Assessment of De-Escalation of Empirical Antimicrobial Therapy in Medical Wards with Recognized Prevalence of Multi-Drug-Resistant Pathogens: A Multicenter Prospective Cohort Study in Non-ICU Patients with Microbiologically Documented Infection
Abstract
1. Introduction
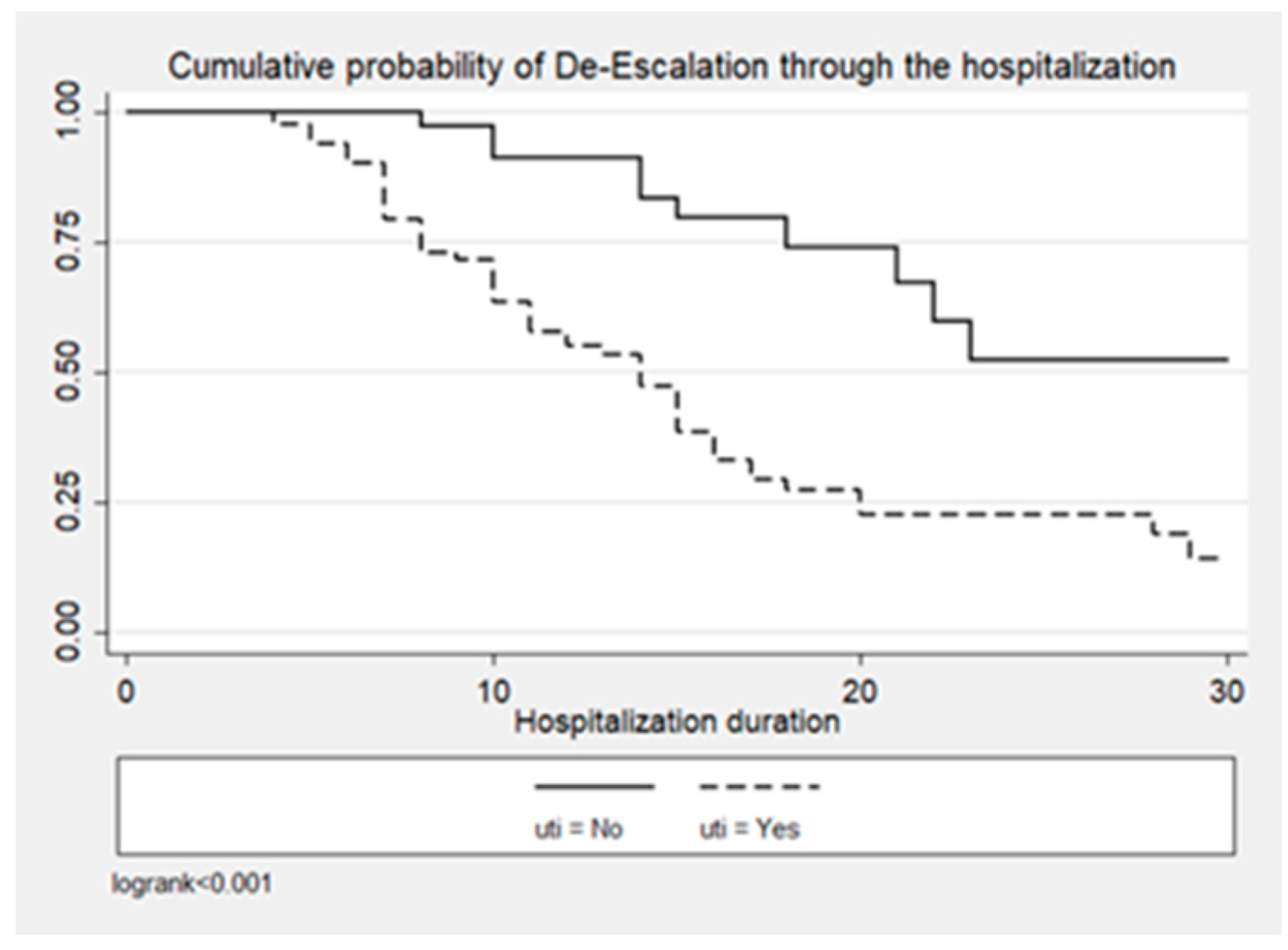
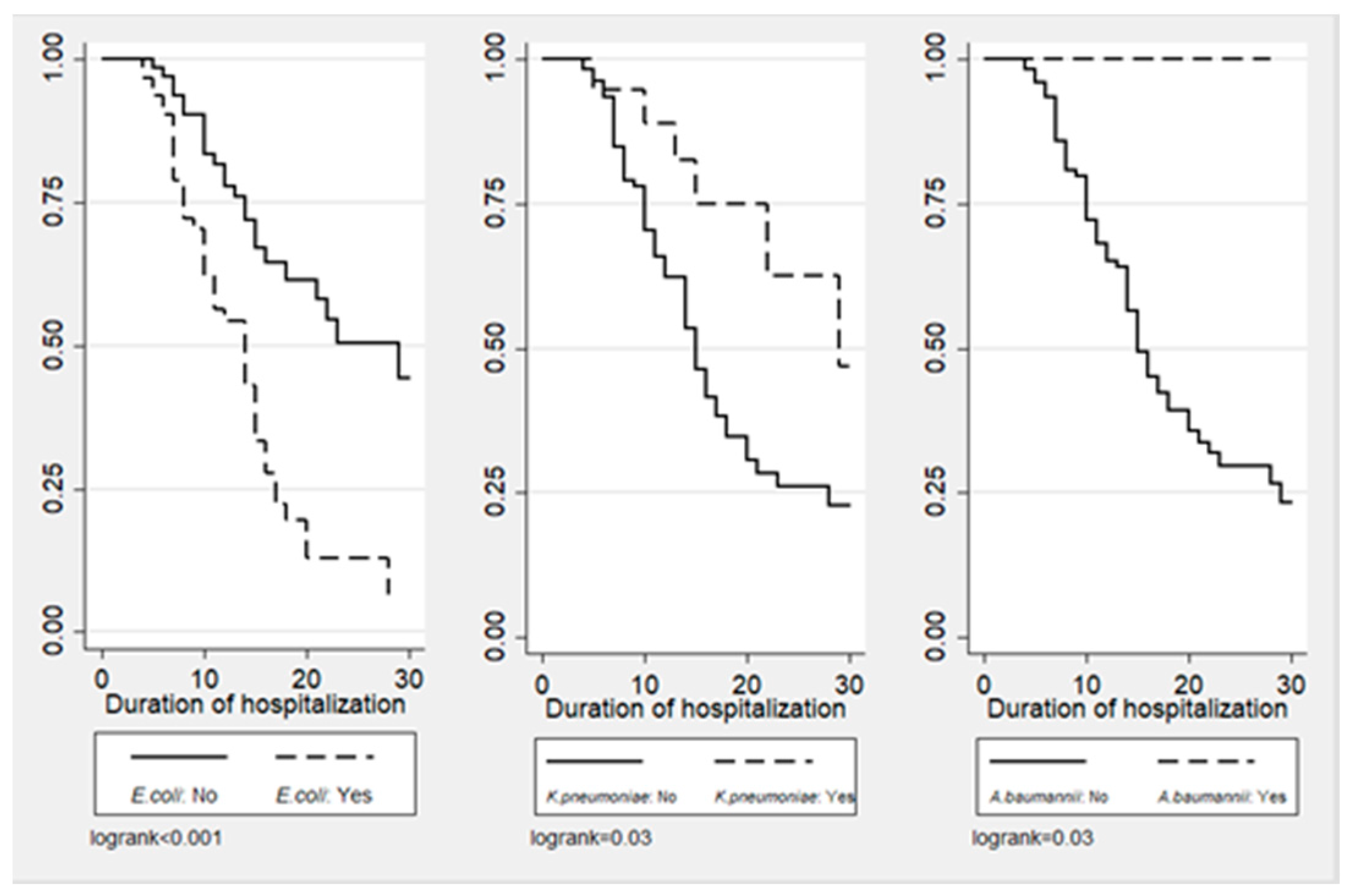
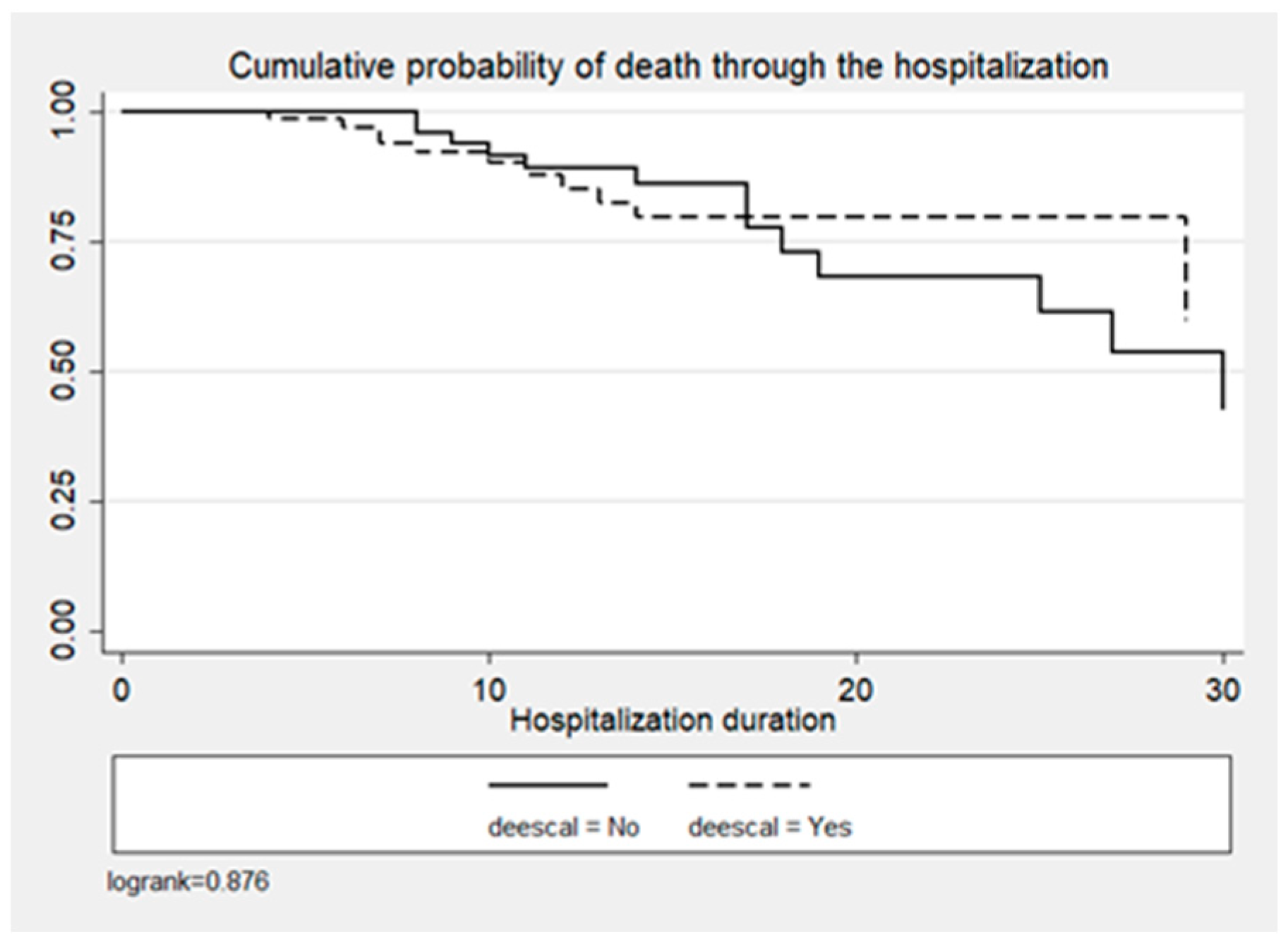
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Setting
4.2. Study Population and Data Collection
4.3. Definitions
4.4. Outcomes
4.5. Statistical Analysis
4.6. Ethical Issues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 6 June 2024).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 6 June 2024).
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society. Policy statement on amtimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Diseases Society (PIDS). Infect. Control Hosp. Epidemiol. 2012, 33, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- De Waele, J.J.; Schouten, J.; Beovic, B.; Tabah, A.; Leone, M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: No simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020, 46, 236–244. [Google Scholar] [CrossRef]
- Tabah, A.; Cotta, M.O.; Garnacho-Montero, J.; Schouten, J.; Roberts, J.A.; Lipman, J.; Tacey, M.; Timsit, J.F.; Leone, M.; Zahar, J.R.; et al. A Systematic Review of the Definitions, Determinants, and Clinical Outcomes of Antimicrobial De-escalation in the Intensive Care Unit. Clin. Infect. Dis. 2016, 62, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Pastene, B.; Cassir, N.; Martin-Loeches, I.; Leone, M. Efficacy and safety of antimicrobial de-escalation as a clinical strategy. Expert Rev. Anti. Infect. Ther. 2019, 17, 79–88. [Google Scholar] [CrossRef]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Kim, R.Y.; Ng, A.M.; Persaud, A.K.; Furmanek, S.P.; Kothari, Y.N.; Price, J.D.; Wiemken, T.L.; Saad, M.A.; Guardiola, J.J.; Cavallazzi, R.S. Antibiotic Timing and Outcomes in Sepsis. Am. J. Med. Sci. 2018, 355, 524–529. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef] [PubMed]
- De Bus, L.; Depuydt, P.; Steen, J.; Dhaese, S.; De Smet, K.; Tabah, A.; Akova, M.; Cotta, M.O.; De Pascale, G.; Dimopoulos, G.; et al. Antimicrobial de-escalation in the critically ill patient and assessment of clinical cure: The DIANA study. Intensive Care Med. 2020, 46, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Routsi, C.; Gkoufa, A.; Arvaniti, K.; Kokkoris, S.; Tourtoglou, A.; Theodorou, V.; Vemvetsou, A.; Kassianidis, G.; Amerikanou, A.; Paramythiotou, E.; et al. De-escalation of antimicrobial therapy in ICU settings with high prevalence of multidrug-resistant bacteria: A multicentre prospective observational cohort study in patients with sepsis or septic shock. J. Antimicrob. Chemother. 2020, 75, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Ambaras Khan, R.; Aziz, Z. Antibiotic de-escalation in patients with pneumonia in the intensive care unit: A systematic review and meta-analysis. Int. J. Clin. Pract. 2018, 72, e13245. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.H.; Huang, L.O.; Cui, Y.H.; Xu, D.; Wu, C.R.; Tang, J.G. Antibiotics De-Escalation in the Treatment of Ventilator-Associated Pneumonia in Trauma Patients: A Retrospective Study on Propensity Score Matching Method. Chin. Med. J. 2018, 131, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Trupka, T.; Fisher, K.; Micek, S.T.; Juang, P.; Kollef, M.H. Enhanced antimicrobial de-escalation for pneumonia in mechanically ventilated patients: A cross-over study. Crit. Care 2017, 21, 180. [Google Scholar] [CrossRef]
- De Bus, L.; Denys, W.; Catteeuw, J.; Gadeyne, B.; Vermeulen, K.; Boelens, J.; Claeys, G.; De Waele, J.J.; Decruyenaere, J.; Depuydt, P.O. Impact of de-escalation of beta-lactam antibiotics on the emergence of antibiotic resistance in ICU patients: A retrospective observational study. Intensive Care Med. 2016, 42, 1029–1039. [Google Scholar] [CrossRef]
- Weiss, E.; Zahar, J.R.; Garrouste-Orgeas, M.; Ruckly, S.; Essaied, W.; Schwebel, C.; Timsit, J.F.; OUTCOMEREA Study Group. De-escalation of pivotal beta-lactam in ventilator-associated pneumonia does not impact outcome and marginally affects MDR acquisition. Intensive Care Med. 2016, 42, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Joffe, A.R.; Muscedere, J.; Marshall, J.C.; Su, Y.; Heyland, D.K.; Canadian Critical Care Trials Group. The safety of targeted antibiotic therapy for ventilator-associated pneumonia: A multicenter observational study. J. Crit. Care 2008, 23, 82–90. [Google Scholar] [CrossRef]
- Alvarez-Lerma, F.; Alvarez, B.; Luque, P.; Ruiz, F.; Dominguez-Roldan, J.M.; Quintana, E.; Sanz-Rodriguez, C.; ADANN Study Group. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: A prospective observational study. Crit. Care 2006, 10, R78. [Google Scholar] [CrossRef]
- Infectious Diseases Society of America (IDSA). Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52, S397–S428. [Google Scholar] [CrossRef]
- Alshareef, H.; Alfahad, W.; Albaadani, A.; Alyazid, H.; Talib, R.B. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J. Infect. Public Health 2020, 13, 985–990. [Google Scholar] [CrossRef]
- Sadyrbaeva-Dolgova, S.; Aznarte-Padial, P.; Jimenez-Morales, A.; Expósito-Ruiz, M.; Calleja-Hernández, M.Á.; Hidalgo-Tenorio, C. Pharmacist recommendations for carbapenem de-escalation in urinary tract infection within an antimicrobial stewardship program. J. Infect. Public Health 2020, 13, 558–563. [Google Scholar] [CrossRef]
- Lee, C.C.; Wang, J.L.; Lee, C.H.; Hung, Y.P.; Hong, M.Y.; Tang, H.J.; Ko, W.C. Clinical benefits of antimicrobial de-escalation in adults with community-onset monomicrobial Escherichia coli, Klebsiella species and Proteus mirabilis bacteremia. Int. J. Antimicrob. Agents 2017, 50, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Dickstein, Y.; Raz-Pasteur, A. Antibiotic de-escalation for bloodstream infections and pneumonia: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2016, 22, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ohl, C.; Johnson, J.; Williamson, J.; Beardsley, J.; Luther, V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established Antimicrobial Stewardship Program. BMC Infect. Dis. 2016, 16, 751. [Google Scholar] [CrossRef]
- Ohji, G.; Doi, A.; Yamamoto, S.; Iwata, K. Is de-escalation of antimicrobials effective? A systematic review and meta-analysis. Int. J. Infect. Dis. 2016, 49, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, F.A.; Karim, A.; Mahmood, T.; Ahmed, S.; Jaffri, S.F.; Tate, M.E.; Mehmood, M. Antibiotic de-escalation in bacteremic urinary tract infections: Potential opportunities and effect on outcome. Infection 2014, 42, 829–834. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Siampanos, A.; Bolanou, A.; Doulou, S.; Kakavoulis, N.; Tsiakos, K.; Katopodis, S.; Schinas, G.; Skorda, L.; Alexiou, Z.; et al. Clarithromycin for early anti-inflammatory responses in community-acquired pneumonia in Greece (ACCESS): A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2024, 12, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Sinapidis, D.; Halvatzis, S.; Velissaris, D.; Alexiou, N.; Kosmas, V.; Adami, M.E.; Kyprianou, M.; Kyprianou, A.; Stefos, A.; et al. Survival benefit associated with clarithromycin in severe community-acquired pneumonia: A matched comparator study. Int. J. Antimicrob. Agents 2020, 55, 105836. [Google Scholar] [CrossRef]
- Karakike, E.; Scicluna, B.P.; Roumpoutsou, M.; Mitrou, I.; Karampela, N.; Karageorgos, A.; Psaroulis, K.; Massa, E.; Pitsoulis, A.; Chaloulis, P.; et al. Effect of intravenous clarithromycin in patients with sepsis, respiratory and multiple organ dysfunction syndrome: A randomized clinical trial. Crit. Care 2022, 26, 183. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Mylona, V.; Antonopoulou, A.; Tsangaris, I.; Koutelidakis, I.; Marioli, A.; Raftogiannis, M.; Kopterides, P.; Lymberopoulou, K.; Mouktaroudi, M.; et al. Effect of clarithromycin in patients with suspected Gram-negative sepsis: Results of a randomized controlled trial. J. Antimicrob. Chemother. 2014, 69, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Tsaganos, T.; Raftogiannis, M.; Pratikaki, M.; Christodoulou, S.; Kotanidou, A.; Papadomichelakis, E.; Armaganidis, A.; Routsi, C.; Giamarellos-Bourboulis, E.J. Clarithromycin Leads to Long-Term Survival and Cost Benefit in Ventilator-Associated Pneumonia and Sepsis. Antimicrob. Agents Chemother. 2016, 60, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Spyridaki, A.; Raftogiannis, M.; Antonopoulou, A.; Tsaganos, T.; Routsi, C.; Baziaka, F.; Karagianni, V.; Mouktaroudi, M.; Koutoukas, P.; Pelekanou, A.; et al. Effect of clarithromycin in inflammatory markers of patients with ventilator-associated pneumonia and sepsis caused by Gram-negative bacteria: Results from a randomized clinical study. Antimicrob. Agents Chemother. 2012, 56, 3819–3825. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Pechère, J.C.; Routsi, C.; Plachouras, D.; Kollias, S.; Raftogiannis, M.; Zervakis, D.; Baziaka, F.; Koronaios, A.; Antonopoulou, A.; et al. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin. Infect. Dis. 2008, 46, 1157–1164. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, W.; Yang, H.; Ma, C.; Sui, S. De-escalation of empiric antibiotics in patients with severe sepsis or septic shock: A meta-analysis. Heart Lung 2016, 45, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; Mornese Pinna, S.; Lupia, T.; Trentalange, A.; Germanò, E.; Cavallo, R.; Lupia, E.; De Rosa, F.G. Antibiotic De-escalation Experience in the Setting of Emergency Department: A Retrospective, Observational Study. J. Clin. Med. 2021, 10, 3285. [Google Scholar] [CrossRef]
- Leone, M.; Bechis, C.; Baumstarck, K.; Lefrant, J.Y.; Albanèse, J.; Jaber, S.; Lepape, A.; Constantin, J.M.; Papazian, L.; Bruder, N.; et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: A multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014, 40, 1399–1408. [Google Scholar] [CrossRef]
- Yamana, H.; Matsui, H.; Tagami, T.; Hirashima, J.; Fushimi, K.; Yasunaga, H. De-escalation versus continuation of empirical antimicrobial therapy in community-acquired pneumonia. J. Infect. 2016, 73, 314–325. [Google Scholar] [CrossRef]
- Moehring, R.W.; Yarrington, M.E.; Warren, B.G.; Lokhnygina, Y.; Atkinson, E.; Bankston, A.; Collucio, J.; David, M.Z.; Davis, A.E.; Davis, J.; et al. Evaluation of an Opt-Out Protocol for Antibiotic De-Escalation in Patients with Suspected Sepsis: A Multicenter, Randomized, Controlled Trial. Clin. Infect Dis. 2023, 76, 433–442. [Google Scholar] [CrossRef]
- Tabah, A.; Bassetti, M.; Kollef, M.H.; Zahar, J.R.; Paiva, J.A.; Timsit, J.F.; Roberts, J.A.; Schouten, J.; Giamarellou, H.; Rello, J.; et al. Antimicrobial de-escalation in critically ill patients: A position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020, 46, 245–265. [Google Scholar] [PubMed]
- Royer, S.; DeMerle, K.M.; Dickson, R.P.; Prescott, H.C. Shorter Versus Longer Courses of Antibiotics for Infection in Hospitalized Patients: A Systematic Review and Meta-Analysis. J. Hosp. Med. 2018, 13, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Seddon, M.M.; Bookstaver, P.B.; Justo, J.A.; Kohn, J.; Rac, H.; Haggard, E.; Mediwala, K.N.; Dash, S.; Al-Hasan, M.N. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin. Infect. Dis. 2019, 69, 414–420. [Google Scholar] [CrossRef]
- Umpleby, H.; Dushianthan, A.; Catton, T.; Saeed, K. Antimicrobial stewardship programmes focused on de-escalation: A narrative review of efficacy and risks. J. Emerg. Crit. Care Med. 2022, 6, 23. [Google Scholar] [CrossRef]
- Lew, K.Y.; Ng, T.M.; Tan, M.; Tan, S.H.; Lew, E.L.; Ling, L.M.; Ang, B.; Lye, D.; Teng, C.B. Safety and clinical outcomes of carbapenem de-escalation as part of an antimicrobial stewardship programme in an ESBL-endemic setting. J. Antimicrob. Chemother. 2015, 70, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Cravoisy, A.; Barraud, D.; Conrad, M.; Nace, L.; Lemarié, J.; Bollaert, P.E.; Gibot, S. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit. Care 2013, 17, R140. [Google Scholar] [CrossRef] [PubMed]
- Polemis, M.; Mandilara, G.; Pappa, O.; Argyropoulou, A.; Perivolioti, E.; Koudoumnakis, N.; Pournaras, S.; Vasilakopoulou, A.; Vourli, S.; Katsifa, H.; et al. COVID-19 and Antimicrobial Resistance: Data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance-WHONET-Greece (January 2018–March 2021). Life 2021, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Palaiopanos, K.; Krystallaki, D.; Mellou, K.; Kotoulas, P.; Kavakioti, C.A.; Vorre, S.; Vertsioti, G.; Gkova, M.; Maragkos, A.; Tryfinopoulou, K.; et al. Healthcare-associated infections and antimicrobial use in acute care hospitals in Greece, 2022; results of the third point prevalence survey. Antimicrob. Resist. Infect. Control 2024, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/antimicrobial-resistance/media/pdfs/ar-threats-2013-508.pdf?CDC_AAref_Val=https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 6 May 2024).

| Variables | ADE Group n = 76 | Non-ADE Group n = 66 | Overall n = 142 | p-Value |
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Mean age, years (SD) | 74.5 (14.7) | 72.8, (16.5) | 73.7 (15.5) | 0.664 |
| Gender | 0.024 | |||
| Male (%) | 33 (43.4) | 42 (63.6) | 75 (52.8) | |
| CCI (median, IQR) | 5 (4, 7) | 5 (3,7) | 5 (3, 7) | 0.542 |
| Residence type, n (%) | 0.068 | |||
| Home | 52 (68.4) | 30 (45.5) | 82 (57.7) | |
| Medical Ward | 2 (2.6) | 3 (4.5) | 5 (3.5) | |
| ICU | 0 (0) | 1 (1.5) | 1 (0.7) | |
| Healthcare facility | 2 (2.6) | 5(7.6) | 7 (4.9) | |
| Long-term care facility | 4 (5.3) | 9 (13.6) | 13 (9.2) | |
| Home with at least 1 hospitalization > 48 h since the last trimester | 16 (21.1) | 18 (27.3) | 34 (24) | |
| Receipt of antimicrobials in the past trimester (%) | 26 (34.2) | 20 (30.3) | 46 (32.4) | 0.193 |
| Times of antimicrobials receipt (median, IQR) | 2.5 (2, 6) | 2 (1, 6) | 2 (1, 6) | 0.378 |
| Symptoms on admission, n (%) | ||||
| Fever | 54 (71) | 46 (69.7) | 100 (70.4) | 0.860 |
| Disorder of Consciousness | 19 (25) | 11 (16.7) | 30 (21.1) | 0.681 |
| Other symptoms | 47 (61.8) | 29 (43.9) | 76 (53.5) | 0.033 |
| Illness severity on admission | ||||
| SOFA score (median, IQR) | 3 (2, 4) | 2 (1, 3.5) | 3 (1, 4) | 0.028 |
| q-SOFA Score (median, IQR) | 1 (0, 1) | 1 (0, 1) | 1 (0, 1) | 0.603 |
| Patients with sepsis, n (%) | 44 (57.9) | 36 (54.5) | 80 (56.3) | 0.622 |
| Patients in septic shock, n (%) | 11 (14.5) | 7 (10.6) | 18 (12.7) | 0.452 |
| Type of Infection, n (%) | ||||
| UTI | 60 (78.9) | 35 (53) | 95 (66.9) | 0.001 |
| LRTI | 3 (3.9) | 6 (9.1) | 9 (6.3) | 0.196 |
| CAP | 4 (5.3) | 5 (7.6) | 9 (6.3) | 0.580 |
| HAP | 0 | 1 (1.5) | 1 (0.7) | 0.283 |
| Osteomyelitis | 0 | 2 (3) | 2 (1.4) | 0.122 |
| SSTI | 3 (3.9) | 3 (4.5) | 6 (4.2) | 0.840 |
| Abdominal | 5 (6.6) | 3 (4.5) | 8 (5.6) | 0.606 |
| BSI | 39 (51.3) | 27 (40.9) | 66 (46.5) | 0.139 |
| Primary | 3 (3.9) | 7 (10.6) | 10 (7) | 0.233 |
| Secondary | 36 (47.4) | 20 (30.3) | 56 (39.4) | 0.027 |
| CLABSI | 2 (2.6) | 3 (4.5) | 5 (3.5) | 0.881 |
| Pathogen, n (%) | ||||
| Escherichia coli | 46 (60.5) | 26 (39.4) | 72 (50.7) | 0.012 |
| Klebsiella pneumoniae | 8 (10.5) | 11 (16.7) | 19 (13.4) | 0.284 |
| Pseudomonas aeruginosa | 4 (5.3) | 7 (10.6) | 11 (7.8) | 0.235 |
| Proteus mirabilis | 4 (5.3) | 4 (6) | 8 (5.6) | 0.837 |
| Acinetobacter baumannii | 0 | 5 (7.6) | 5 (3.5) | 0.015 |
| Other | 16 (21) | 13 (19.7) | 29 (20.4) | 0.842 |
| Clinical specimens of pathogen isolation, n (%) | ||||
| Blood | 42 (58.3) | 29 (45.3) | 71 (52.2) | 0.129 |
| Urine | 47 (61.8) | 39 (59) | 86 (60.6) | 0.738 |
| Sputum | 1 (1.4) | 3 (4.6) | 4 (2.9) | 0.257 |
| Skin and soft-tissue | 0 | 2 (3.1) | 2 (1.4) | 0.129 |
| Other | 5 (6.6) | 3 (4.5) | 8 (5.6) | 0.600 |
| Empirical antimicrobial therapy | 0.064 | |||
| Ampicillin-sulbactam or Ampicillin/sulbactam- based antimicrobial therapy | 3 (3.9) | 8 (12.1) | 11 (7.7) | |
| Fluoroquinolones monotherapy or Fluoroquinolones-based antimicrobial therapy | 6 (7.9) | 12 (18.2) | 18 (12.7) | |
| Piperacillin/tazobactam monotherapy or Piperacillin/tazobactam-based antimicrobial therapy | 39 (51.3) | 17 (25.7) | 56 (39.4) | |
| Meropenem or Meropenem-based antimicrobial therapy | 11 (14.5) | 5 (7.6) | 16 (11.3) | |
| Other | 17 (22.4) | 24 (36.4) | 41 (28.9) | |
| Amikacin co-administration | 29 (38.1) | 15 (22.7) | 44 (31) | |
| Vancomycin co-administration | 15 (19.7) | 8 (12.1) | 23 (16.2) | |
| Metronidazole co-administration | 4 (5.3) | 1 (1.5) | 5 (3.5) | |
| Exploratory end-points | ||||
| Mortality (n, %) | 14 (18.4) | 20 (30.3) | 34 (23.9) | <0.1 |
| LOS (median, IQR) | 11 (7–15) | 14 (8–19) | 12.5 (8, 6) | 0.047 |
| Duration of antibiotic treatment (median, IQR) | 12 (10, 14) | 13 (9, 15) | 12.5 (10, 15) | 0.9 |
| Antibiotic-free days (median, IQR) | 4 (0, 14) | 4(0, 14) | 4 (0, 14) | 0.8 |
| Hydrocortisone administration (median, IQR) | 0 (0, 0) | 0(0, 0) | 3.5 (3, 3.5) | 0.7 |
| Vasopressors administration (median, IQR) | 0 (0, 0) | 0(0, 0) | 3.5 (2, 4.5) | 0.3 |
| AKI | 3 (3.9) | 6 (9.1) | 9 (6.3) | 0.6 |
| Multi-organ failure | 4 (5.3) | 2 (3) | 6 (4.2) | 0.5 |
| Superinfection | 5 (6.6) | 4 (6.1) | 9 (6.3) | 0.3 |
| Clostridium difficile infection | 1 (1.3) | 1 (1.5) | 2 (1.4) | 0.9 |
| XDR bacteria colonization | 1 (1.3) | 1 (1.5) | 2 (1.4) | 0.9 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Females vs. males | 2.65 | 1.09–6.43 | 0.031 |
| Symptoms on admission other than fever and disorder of consciousness | 3.44 | 1.37–8.67 | 0.009 |
| Previously been at home | 2.43 | 0.10–5.92 | 0.051 |
| SOFA on admission | 1.22 | 1.00–1.51 | 0.064 |
| UTI | 10.04 | 2.91–34.57 | <0.001 |
| SSTI | 16.28 | 1.68–158.08 | 0.016 |
| Abdominal Infection | 5.50 | 0.82–36.74 | 0.078 |
| BSI | 2.52 | 1.00–6.36 | 0.05 |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| 0.0002 | |||
| Age | 1.01 | 1.00–1.09 | 0.031 |
| ADE performance | 1.49 | 0.55–4.01 | 0.432 |
| Moxifloxacin administration | 36.50 | 2.39–556.6 | 0.010 |
| Piperacillin/Tazobactam administration | 4.36 | 1.47–12.99 | 0.008 |
| Cefuroxime administration | 26.49 | 5.75–122 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapti, V.; Poulakou, G.; Mousouli, A.; Kakasis, A.; Pagoni, S.; Pechlivanidou, E.; Masgala, A.; Sympardi, S.; Apostolopoulos, V.; Giannopoulos, C.; et al. Assessment of De-Escalation of Empirical Antimicrobial Therapy in Medical Wards with Recognized Prevalence of Multi-Drug-Resistant Pathogens: A Multicenter Prospective Cohort Study in Non-ICU Patients with Microbiologically Documented Infection. Antibiotics 2024, 13, 812. https://doi.org/10.3390/antibiotics13090812
Rapti V, Poulakou G, Mousouli A, Kakasis A, Pagoni S, Pechlivanidou E, Masgala A, Sympardi S, Apostolopoulos V, Giannopoulos C, et al. Assessment of De-Escalation of Empirical Antimicrobial Therapy in Medical Wards with Recognized Prevalence of Multi-Drug-Resistant Pathogens: A Multicenter Prospective Cohort Study in Non-ICU Patients with Microbiologically Documented Infection. Antibiotics. 2024; 13(9):812. https://doi.org/10.3390/antibiotics13090812
Chicago/Turabian StyleRapti, Vasiliki, Garyfallia Poulakou, Anastasia Mousouli, Athanasios Kakasis, Stamata Pagoni, Evmorfia Pechlivanidou, Aikaterini Masgala, Styliani Sympardi, Vasileios Apostolopoulos, Charalampos Giannopoulos, and et al. 2024. "Assessment of De-Escalation of Empirical Antimicrobial Therapy in Medical Wards with Recognized Prevalence of Multi-Drug-Resistant Pathogens: A Multicenter Prospective Cohort Study in Non-ICU Patients with Microbiologically Documented Infection" Antibiotics 13, no. 9: 812. https://doi.org/10.3390/antibiotics13090812
APA StyleRapti, V., Poulakou, G., Mousouli, A., Kakasis, A., Pagoni, S., Pechlivanidou, E., Masgala, A., Sympardi, S., Apostolopoulos, V., Giannopoulos, C., Alexiou, N., Arvaniti, K., Trakatelli, C., Prionas, A., Samarkos, M., Daikos, G. L., & Giamarellou, H. (2024). Assessment of De-Escalation of Empirical Antimicrobial Therapy in Medical Wards with Recognized Prevalence of Multi-Drug-Resistant Pathogens: A Multicenter Prospective Cohort Study in Non-ICU Patients with Microbiologically Documented Infection. Antibiotics, 13(9), 812. https://doi.org/10.3390/antibiotics13090812






