Antimicrobial Properties and Cytotoxicity of LL-37-Derived Synthetic Peptides to Treat Orthopedic Infections
Abstract
1. Introduction
2. Results
2.1. Synthesis and Purification of AMPs
2.2. Screening of Synthetic AMPs for Antimicrobial Activity against ATCC Strains
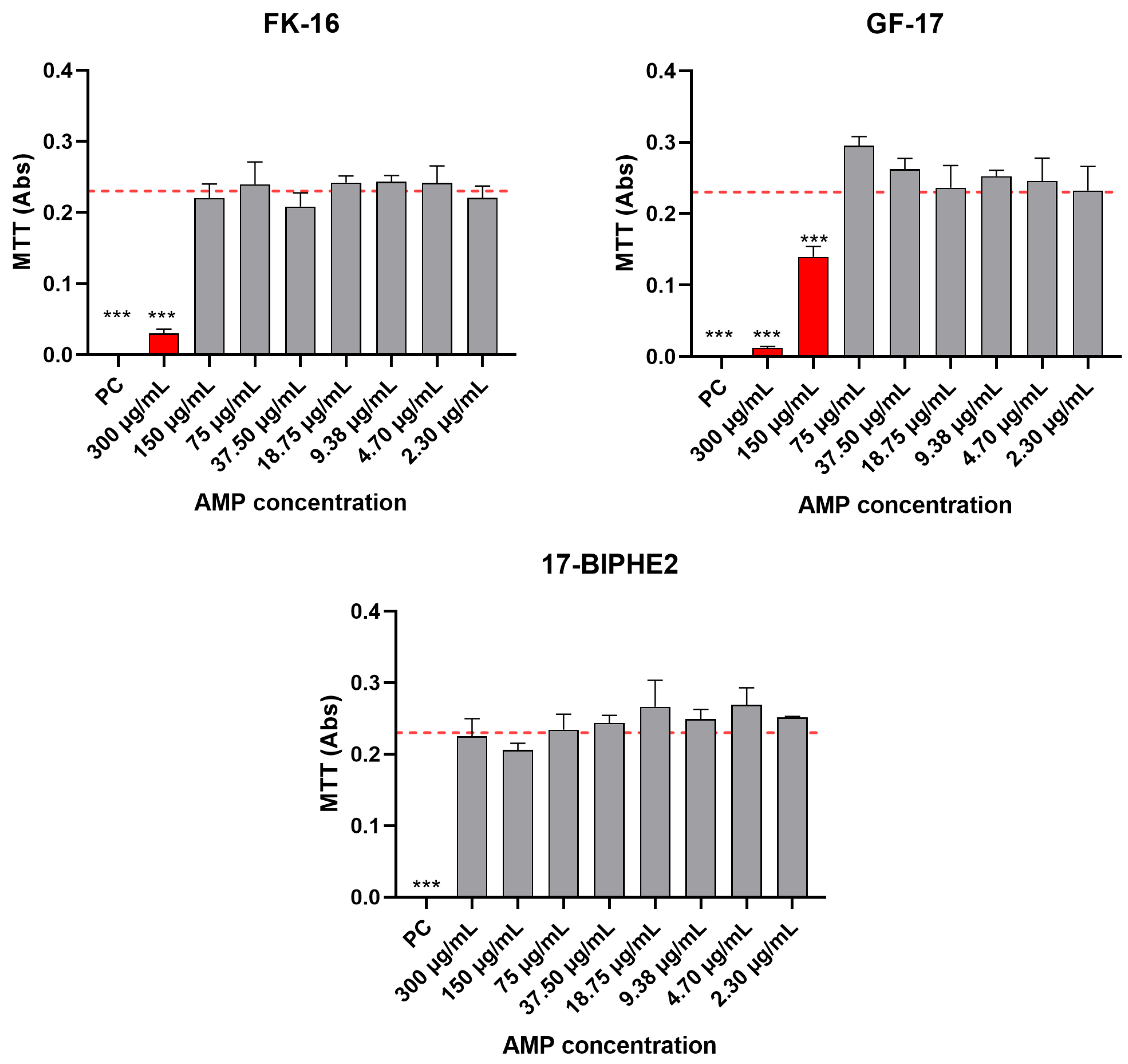
2.3. Biocompatibility Evaluation on Eukaryotic Cells of Selected Synthetic AMPs
2.4. Hemolytic Activity of Selected Synthetic AMPs
2.5. MIC and MBC of Selected AMPs against Clinical Isolates
2.6. Synergy between Selected AMPs and Vancomycin
2.7. Selection for Resistance
2.8. Antibiofilm Activity
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. AMPs Synthesis and Purification
4.3. Bacterial Strains and Culture Conditions
4.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.5. Citotoxicity on Eukaryotic Cells
4.6. Hemolytic Activity Assessment
4.7. Checkerboard Assays to Assess the Presence of Synergy between Selected AMPs and Vancomycin
4.8. Selection for Resistance
4.9. Biofilm Removal Activity
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cha, J.-O.; Yoo, J.I.; Yoo, J.S.; Chung, H.-S.; Park, S.-H.; Kim, H.S.; Lee, Y.S.; Chung, G.T. Investigation of Biofilm Formation and its Association with the Molecular and Clinical Characteristics of Methicillin-resistant Staphylococcus aureus. Osong Public Health Res. Perspect. 2013, 4, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cao, X.; Qian, J.; Liu, Z.; Wang, X.; Su, Q.; Wang, Y.; Xie, R.; Li, X. Evaluation of antimicrobial peptide LL-37 for treatment of Staphylococcus aureus biofilm on titanium plate. Medicine 2021, 100, e27426. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Drago, L.; Monti, L.; De Vecchi, E.; Previdi, S.; Banfi, G.; Romanò, C.L. Diabetic mouse model of orthopaedic implant-related Staphylococcus aureus infection. PLoS ONE 2013, 8, e67628. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Romanò, C.L.; Monti, L.; Vassena, C.; Previdi, S.; Drago, L. Does PGE1 vasodilator prevent orthopaedic implant-related infection in diabetes? Preliminary results in a mouse model. PLoS ONE 2014, 9, e94758. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Wang, G. Titanium surfaces immobilized with the major antimicrobial fragment FK-16 of human cathelicidin LL-37 are potent against multiple antibiotic-resistant bacteria. Biofouling 2017, 33, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.; Thangamani, S.; Seleem, M.N. Antimicrobial peptides and peptidomimetics—Potent therapeutic allies for staphylococcal infections. Curr. Pharm. Des. 2015, 21, 2073–2088. [Google Scholar] [CrossRef] [PubMed]
- Pennone, V.; Rosini, E.; Mascheroni, E.; Gianola, S.; Castellini, G.; Bargeri, S.; Lovati, A.B. Revolutionizing orthopedic healthcare: A systematic review unveiling recombinant antimicrobial peptides. Front. Microbiol. 2024, 15, 1370826. [Google Scholar] [CrossRef] [PubMed]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Tajer, L.; Paillart, J.-C.; Dib, H.; Sabatier, J.-M.; Fajloun, Z.; Abi Khattar, Z. Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review. Microorganisms 2024, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Yang, P.; Lei, J.; Zhao, J. Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity. Antibiotics 2023, 12, 1037. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2012, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Epand, R.F.; Mishra, B.; Lushnikova, T.; Thomas, V.C.; Bayles, K.W.; Epand, R.M. Decoding the functional roles of cationic side chains of the major antimicrobial region of human cathelicidin LL-37. Antimicrob. Agents Chemother. 2012, 56, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Hancock, R.E.W. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 2007, 64, 922. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Epand, R.F.; Epand, R.M.; Wang, G. Structural location determines functional roles of the basic amino acids of KR-12, the smallest antimicrobial peptide from human cathelicidin LL-37. RSC Adv. 2013, 3, 19560–19571. [Google Scholar] [CrossRef]
- Wang, G.; Hanke, M.L.; Mishra, B.; Lushnikova, T.; Heim, C.E.; Thomas, V.C.; Bayles, K.W.; Kielian, T. Transformation of human cathelicidin LL-37 into selective, stable, and potent antimicrobial compounds. ACS Chem. Biol. 2014, 9, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gong, W.; Huang, J.; Yoshimura, T.; Wang, J.M. The potentials of short fragments of human anti-microbial peptide LL-37 as a novel therapeutic modality for diseases. Front. Biosci. 2021, 26, 1362–1372. [Google Scholar] [CrossRef]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Alanine and Lysine Scans of the LL-37-Derived Peptide Fragment KR-12 Reveal Key Residues for Antimicrobial Activity. Chembiochem 2018, 19, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, G.; Kim, E.Y.; Shin, S.Y. LL-37-Derived Membrane-Active FK-13 Analogs Possessing Cell Selectivity, Anti-Biofilm Activity and Synergy with Chloramphenicol and Anti-Inflammatory Activity. Biochim. Biophys. Acta BBA-Biomembr. 2017, 1859, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Golla, R.M.; Lau, K.; Lushnikova, T.; Wang, G. Anti-Staphylococcal Biofilm Effects of Human Cathelicidin Peptides. ACS Med. Chem. Lett. 2015, 7, 117–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Lakshmaiah Narayana, J.; Wu, Q.; Dang, X.; Wang, G. Structure and Activity of a Selective Antibiofilm Peptide SK-24 Derived from the NMR Structure of Human Cathelicidin LL-37. Pharmaceuticals 2021, 14, 1245. [Google Scholar] [CrossRef]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mishra, B.; Epand, R.F.; Epand, R.M. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta 2014, 1838, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. In Antimicrobial Peptides; Matsuzaki, K., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1117, pp. 215–240. [Google Scholar] [CrossRef]
- Jacob, B.; Park, I.; Bang, J.; Shin, S.Y. Short KR-12 Analogs Designed from Human Cathelicidin LL-37 Possessing Both Antimicrobial and Antiendotoxic Activities without Mammalian Cell Toxicity. J. Pept. Sci. 2013, 19, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Shen, J.; Cheng, A.S.L.; Lu, L.; Chan, R.L.Y.; Li, Z.J.; Wang, X.J.; Wong, C.C.M.; Zhang, L.; Ng, S.S.M.; et al. FK-16 Derived from the Anticancer Peptide LL-37 Induces Caspase-Independent Apoptosis and Autophagic Cell Death in Colon Cancer Cells. PLoS ONE 2013, 8, e63641. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, H.; Ganjali Koli, M.; Ranjbar, R.; Pooshang Bagheri, K. Interactions of GF-17 Derived from LL-37 Antimicrobial Peptide with Bacterial Membranes: A Molecular Dynamics Simulation Study. J. Comput. Aided Mol. Des. 2020, 34, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Camesano, T.A. LL37-Derived Fragments Improve the Antibacterial Potential of Penicillin G and Ampicillin against Methicillin-Resistant Staphylococcus aureus. Antibiotics 2023, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Lakshmaiah Narayana, J.; Lushnikova, T.; Wang, X.; Wang, G. Low cationicity is important for systemic in vivo efficacy of database-derived peptides against drug-resistant Gram-positive pathogens. Proc. Natl. Acad. Sci. USA 2019, 116, 13517–13522. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnology 2020, 18, 3. [Google Scholar] [CrossRef]
- Bottagisio, M.; Barbacini, P.; Bidossi, A.; Torretta, E.; Delancey-Pulcini, E.; Gelfi, C.; James, G.A.; Lovati, A.B.; Capitanio, D. Phenotypic Modulation of Biofilm Formation in a Staphylococcus epidermidis Orthopedic Clinical Isolate Grown under Different Mechanical Stimuli: Contribution from a Combined Proteomic Study. Front. Microbiol. 2020, 11, 565914. [Google Scholar] [CrossRef] [PubMed]
- BBottagisio, M.; Soggiu, A.; Lovati, A.B.; Toscano, M.; Piras, C.; Romanò, C.L.; Bonizzi, L.; Roncada, P.; Drago, L. Draft Genome Sequence of Staphylococcus epidermidis Clinical Strain GOI1153754-03-14 Isolated from an Infected Knee Prosthesis. Genome Announc. 2017, 5, e00378-17. [Google Scholar] [CrossRef] [PubMed]
- CLSI Standard M07-Ed12; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical Laboratory Standards Institute: Wayne, PA, USA, 2024.
- ISO 20776-1:2019; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. ISO: Geneva, Switzerland, 2019.
- Ferrari, M.; Fornasiero, M.C.; Isetta, A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods 1990, 131, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.S. (Ed.) Synergism testing: Broth microdilution checkerboard and broth macrodilution methods. In Clinical Microbiology Procedures Handbook, 3rd ed.; American Society of Microbiology Press: Washington, DC, USA, 2010; pp. 140–162. [Google Scholar]
- CLSI Document M2-Ed14; Performance Standards for Antimicrobial Disk Susceptibility Tests. Clinical Laboratory Standards Institute: Wayne, PA, USA, 2024.





| Peptide | Sequence | Molecular Weight |
|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 4493.3 |
| KR-12 | KRIVQRIKDFLR | 1571.9 |
| FK-13 | FKRIVQRIKDFLR | 1719.1 |
| FK-16 | FKRIVQRIKDFLRNLV | 2045.5 |
| GF-17 a | GFKRIVQRIKDFLRNLV | 2102.5 |
| 17BIPHE2 a | GBKR{D-LEU}VQR{D-LEU}KDB{D-LEU}RNLV | 2101.5 |
| SK-24 | SKEKIGKEFKRIVQRIKDFLRNLV | 2944.6 |
| Minimum Inhibitory Concentration (MIC) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP (µg/mL) | |||||||||||
| Bacteria | Strain | cLL-37 | Van | Gen | KR-12 | FK-13 | FK-16 | GF-17 | 17BIPHE2 | SK-24 | Na-TFA |
| S. epidermidis | 14990 | 75.00 | >300.00 | 150.00 | 4.69 | 4.69 | 18.75 | 18.75 | >200.00 | ||
| S. epidermidis | 35984 | 9.38 | >300.00 | 150.00 | 9.38 | 2.34 | 37.50 | 37.50 | >200.00 | ||
| S. aureus | 25923 | 37.50 | 0.6S | 0.6S | >300.00 | 300.00 | 18.75 | 2.34 | 300.00 | 150.00 | >200.00 |
| S. aureus | 43300 | 75.00 | >300.00 | 300.00 | 9.38 | 4.69 | 150.00 | 150.00 | >200.00 | ||
| S. aureus | 49230 | 75.00 | >300.00 | >300.00 | 18.75 | 4.69 | 300.00 | 75.00 | >200.00 | ||
| P. aeruginosa | 27853 | 37.50 | >40R | 0.6S | >300.00 | 300.00 | 150.00 | 18.75 | 75.00 | 150.00 | >200.00 |
| E. coli | 25922 | 37.50 | >40R | 0.6S | >300.00 | 75.00 | 18.75 | 9.38 | 37.50 | 75.00 | >200.00 |
| Minimum Bactericidal Concentration (MBC) | |||||||||||
| AMP (µg/mL) | |||||||||||
| Bacteria | Strain | cLL-37 | Van | Gen | KR-12 | FK-13 | FK-16 | GF-17 | 17BIPHE2 | SK-24 | Na-TFA |
| S. epidermidis | 14990 | 75.00 | >300.00 | 150.00 | 9.38 | 75.00 | 300.00 | 150.00 | >200.00 | ||
| S. epidermidis | 35984 | >300.00 | >300.00 | 150.00 | 18.75 | 4.69 | 75.00 | 300.00 | >200.00 | ||
| S. aureus | 25923 | >300.00 | 0.6 | 2.5 | >300.00 | >300.00 | 18.75 | 37.50 | >300.00 | >300.00 | >200.00 |
| S. aureus | 43300 | >300.00 | >300.00 | >300.00 | 75.00 | 150.00 | 300.00 | >300.00 | >200.00 | ||
| S. aureus | 49230 | >300.00 | >300.00 | >300.00 | 37.50 | 75.00 | >300.00 | >300.00 | >200.00 | ||
| P. aeruginosa | 27853 | 75.00 | >40 | 2.5 | >300.00 | 300.00 | 300.00 | 75.00 | 150.00 | 150.00 | >200.00 |
| E. coli | 25922 | 75.00 | >40 | 2.5 | >300.00 | 150.00 | 75.00 | 300.00 | 37.50 | 75.00 | >200.00 |
| Minimum Inhibitory Concentration (MIC) | ||||||
|---|---|---|---|---|---|---|
| Microorganism Tested | AMP (µg/mL) | |||||
| Bacteria | Strain | Van | Gen | cLL-37 | FK-16 | GF-17 |
| S. epidermidis | GOI1153754-03-14 | 1.25S | >20R | 75.00 | 9.38 | 2.34 |
| S. aureus | Sau39 | 150.00 | 4.69 | 2.34 | ||
| S. aureus | Sau89 | >300.00 | 18.75 | 4.69 | ||
| S. aureus | Sau221 | >300.00 | 18.75 | 9.38 | ||
| P. aeruginosa | Pae2 | 75.00 | 150.00 | 150.00 | ||
| P. aeruginosa | Pae61 | 37.50 | 150.00 | 37.50 | ||
| P. aeruginosa | Pae82 | 37.50 | 150.00 | 75.00 | ||
| E. coli | Eco53 | 37.50 | 18.75 | 18.75 | ||
| E. coli | Eco61 | 37.50 | 9.38 | 9.38 | ||
| E. coli | Eco75 | 37.50 | 18.75 | 18.75 | ||
| Minimum Bactericidal Concentration (MBC) | ||||||
| Microorganism Tested | AMP (µg/mL) | |||||
| Bacteria | Strain | Van | Gen | cLL-37 | FK-16 | GF-17 |
| S. epidermidis | GOI1153754-03-14 | 2.5 | >20 | >300.00 | 18.75 | 37.50 |
| S. aureus | Sau39 | >300.00 | 75.00 | 9.38 | ||
| S. aureus | Sau89 | >300.00 | 75.00 | 4.69 | ||
| S. aureus | Sau221 | >300.00 | 75.00 | 18.75 | ||
| P. aeruginosa | Pae2 | 75.00 | 300.00 | 150.00 | ||
| P. aeruginosa | Pae61 | 150.00 | 300.00 | 150.00 | ||
| P. aeruginosa | Pae82 | 75.00 | 150.00 | 300.00 | ||
| E. coli | Eco53 | 300.00 | 18.75 | 75.00 | ||
| E. coli | Eco61 | 75.00 | 37.50 | 18.75 | ||
| E. coli | Eco75 | 300.00 | 37.50 | 75.00 | ||
| FK-16 + GF-17 | GF-17 + Van | FK-16 + Van | |
|---|---|---|---|
| S. epidermidis GOI1153754-03-14 | 0.65 | 1.02 | 2 |
| S. aureus 43300 | 0.55 | 2 | 2 |
| S. epidermidis 35984 | 0.77 | 2 | 2 |
| S. aureus 29213 | 0.99 | 2 | 2 |
| Bacteria | Strain | Source | Resistance or Predicted Phenotype |
|---|---|---|---|
| S. epidermidis | ATCC 14990 | Nose | Ampicillin, fosfomycin, tetracycline * |
| S. epidermidis | ATCC 35984 | Catheter sepsis | Amikacin, gentamicin, tobramycin, streptomycin, spectinomycin, kanamycin, ampicillin, erythromycin, azithromycin, fosfomycin, penicillin * |
| S. epidermidis | GOI1153754-03-14 (CI) | Orthopedic infection | Benzyl penicillin/oxacillin/gentamicin/cefazolin/rifampin/levofloxacin |
| S. aureus | ATCC 25923 | Wound | Susceptible * |
| S. aureus | ATCC 43300 | Human clinical isolate | Amikacin, gentamicin, tobramycin, spectinomycin, ampicillin, erythromycin, azithromycin, penicillin * |
| S. aureus | ATCC 49230 | Chronic osteomyelitis | No genome available * |
| S. aureus | ATCC 29213 | Wound | Ampicillin * |
| S. aureus | Sau39 (CI) | Wound swab | Benzylpenicillin/gentamicin/levofloxacin |
| S. aureus | Sau89 (CI) | Synovial fluid | Benzylpenicillin/erythromycin/teicoplanin/fusidic acid |
| S. aureus | Sau221 (CI) | Biopsy | Gentamicin |
| P. aeruginosa | ATCC 27853 | Blood culture | Kanamycin, ampicillin, amoxicillin/clavulanic acid, cefoxitin, ceftriaxone, chloramphenicol, Fosfomycin * |
| P. aeruginosa | Pae2 (CI) | Orthopedic infection | N/D |
| P. aeruginosa | Pae61 (CI) | Fixation device | Amoxicillin+clavulanic acid/cefazolin/cefotaxime/ceftriaxone/ciprofloxacin/levofloxacin/tigecyclin/trimetoprim |
| P. aeruginosa | Pae82 (CI) | Osteoarticular tissue | Cefotaxime/tigecyclin/fosfomycin/trimetoprim |
| E. coli | ATCC 25922 | Human clinical isolate | Susceptible * |
| E. coli | Eco53 (CI) | Osteoarticular tissue | ESBL/cefotaxime/ciprofloxacin/levofloxacin |
| E. coli | Eco61 (CI) | Synovial fluid | ESBL/amoxicillin+clavulanic acid/ceftazidine/gentamicin/cefotaxime/ciprofloxacin/levofloxacin/trimetoprim |
| E. coli | Eco75 (CI) | Synovial fluid | Susceptible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennone, V.; Angelini, E.; Sarlah, D.; Lovati, A.B. Antimicrobial Properties and Cytotoxicity of LL-37-Derived Synthetic Peptides to Treat Orthopedic Infections. Antibiotics 2024, 13, 764. https://doi.org/10.3390/antibiotics13080764
Pennone V, Angelini E, Sarlah D, Lovati AB. Antimicrobial Properties and Cytotoxicity of LL-37-Derived Synthetic Peptides to Treat Orthopedic Infections. Antibiotics. 2024; 13(8):764. https://doi.org/10.3390/antibiotics13080764
Chicago/Turabian StylePennone, Vincenzo, Elisa Angelini, David Sarlah, and Arianna B. Lovati. 2024. "Antimicrobial Properties and Cytotoxicity of LL-37-Derived Synthetic Peptides to Treat Orthopedic Infections" Antibiotics 13, no. 8: 764. https://doi.org/10.3390/antibiotics13080764
APA StylePennone, V., Angelini, E., Sarlah, D., & Lovati, A. B. (2024). Antimicrobial Properties and Cytotoxicity of LL-37-Derived Synthetic Peptides to Treat Orthopedic Infections. Antibiotics, 13(8), 764. https://doi.org/10.3390/antibiotics13080764









