1076 | PO2- symmetrical stretching | DNA/RNA | [63] |
| 1084; 1085 | C–C stretching | Lipids | [64] |
| 1102–1105 | PO2− symmetrical stretching | DNA/RNA | [70] |
| 1123–1129 | Glucose; C–C, C–N stretching | Carbohydrates; protein | [64,66] |
| 1159–1166 (1183) | C–C stretching; CH2 deformation | Lipids | [64] |
| 1222; 1233 | N–H bending and CO stretching (amide III) and CN amide (stretching) | Protein | [62] |
| 1241 | Amide III | Protein | [65] |
| 1258 | CH2 deformation | Protein, lipids | [66] |
| 1286 | C–N, N–H, Amide III | Protein | [63] |
| 1296–1306 | CH2 deformation | Protein, lipids | [64] |
| 1319–1326 | Tyrosine, CH2 deformation | Lipid, protein | [66] |
| 1338 | Adenine ring mode and CH2 deformation modes (non-aromatic residues), α-helices | DNA/RNA protein | [66] |
| 1346–1370 | C–OH stretching | Carbohydrates | [64] |
| 1446–1459 | CH2 deformation | Lipids, protein | [62] |
| 1501–1506 | CH2 deformation | Lipids, protein | [64] |
| 1577–1591 | A,G | DNA/RNA | [62,63] |
| 1609 | Protein | [62] | |
| 1653 | Amide I | Protein | [65] |
| 1664–1666 | C=C, C=O, C–N stretching, N–H binding | Protein | [64,66] |
| 1688–1702 | Amide I | Protein | [64] |
| 1744; 1747 | C=C, C=O stretching | Carbohydrates, lipids | [68] |
4. Materials and Methods
4.1. Chemicals, Reagents, and Media
4.2. Bacterial Strains
4.3. Microdilution Assay
4.4. Antibiofilm Assay
4.4.1. The Effect on Biofilm Formation
4.4.2. The Effect on Pre-Formed Biofilm
4.5. Biofilm Matrix EPS Quantification
4.6. Biofilm Matrix eDNA Quantification
4.7. Biofilm Matrix Protein Quantification
4.8. Motility Assay
4.9. RNA Isolation
4.10. RT-qPCR
4.11. Raman Spectroscopy
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed, E.A.; Raafat, M.M.; Samir Mohamed, R.; Ali, A.E.E. Acinetobacter baumannii biofilm and its potential therapeutic targets. Future J. Pharm. Sci. 2023, 9, 82. [Google Scholar] [CrossRef]
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: A review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Mea, H.J.; Yong, P.V.C.; Wong, E.H. An overview of Acinetobacter baumannii pathogenesis: Motility, adherence and biofilm formation. Microbiol. Res. 2021, 247, 126722. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, Y.; Wang, X.; Ding, Y.; Sun, X.; Ni, Z. Contribution of the AbaI/AbaR quorum sensing system to resistance and virulence of Acinetobacter baumannii clinical strains. Infect. Drug Resist. 2020, 13, 4273–4281. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- El Kheloui Raja, E.M.S.; Asma, L.; Rachida, M.; Fatima, H. Acinetobacter baumannii Extracellular Matrix as An Antibiofilm and Anti-Infection Target. World J. Pharm. Res. 2022, 11, 10–35. [Google Scholar]
- Reena, A.A.A.; Subramaniyan, A.; Kanungo, R. Biofilm formation as a virulence factor of Acinetobacter baumannii: An emerging pathogen in critical care units. J. Curr. Res. Sci. Med. 2017, 3, 74–78. [Google Scholar] [CrossRef]
- De Gregorio, E.; Del Franco, M.; Martinucci, M.; Roscetto, E.; Zarrilli, R.; Di Nocera, P.P. Biofilm-associated proteins: News from Acinetobacter. BMC Genom. 2015, 16, 933. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int. J. Mol. Sci. 2021, 22, 9100. [Google Scholar] [CrossRef]
- Pancu, D.F.; Scurtu, A.; Macasoi, I.G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics: Conventional therapy and natural compounds with antibacterial activity—A pharmaco-toxicological screening. Antibiotics 2021, 10, 401. [Google Scholar] [CrossRef]
- Błaszczyk, N.; Rosiak, A.; Kałużna-Czaplińska, J. The potential role of cinnamon in human health. Forests 2021, 12, 648. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Ganić, T.; Vuletić, S.; Nikolić, B.; Stevanović, M.; Kuzmanović, M.; Kekić, D.; Cvetković, S.; Mitić-Ćulafić, D. Cinnamon essential oil and its emulsion as efficient antibiofilm agents to combat Acinetobacter baumannii. Front. Microbiol. 2022, 13, 989667. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.B.S.; Marques, L.A.; Röder, D.D.B. Diagnosis of biofilm infections: Current methods used, challenges and perspectives for the future. J. Appl. Microbiol. 2021, 131, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Alvarez, M.; Lopez, E.N.V.; Franyuti-Kelly, G.; Alvarez-Hernandez, D.A.; et al. Acinetobacter baumannii resistance: A real challenge for clinicians. Antibiotics. 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Teixeira, J.A.; Pereira, M.O.; Rocha, C.M.; Sousa, A.M. Evolving biofilm inhibition and eradication in clinical settings through plant-based antibiofilm agents. Phytomedicine 2023, 119, 154973. [Google Scholar] [CrossRef]
- Firmino, D.F.; Cavalcante, T.T.; Gomes, G.A.; Firmino, N.C.; Rosa, L.D.; de Carvalho, M.G.; Catunda Jr, F.E. Antibacterial and antibiofilm activities of Cinnamomum sp. essential oil and cinnamaldehyde: Antimicrobial activities. Sci. World J. 2018, 1, 7405736. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An overview of biofilm formation–combating strategies and mechanisms of action of antibiofilm agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Intorasoot, A.; Chornchoem, P.; Sookkhee, S.; Intorasoot, S. Bactericidal activity of herbal volatile oil extracts against multidrug-resistant Acinetobacter baumannii. J. Intercult. Ethnopharmacol. 2017, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.H.; Salem, D.; Azmy, M.; Fam, N.S. Antibacterial and antibiofilm activity of cinnamaldehyde against carbapenem-resistant Acinetobacter baumannii in Egypt: In vitro study. J. Appl. Pharm. Sci. 2018, 8, 151–156. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; López-Malo, A.; Sosa-Morales, M.E. Cinnamon (Cinnamomum zeylanicum) essential oils. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 38; pp. 339–347. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.M. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. 2011, 51, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef] [PubMed]
- Millezi, A.F.; Costa, K.A.D.; Oliveira, J.M.; Lopes, S.P.; Pereira, M.O.; Piccoli, R.H. Antibacterial and anti-biofilm activity of cinnamon essential oil and eugenol. Cienc. Rural 2019, 49, e20180314. [Google Scholar] [CrossRef]
- Liu, F.; Jin, P.; Sun, Z.; Du, L.; Wang, D.; Zhao, T.; Doyle, M.P. Carvacrol oil inhibits biofilm formation and exopolysaccharide production of Enterobacter cloacae. Food Control 2021, 119, 107473. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Kim, S.I.; Baek, K.H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Baskaran, R.; Nithyanand, P.; Vadivel, V. Effect of nanoemulsification on the antibacterial and anti-biofilm activities of selected spice essential oils and their major constituents against Salmonella enterica Typhimurium. J. Clust. Sci. 2020, 31, 1123–1135. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Cantu-Soto, E.U.; Vazquez-Armenta, F.J.; Bernal-Marcado, A.T.; Ayala-Zavala, J.F. Inhibition of Acinetobacter baumannii biofilm formation by terpenes from Oregano (Lippia graveolens) essential oil. Antibiotics 2023, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Shrivastava, R.; Vashistt, J. Eugenol and geraniol impede Csu-pilus assembly and evades multidrug-resistant Acinetobacter baumannii biofilms: In-vitro and in-silico evidence. Biochem. Biophys. Res. Commun. 2022, 636, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Artini, M.; Papa, R.; Barbato, G.; Scoarughi, G.L.; Cellini, A.; Morazzoni, P.; Bombardelli, E.; Selan, L. Bacterial biofilm formation inhibitory activity revealed for plant derived natural compounds. Bioorg. Med. Chem. 2012, 20, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.; Crulhas, B.P.; Alves, F.C.B.; Pereira, A.F.M.; Andrade, B.F.M.T.; Barbosa, L.N.; Furlanetto, A.; Lyra, L.P.S.; Rall, V.L.M.; Júnior, A.F. Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb. Pathog. 2019, 126, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Subhaswaraj, P.; Barik, S.; Macha, C.; Chiranjeevi, P.V.; Siddhardha, B. Anti quorum sensing and anti biofilm efficacy of cinnamaldehyde encapsulated chitosan nanoparticles against Pseudomonas aeruginosa PAO1. LWT 2018, 97, 752–759. [Google Scholar] [CrossRef]
- Fleming, D.; Chahin, L.; Rumbaugh, K. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob. Agents Chemother. 2017, 61, e01988-16. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.N.; Yildiz, F.H. Biofilm matrix proteins. In Microbial Biofilms, 2nd ed.; Ghannoum, M., Parsek, M., Whiteley, M., Mukherjee, P.K., Eds.; ASM Press: Washington, DC, USA, 2015; Chapter 10; pp. 201–222. [Google Scholar] [CrossRef]
- Goh, H.S.; Beatson, S.A.; Totsika, M.; Moriel, D.G.; Phan, M.D.; Szubert, J.; Runnegar, N.; Sidjabat, H.E.; Paterson, D.L.; Nimmo, G.R.; et al. Molecular analysis of the Acinetobacter baumannii biofilm-associated protein. Appl. Environ. Microbiol. 2013, 79, 6535–6543. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Tiwari, D.; Patel, V.; Tiwari, M. Effect of secondary metabolite of Actinidia deliciosa on the biofilm and extra-cellular matrix components of Acinetobacter baumannii. Microb. Pathog. 2017, 110, 345–351. [Google Scholar] [CrossRef]
- Banerji, R.; Mahamune, A.; Saroj, S.D. Aqueous extracts of spices inhibit biofilm in Listeria monocytogenes by downregulating release of eDNA. LWT 2022, 154, 112566. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition mechanism of cardamom essential oil on methicillin-resistant Staphylococcus aureus biofilm. LWT 2020, 122, 109057. [Google Scholar] [CrossRef]
- Yamabe, K.; Arakawa, Y.; Shoji, M.; Miyamoto, K.; Tsuchiya, T.; Minoura, K.; Akeda, Y.; Tomono, K.; Onda, M. Enhancement of Acinetobacter baumannii biofilm growth by cephem antibiotics via enrichment of protein and extracellular DNA in the biofilm matrices. J. Appl. Microbiol. 2022, 133, 2002–2013. [Google Scholar] [CrossRef]
- Xi, C.; Wu, J. dATP/ATP, a multifunctional nucleotide, stimulates bacterial cell lysis, extracellular DNA release and biofilm development. PLoS ONE 2010, 5, e13355. [Google Scholar] [CrossRef]
- Nait Chabane, Y.; Mlouka, M.B.; Alexandre, S.; Nicol, M.; Marti, S.; Pestel-Caron, M.; Vila, J.; Jouenne, T.; Dé, E. Virstatin inhibits biofilm formation and motility of Acinetobacter baumannii. BMC Microbiol. 2014, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Lee, J.H.; Kim, Y.G.; Rajasekharan, S.K.; García-Contreras, R.; Lee, J. Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front. Microbiol. 2019, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- McQueary, C.N.; Kirkup, B.C.; Si, Y.; Barlow, M.; Actis, L.A.; Craft, D.W.; Zurawski, D.V. Extracellular stress and lipopolysaccharide modulate Acinetobacter baumannii surface-associated motility. J. Microbiol. 2012, 50, 434–443. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lu, F.; Yuan, F.; Jiang, D.; Zhao, P.; Zhu, J.; Cheng, H.; Cao, J.; Lu, G. Biofilm formation caused by clinical Acinetobacter baumannii isolates is associated with overexpression of the AdeFGH efflux pump. Antimicrob. Agents Chemother. 2015, 59, 4817–4825. [Google Scholar] [CrossRef]
- Ramezanalizadeh, F.; Owlia, P.; Rasooli, I. Type I pili, CsuA/B and FimA induce a protective immune response against Acinetobacter baumannii. Vaccine 2020, 38, 5436–5446. [Google Scholar] [CrossRef]
- Quinn, B.; Rodman, N.; Jara, E.; Fernandez, J.S.; Martinez, J.; Traglia, G.M.; Montana, S.; Cantera, V.; Place, K.; Bonomo, R.A.; et al. Human serum albumin alters specific genes that can play a role in survival and persistence in Acinetobacter baumannii. Sci. Rep. 2018, 8, 14741. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, X.; Chen, C.; Qi, J.; Wang, Y. Integrating transcriptomics and metabolomics analysis on kojic acid combating Acinetobacter baumannii biofilm and its potential roles. Microbiol. Res. 2022, 254, 126911. [Google Scholar] [CrossRef]
- Moon, K.H.; Weber, B.S.; Feldman, M.F. Subinhibitory concentrations of trimethoprim and sulfamethoxazole prevent biofilm formation by Acinetobacter baumannii through inhibition of Csu pilus expression. Antimicrob. Agents Chemoter. 2017, 61, e00778-17. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.S.; Tuttobene, M.R.; Montaña, S.; Subils, T.; Cantera, V.; Iriarte, A.; Tuchscherr, L.; Ramirez, M.S. Staphylococcus aureus α-toxin effect on Acinetobacter baumannii behavior. Biology 2022, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, B.A.; Stroeher, U.H.; Hassan, K.A.; Papadimitrious, M.S.; Paulsen, I.T.; Brown, M.H.; Lo, R. Adherence and motility characteristics of clinical Acinetobacter baumannii isolates. FEMS Microbiol. Lett. 2011, 323, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Corral, J.; Pérez-Varela, M.; Sánchez-Osuna, M.; Cortés, P.; Barbé, J.; Aranda, J. Importance of twitching and surface-associated motility in the virulence of Acinetobacter baumannii. Virulence 2021, 12, 2201–2213. [Google Scholar] [CrossRef]
- Li, M.; Aye, S.M.; Ahmed, M.U.; Han, M.L.; Li, C.; Song, J.; Boyce, J.D.; Powell, D.R.; Azad, M.A.K.; Velkov, T.; et al. Pan-transcriptomic analysis identified common differentially expressed genes of Acinetobacter baumannii in response to polymyxin treatments. Mol. Omics 2020, 16, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Dhabaan, G.N.; AbuBakar, S.; Cerqueira, G.M.; Al-Haroni, M.; Pang, S.P.; Hassan, H. Imipenem treatment induces expression of important genes and phenotypes in a resistant Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 2016, 60, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Fernandez, J.S.; Liu, C.; Hoard, A.; Mendoza, A.; Nakanouchi, J.; Rodman, N.; Courville, R.; Tuttobene, M.R.; Lopez, C.; et al. Human pleural fluid triggers global changes in the transcriptional landscape of Acinetobacter baumannii as an adaptive response to stress. Sci. Rep. 2019, 9, 17251. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zuo, R.; González Barrios, A.F.; Bedzyk, L.A.; Eldridge, G.R.; Pasmore, M.E.; Wood, T.K. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005, 71, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Kusić, D.; Kampe, B.; Ramoji, A.; Neugebauer, U.; Rösch, P.; Popp, J. Raman spectroscopic differentiation of planktonic bacteria and biofilms. Anal. Bioanal. Chem. 2015, 407, 6803–6813. [Google Scholar] [CrossRef]
- Shakeel, M.; Majeed, M.I.; Nawaz, H.; Rashid, N.; Ali, A.; Haque, A.; Akbar, M.U.; Tahir, M.; Munir, S.; Ali, Z.; et al. Surface-enhanced Raman spectroscopy for the characterization of pellets of biofilm forming bacterial strains of Staphylococcus epidermidis. Photodiagn. Photodyn. Ther. 2022, 40, 103145. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Krysa, M.; Wojtowicz, K.; Wiater, A.; Pleszczyńska, M.; Tomczyk, M.; Sroka-Bartnicka, A. The FT-IR and Raman spectroscopies as tools for biofilm characterization created by cariogenic streptococci. Int. J. Mol. Sci. 2020, 21, 3811. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Zhang, P.; Guo, J.S.; Fang, F.; Gao, X.; Li, C. Functional groups characteristics of EPS in biofilm growing on different carriers. Chemosphere 2013, 92, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Kusić, D.; Kampe, B.; Rösch, P.; Popp, J. Identification of water pathogens by Raman microspectroscopy. Water Res. 2014, 48, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.B.; Nam, S.W.; Choi, S.; Lee, G.J.; Park, H.K. Evaluation of antibiotic effects on Pseudomonas aeruginosa biofilm using Raman spectroscopy and multivariate analysis. Biomed. Opt. Express 2014, 5, 3238–3251. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P.; Wagner, M.; Horn, H.; Niessner, R.; Haisch, C. Towards a nondestructive chemical characterization of biofilm matrix by Raman microscopy. Anal. Bioanal. Chem. 2009, 393, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Ofuji, S.; Imamura, H.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Ohgitani, E.; Marin, E.; Zhu, W.; Mazda, O.; et al. In Situ Raman Analysis of Biofilm Exopolysaccharides Formed in Streptococcus mutans and Streptococcus sanguinis Commensal Cultures. Int. J. Mol. Sci. 2023, 24, 6694. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Mora, T.; Dávila-Pérez, C.; Torres-Méndez, F.; Valle-Bourrouet, G. Raman spectroscopic characterization of endodontic biofilm matrices. J. Spectrosc. 2019, 1, 1307397. [Google Scholar] [CrossRef]
- Lukovic, B.; Gajic, I.; Dimkic, I.; Kekic, D.; Zornic, S.; Pozder, T.; Radisavljevic, S.; Opavski, N.; Kojic, M.; Ranin, L. The first nationwide multicenter study of Acinetobacter baumannii recovered in Serbia: Emergence of OXA-72, OXA-23 and NDM-1-producing isolates. Antimicrob. Resist. Infect. Control 2020, 9, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Tomić, N.; Stevanović, M.M.; Filipović, N.; Ganić, T.; Nikolić, B.; Gajić, I.; Ćulafić, D.M. Resveratrol/Selenium Nanocomposite with Antioxidative and Antibacterial Properties. Nanomaterials 2024, 14, 368. [Google Scholar] [CrossRef]
- Rubini, D.; Banu, S.F.; Nisha, P.; Murugan, R.; Thamotharan, S.; Percino, M.J.; Subramani, P.; Nithyanand, P. Essential oils from unexplored aromatic plants quench biofilm formation and virulence of Methicillin resistant Staphylococcus aureus. Microb. Pathog. 2018, 122, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Vuletić, S.; Ganić, T.; Lončarević, B.; Cvetković, S.; Nikolić, B.; Lješević, M.; Mitić-Ćulafić, D. New insights into underlying mechanism involved in the Frangula alnus antivirulence potential directed toward Staphylococcus aureus. Bot. Serbica 2025, in press. [Google Scholar]
- Đukanović, S.; Ganić, T.; Lončarević, B.; Cvetković, S.; Nikolić, B.; Tenji, D.; Randjelović, D.; Mitić-Ćulafić, D. Elucidating the antibiofilm activity of Frangula emodin against Staphylococcus aureus biofilms. J. Appl. Microbiol. 2022, 132, 1840–1855. [Google Scholar] [CrossRef] [PubMed]
- Selasi, G.N.; Nicholas, A.; Jeon, H.; Na, S.H.; Kwon, H.I.; Kim, Y.J.; Heo, S.T.; Oh, M.H.; Lee, J.C. Differences in biofilm mass, expression of biofilm-associated genes, and resistance to desiccation between epidemic and sporadic clones of carbapenem-resistant Acinetobacter baumannii sequence type 191. PLoS ONE 2016, 11, e0162576. [Google Scholar] [CrossRef]
- Lannan, F.M.; O’conor, D.K.; Broderick, J.C.; Tate, J.F.; Scoggin, J.T.; Moran, N.A.; Hussan, C.M.; Hegeman, E.M.; Ogrydziak, C.E.; Singh, S.A.; et al. Evaluation of virulence gene expression patterns in Acinetobacter baumannii using quantitative real-time polymerase chain reaction array. Mil. Med. 2016, 181, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, J.V.; Girija, A.S.; Paramasivam, A. In silico analysis of virulence genes in an emerging dental pathogen A. baumannii and related species. Arch. Oral Biol. 2018, 94, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Wang, J.; Wang, X. FleQ regulates both the type VI secretion system and flagella in Pseudomonas putida. Biotechnol. Appl. Biochem. 2018, 65, 419–427. [Google Scholar] [CrossRef]
- Menges, F. Spectragryph Optical Spectroscopy Software, Version 1.2.14. Available online: http://www.effemm2.de/spectragryph/ (accessed on 27 October 2022).
- Hammer, O.Y.V.I.N.D.; Harper, D.A.; Ryan, P.D. Palaeontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]






| Control | DMSO | CEO | EM | |
|---|---|---|---|---|
| GN 189 | 96.15 ± 59.75 | 141.55 ± 36.13 | 162.05 ± 6.57 | 225.85 ± 29.63 |
| GN 242 | 391.5 ± 69.01 | 390.6 ± 5.09 | 318.95 ± 17.89 | 600.45 ± 98.92 |
| GN 1105 | 38.5 ± 6.65 | 33.8 ± 3.73 | 53.3 ± 8.63 | 35.6 ± 2.55 |
| Gene | Sequence | References |
|---|---|---|
| abaI | Forward—CCG CCT TCC TCT AGC AGT CA Reverse—AAA ACC CGC AGC ACG TAA TAA | [76] |
| csuA | Forward—TGG TAC AGC AGT AGC TTG GC Reverse—GAC GGT GGT GAA CGT ACA GA | [77] |
| pilA | Forward—TGT GGA TGA TGT GCC GGA AA Reverse—ATC CGG TAA GCA TCG GTG TG | [78] |
| 16S rRNA | Forward—GCA ACG CGA AGA ACC TTA Reverse—AAC CCA ACA TCT CAC GAC AC | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganić, T.; Pećinar, I.; Nikolić, B.; Kekić, D.; Tomić, N.; Cvetković, S.; Vuletić, S.; Mitić-Ćulafić, D. Evaluation of Cinnamon Essential Oil and Its Emulsion on Biofilm-Associated Components of Acinetobacter baumannii Clinical Strains. Antibiotics 2025, 14, 106. https://doi.org/10.3390/antibiotics14010106
Ganić T, Pećinar I, Nikolić B, Kekić D, Tomić N, Cvetković S, Vuletić S, Mitić-Ćulafić D. Evaluation of Cinnamon Essential Oil and Its Emulsion on Biofilm-Associated Components of Acinetobacter baumannii Clinical Strains. Antibiotics. 2025; 14(1):106. https://doi.org/10.3390/antibiotics14010106
Chicago/Turabian StyleGanić, Tea, Ilinka Pećinar, Biljana Nikolić, Dušan Kekić, Nina Tomić, Stefana Cvetković, Stefana Vuletić, and Dragana Mitić-Ćulafić. 2025. "Evaluation of Cinnamon Essential Oil and Its Emulsion on Biofilm-Associated Components of Acinetobacter baumannii Clinical Strains" Antibiotics 14, no. 1: 106. https://doi.org/10.3390/antibiotics14010106
APA StyleGanić, T., Pećinar, I., Nikolić, B., Kekić, D., Tomić, N., Cvetković, S., Vuletić, S., & Mitić-Ćulafić, D. (2025). Evaluation of Cinnamon Essential Oil and Its Emulsion on Biofilm-Associated Components of Acinetobacter baumannii Clinical Strains. Antibiotics, 14(1), 106. https://doi.org/10.3390/antibiotics14010106







