Evaluation of Gentamicin Release of PMMA Cements Using Different Methods: HPLC, Elution and Inhibition Zone Testing
Abstract
1. Introduction
2. Results
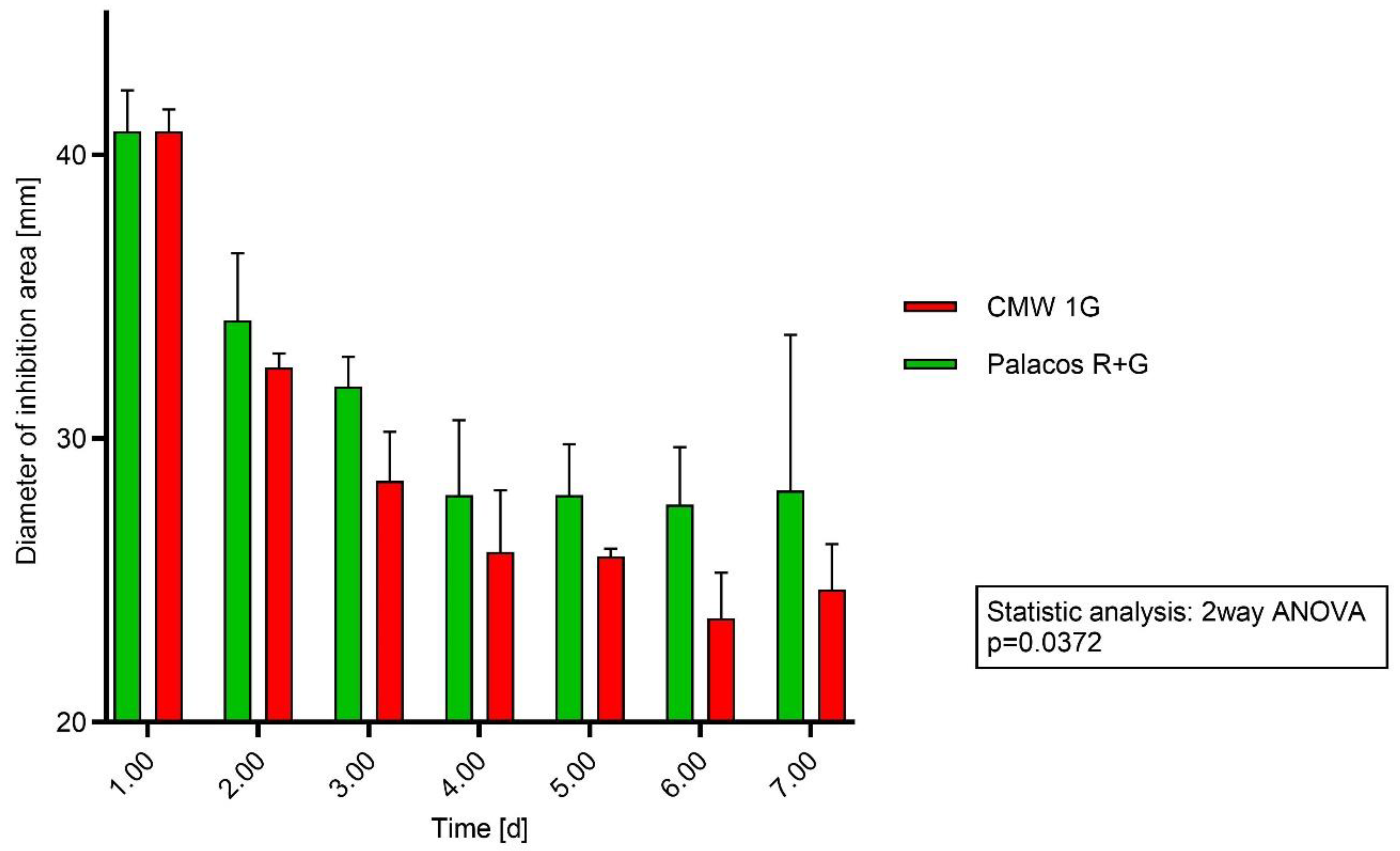
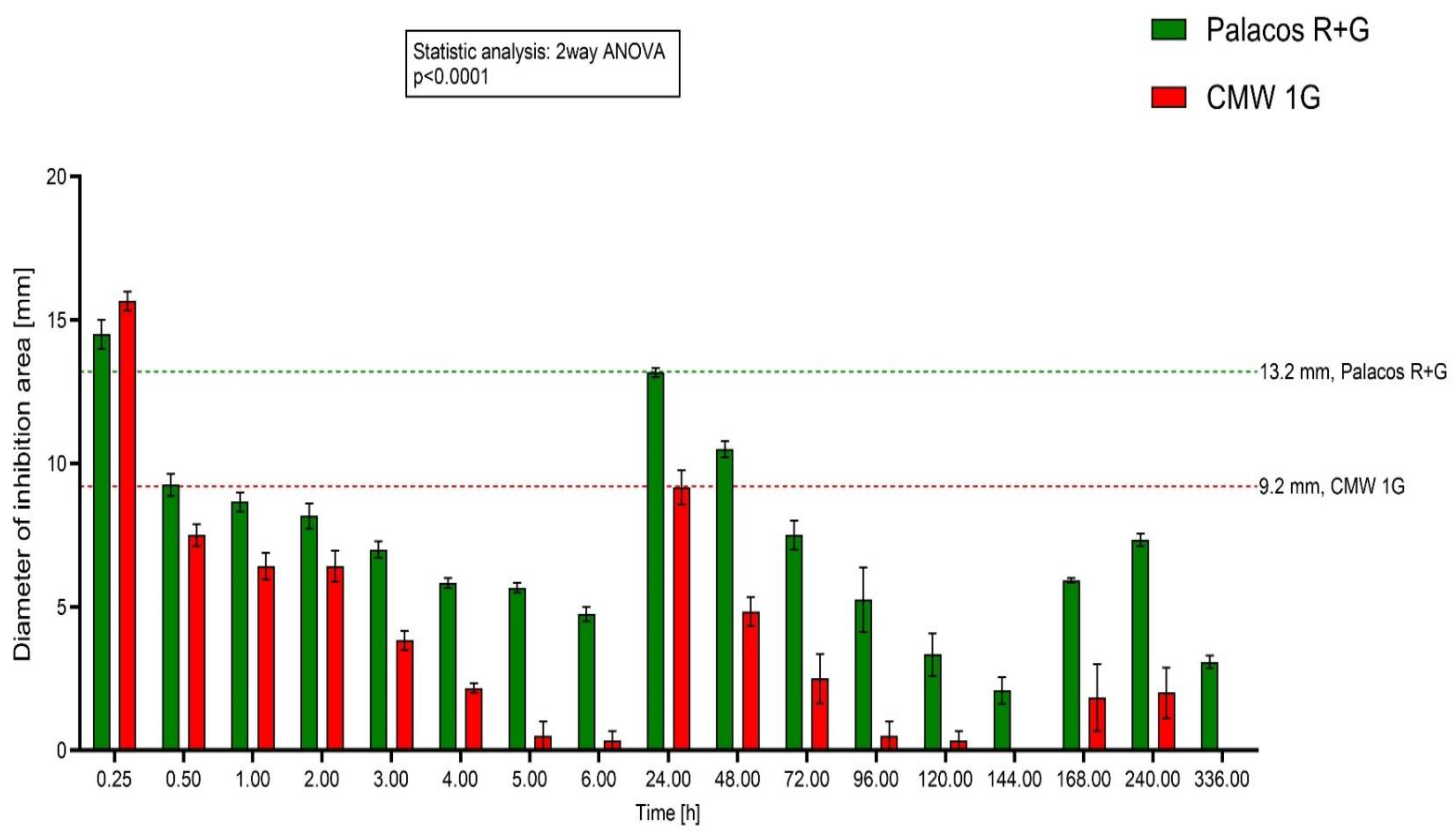
2.1. Testing PMMA Discs on Agar Plates with S. aureus
2.2. Testing PMMA Discs on Agar Plates with S. epidermidis
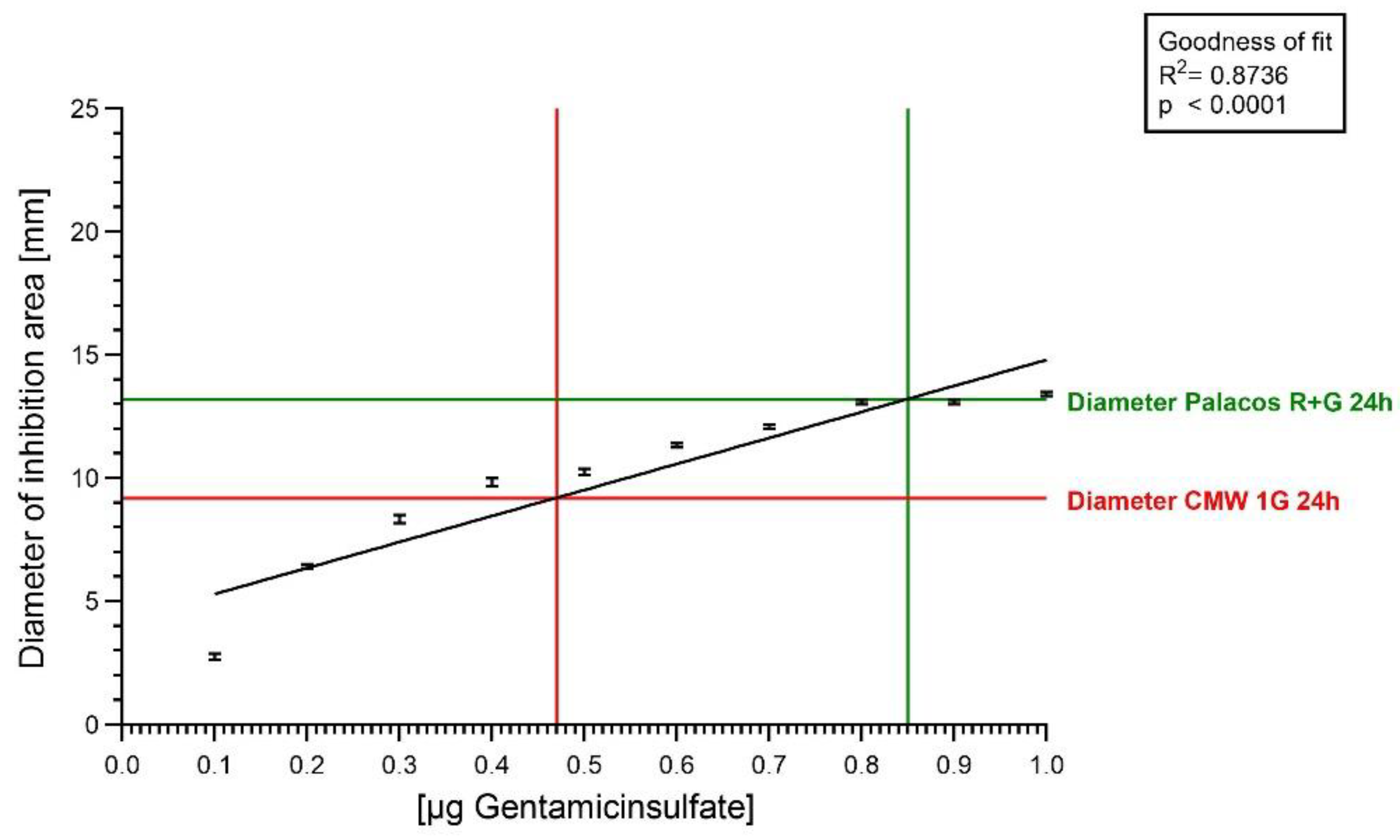
2.3. Agar Diffusion Test with Gentamicin Sulfate Solution
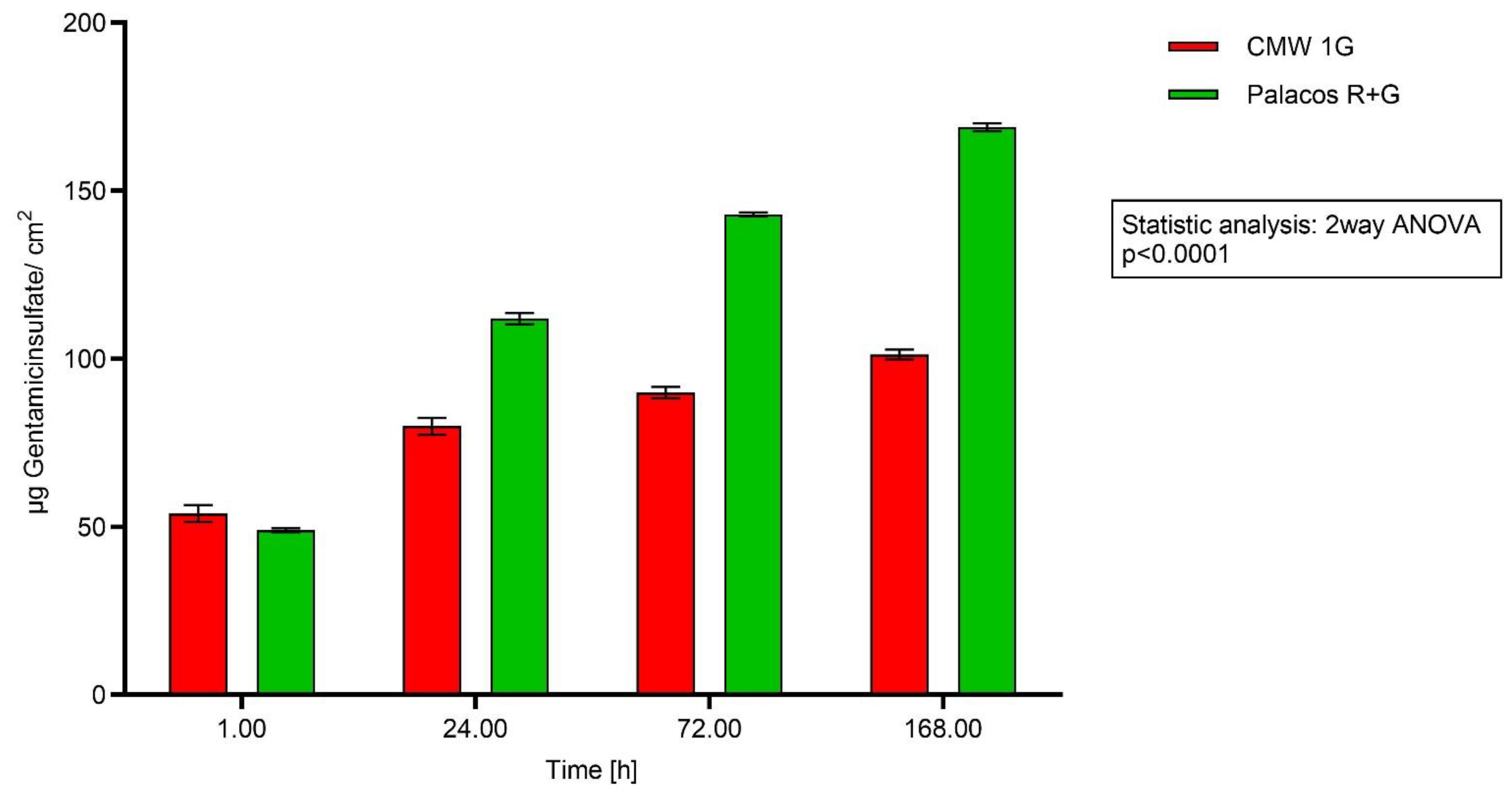
2.4. Analysis of Bone Cement Eluates with HPLC
3. Materials and Methods
3.1. PMMA Bone Cements
3.2. PMMA Cement Discs
3.3. Bacterial Strains
3.4. Preparation of Bacterial Suspension
3.5. Agar Diffusion
3.6. Elution Tests
3.7. Agar Diffusion Test with Gentamicin Sulfate Solution
3.8. Measurement of the Inhibition Areas
3.9. HPLC (Carried Out at Analytic Center Berlin, AZB)
3.10. Data Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, J.; Newman, J.M.; Klika, A.K.; Miller, E.M.; Tan, T.L.; Parvizi, J.; Higuera, C.A. Changes in antibiotic susceptibility of staphylococcus aureus between the stages of 2-stage revision arthroplasty. J. Arthroplast. 2018, 33, 1844–1849. [Google Scholar] [CrossRef]
- Choe, H.; Maruo, A.; Hieda, Y.; Abe, K.; Kobayashi, N.; Ike, H.; Kumagai, K.; Takeyama, M.; Kawabata, Y.; Inaba, Y. Novel local antibiotic antifungal treatment for fungal periprosthetic joint infection with continous local antibiotic perfusion: A surgical technique. Arthroplast. Today 2023, 24, 101245. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.C.; Gbejuade, H.; Lovering, A.; Spencer, R. Characterisation of in vivo release of gentamicin from polymethyl methacrylate cement using a novel method. Int. Orthop. 2013, 37, 2031–2036. [Google Scholar] [CrossRef]
- Miyake, Y.; Takagi, T. Treatment experience with continous local antibiotic perfusion for periprosthetic joint infection. J. Orthop. Sci. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Stuhlert, M.G.W.; Lieb, E.; Müller, M.; von Roth, P.; Preininger, B.; Trampuz, A.; Perka, C.F. Outcome of short versus long interval in two-stage exchange of periprosthetic joint infection: A prospective cohort study. Arch. Orthop. Trauma Surg. 2019, 139, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Carvalho, A.; Soares, D.; Abreu, M. Interval between two-stage exchanges: What is optimal and how do we know? Arthroplasty 2023, 5, 33. [Google Scholar] [CrossRef]
- Frank, B.J.H.; Simon, S.; Aichmair, A.; Dominkus, M.; Hofstaetter, J.G. Clinical impact of microbiology results in two-stage revision arthroplasty with spacer exchange. Arch. Orthop. Trauma Surg. 2023, 143, 4741–4754. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ruiz, P.; Matas-Diez, J.A.; Villanueva-Martínez, M.; Blanco AD, S.V.; Vaquero, J. Is dual antibiotic-loaded bone cement more effective and cost-efficient than a single antibiotic-loaded bone cement to reduce the risk of periprosthetic joint infection in aseptic revision knee arthroplasty? J. Arthroplast. 2020, 35, 3724–3729. [Google Scholar] [CrossRef]
- Blersch, B.P.; Barthels, M.; Schuster, P.; Fink, B. A low rate of periprosthetic infections after aseptic knee prosthesis revision using dual-antibiotic-impregnated bone cement. Antibiotics 2023, 12, 1368. [Google Scholar] [CrossRef]
- Hansen, E.; Kühn, K.D. (Eds.) Essentials of Cemented Knee Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Wall, V.; Nguyen, T.-H.; Nguyen, N.; Tran, P.A. Controlling Antibiotic Release from Polymethylmethacrylate Bone Cement. Biomedicines 2021, 9, 26. [Google Scholar] [CrossRef]
- Köster, U.; Jaeger, R.; Bardts, M.; Wahnes, C.; Büchner, H.; Kühn, K.-D.; Vogt, S. Creep and fatigue behavior of a novel 2-component paste-like formulation of acrylic bone cements. J. Mater. Sci. Mater. Med. 2013, 24, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.D.; Lieb, E.; Berberich, C. PMMA bone cement: What is the role of local antibiotics. Matrise Orthop. 2016, 243, 12–18. [Google Scholar]
- Czuban, M.; Wulsten, D.; Wang, L.; Di Luca, M.; Trampuz, A. Release of different amphotercin B formulations from PMMA bone cements and their activity against Candida biofilm. Colloids Surf. B Biointerfaces 2019, 183, 110406. [Google Scholar] [CrossRef]
- Krampitz, B.; Steiner, J.; Trampuz, A.; Kühn, K.-D. Voriconazole Admixed with PMMA-Impact on Mechanical Properties and Efficacy. Antibiotics 2023, 12, 848. [Google Scholar] [CrossRef]
- Humez, M.; Domann, E.; Thormann, K.M.; Fölsch, C.; Strathausen, R.; Vogt, S.; Alt, V.; Kühn, K.-D. Daptomycin-Impregnated PMMA Cement against Vancomycin-Resistant Germs: Dosage, Handling, El tion, Mechanical Stability, and Effectiveness. Antibiotics 2023, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- von Hertzberg-Boelch, S.P.; Luedemann, M.; Rudert, M.; Steinert, A.F. PMMA Bone Cement: Antibiotic Elution and Mechanical Properties in the Context of Clinical Use. Biomedicines 2022, 10, 1830. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Jt. Surg. Am. 2006, 88, 2487–2500. [Google Scholar] [CrossRef]
- Kuehn, K.-D.; Ege, W.; Gopp, U. Acrylic bone cements: Composition and properties. Orthop. Clin. N. Am. 2005, 36, 17–28. [Google Scholar] [CrossRef]
- Neut, D.; Choe, H.D.; Maruo, A.; Hieda, Y.; Abe, K.; Kobayashi, N.; Ike, H.; Kumagai, K.; Takeyama, M.; Kawabata, Y.; et al. Antibacterial efficacy of a new gentamicin-coating for cementless prostheses compared to gentamicin-loaded bone cement. J. Orthop. Res. 2011, 29, 1654–1661. [Google Scholar] [CrossRef]
- Wahlig, H.; Dingeldein, E. Antibiotics and bone cements. Experimental and clinical long-term observations. Acta Orthop. Scand. 1980, 51, 49–56. [Google Scholar] [CrossRef]
- Young, B.C.; Dudareva, M.; Vicentine, M.P.; Hotchen, A.J.; Ferguson, J.; McNally, M. Microbial Persistence, Replacement and Local Antimicrobial Therapy in Recurrent Bone and Joint Infection. Antibiotics 2023, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Liawrungrueang, W.; Ungphaiboon, S.; Jitsurong, A.; Ingviya, N.; Tangtrakulwanich, B.; Yuenyongviwat, V. In vitro elution characteristics of gentamicin-impregnated Polymethylmethacrylate: Premixed with a second powder vs. liquid Lyophilization. BMC Musculoskelet. Disord. 2021, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.D. Antibiotic—Loaded Bone Cements—Antibiotic Release and Influence on Mechanical Properties. In Local Antibiotics in Arthroplasty; Walenkamp, G., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 2007; pp. 47–57. [Google Scholar]
- van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- Cherednichenko, K.; Sayfutdinova, A.; Rimashevskiy, D.; Malik, B.; Panchenko, A.; Kopitsyna, M.; Ragnaev, S.; Vinokurov, V.; Voronin, D.; Kopitsyn, D. Composite bone cements with enhanced drug elution. Polymers 2023, 15, 3757. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Not all approved antibiotic-loaded PMMA bone cement brands are the same: Ranking using the utility materials selection concept. J. Mater. Sci. Mater. Med. 2015, 26, 48. [Google Scholar] [CrossRef] [PubMed]
- Meeker, D.G.; Cooper, K.B.; Renard, R.L.; Mears, S.C.; Smeltzer, M.S.; Barnes, C.L. Comparative study of antibiotic elution profiles from alternative formulations of polymethylmethacrylate bone cement. J. Arthroplast. 2019, 34, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Weisman, D.L.; Olmstead, M.L.; Kowalski, J.J.; Dvm, D.L.W.; Dvm, M.L.O.; Dvm, J.J.K. In vitro evaluation of antibiotic elution from polymethylmethacrylate (PMMA) and mechanical assessment of antibiotic-PMMA composites. Vet. Surg. 2000, 29, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.N.; Peggins, J.O.; Nochetto, C.B.; Smith, M.L.; Chiesa, O.A.; Moulton, K. LC/MS/MS measurement of gentamicin in bovine plasma, urine, milk, and biopsy samples taken from kidneys of standing animals. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 821, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Carlos, F.-A.J.; Rene, G.-C.; Germán, V.-S.; Susana, A.-T.L. Antimicrobial poly (methyl methacrylate) with silver nanoparticles for dentistry: A systemic review. Appl. Sci. 2020, 10, 4007. [Google Scholar] [CrossRef]
- Chen, I.-C.; Su, C.-Y.; Nien, W.-H.; Huang, T.-T.; Huang, C.-H.; Lu, Y.-C.; Chen, Y.-J.; Huang, G.-C.; Fang, H.-W. Influence of antibiotic-loaded acrylic bone cement composition on drug release behavior and mechnism. Polymers 2021, 13, 2240. [Google Scholar] [CrossRef]
- Meyer, J.; Piller, G.; Spiegel, C.A.; Hetzel, S.; Squire, M. Vacuum-mixing significantly changes antibiotic elution characteristics of commercially available antibiotic-impregnated bone cements. J. Bone Jt. Surg. Am. 2011, 93, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Squire, M.W.; Ludwig, B.J.; Thompson, J.R.; Jagodzinski, J.; Hall, D.; Andes, D. Premixed antibiotic bone cement: An in vitro comparison of antimicrobial efficacy. J. Arthroplast. 2008, 23, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Anguita-Alonso, P.; Rouse, M.S.; E Piper, K.; Jacofsky, D.J.; Osmon, D.R.; Patel, R. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin. Orthop. Relat. Res. 2006, 445, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Bogdanová, K.; Šiller, M.; Švábová, M.; Lošťák, J.; Kolář, M. Microbial and pharmacological characteristics of VancogenX. Acta Chir. Orthop. Traumatol. Cechoslov. 2013, 80, 69–76. [Google Scholar] [CrossRef]
- Lunz, A.; Lehner, B.; Voss, M.N.; Knappe, K.; Jaeger, S.; Innmann, M.M.; Renkawitz, T.; Omlor, G.W. Impact and Modification of the New PJI-TNM Classification for Periprosthetic Joint Infections. J. Clin. Med. 2023, 12, 1262. [Google Scholar] [CrossRef]

| CMW 1G | Palacos R+G | Gentamicin | |
|---|---|---|---|
| Procedure | |||
| SA SE | SA SE | ||
| Agar diffusion with discs | 1–7 d | 1–7 d | |
| Inhibition zone elution with eluates | 0.25 h–14 d | 0.25 h–14 d | |
| HPLC elution | 1 h–7 d | 1 h–7 d | |
| Agar diffusion-inhibition zone | 0.0–1.0 µg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittinger, C.; Stadler, J.; Kühn, K.D. Evaluation of Gentamicin Release of PMMA Cements Using Different Methods: HPLC, Elution and Inhibition Zone Testing. Antibiotics 2024, 13, 754. https://doi.org/10.3390/antibiotics13080754
Kittinger C, Stadler J, Kühn KD. Evaluation of Gentamicin Release of PMMA Cements Using Different Methods: HPLC, Elution and Inhibition Zone Testing. Antibiotics. 2024; 13(8):754. https://doi.org/10.3390/antibiotics13080754
Chicago/Turabian StyleKittinger, Clemens, Johannes Stadler, and Klaus Dieter Kühn. 2024. "Evaluation of Gentamicin Release of PMMA Cements Using Different Methods: HPLC, Elution and Inhibition Zone Testing" Antibiotics 13, no. 8: 754. https://doi.org/10.3390/antibiotics13080754
APA StyleKittinger, C., Stadler, J., & Kühn, K. D. (2024). Evaluation of Gentamicin Release of PMMA Cements Using Different Methods: HPLC, Elution and Inhibition Zone Testing. Antibiotics, 13(8), 754. https://doi.org/10.3390/antibiotics13080754






