Impact of Combined Pollution of Ciprofloxacin and Copper on the Diversity of Archaeal Communities and Antibiotic-Resistance Genes
Abstract
1. Introduction
2. Result and Discussion
2.1. Variations of Archaeal Community Structures
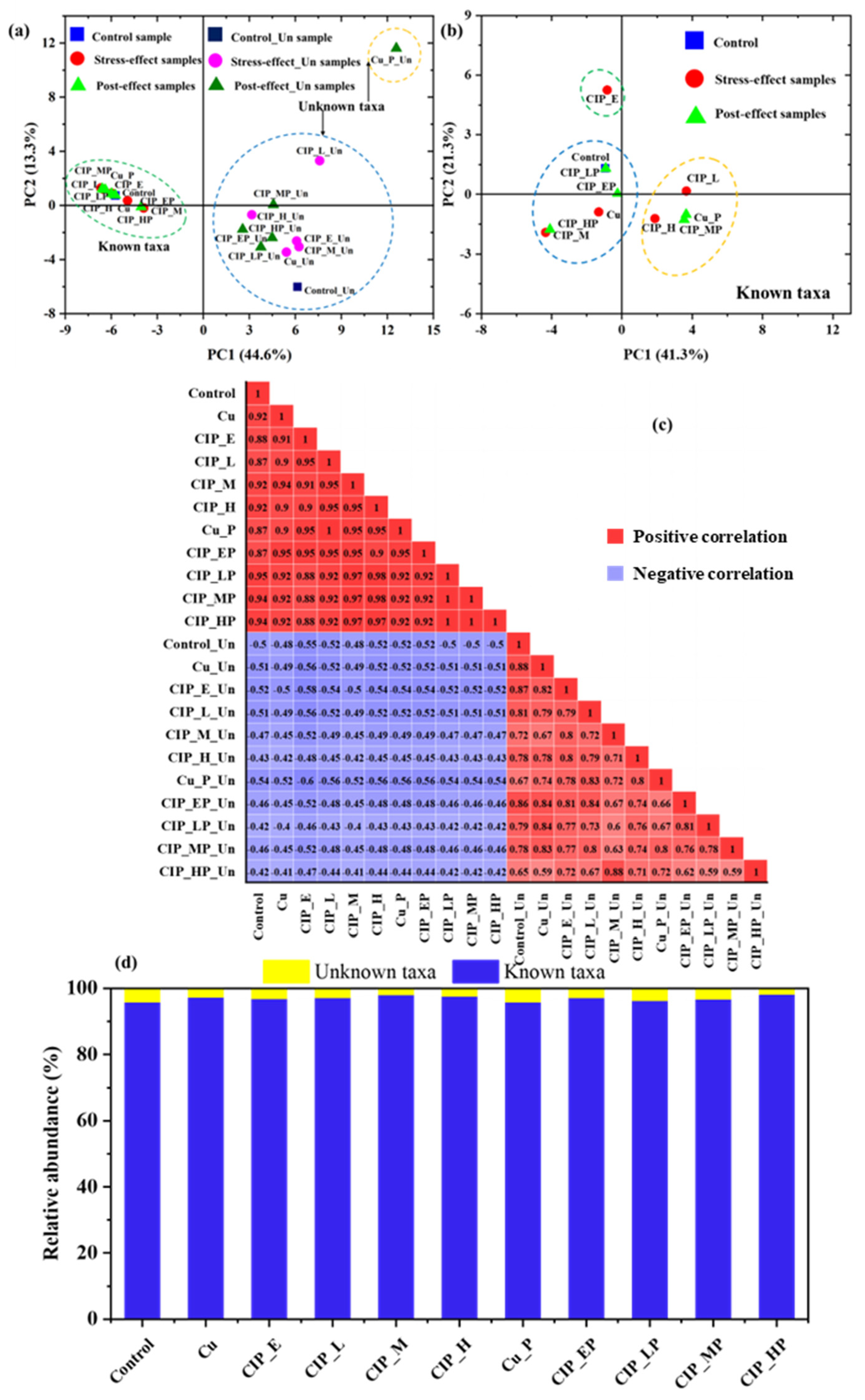
2.2. The Significant Fraction of Unknown Taxa in the Microbial Community
2.3. The Response of Archaea Genera Based on Full-Scale Classification
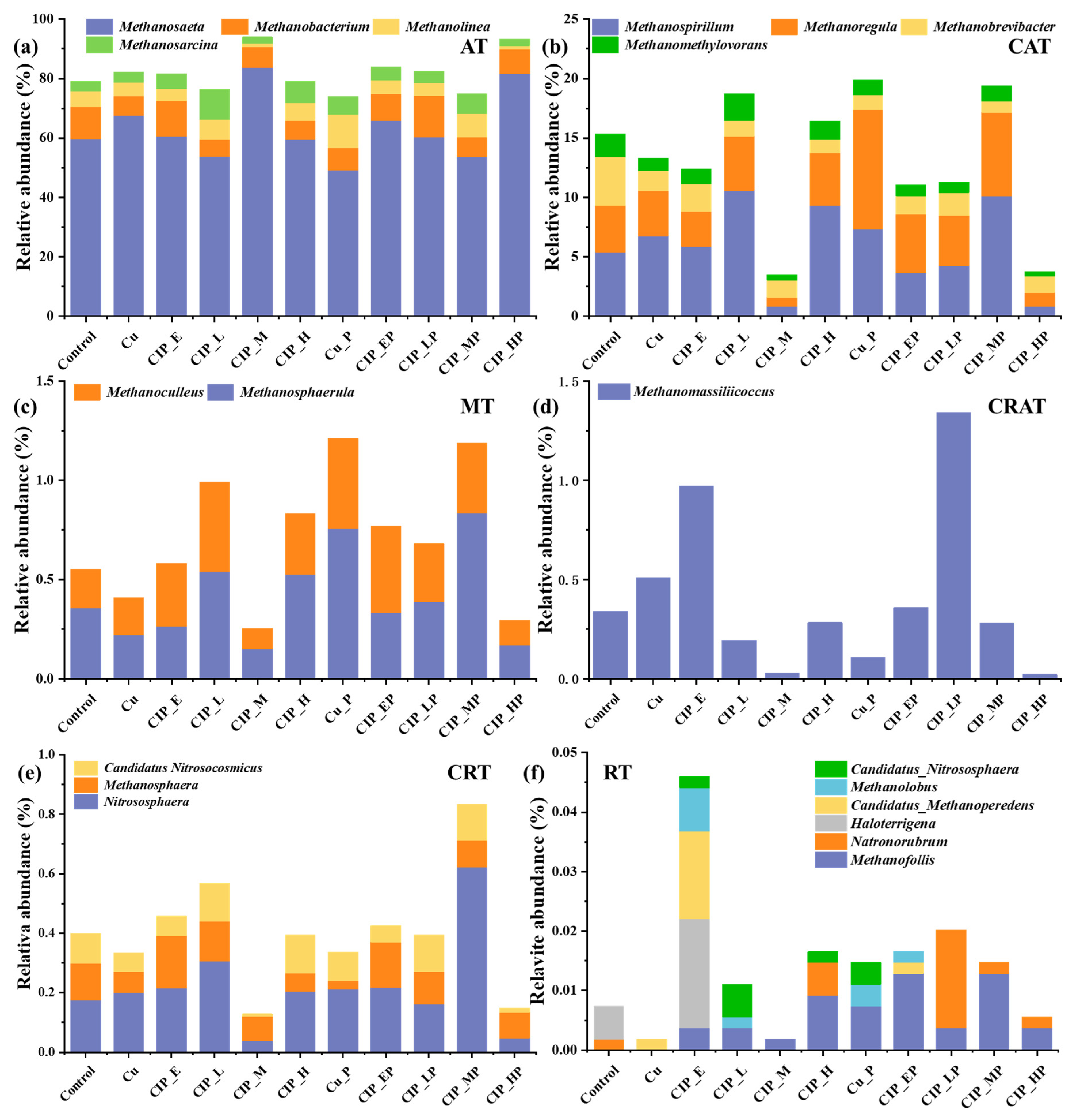
2.3.1. Abundant Taxa
2.3.2. Rare Taxa
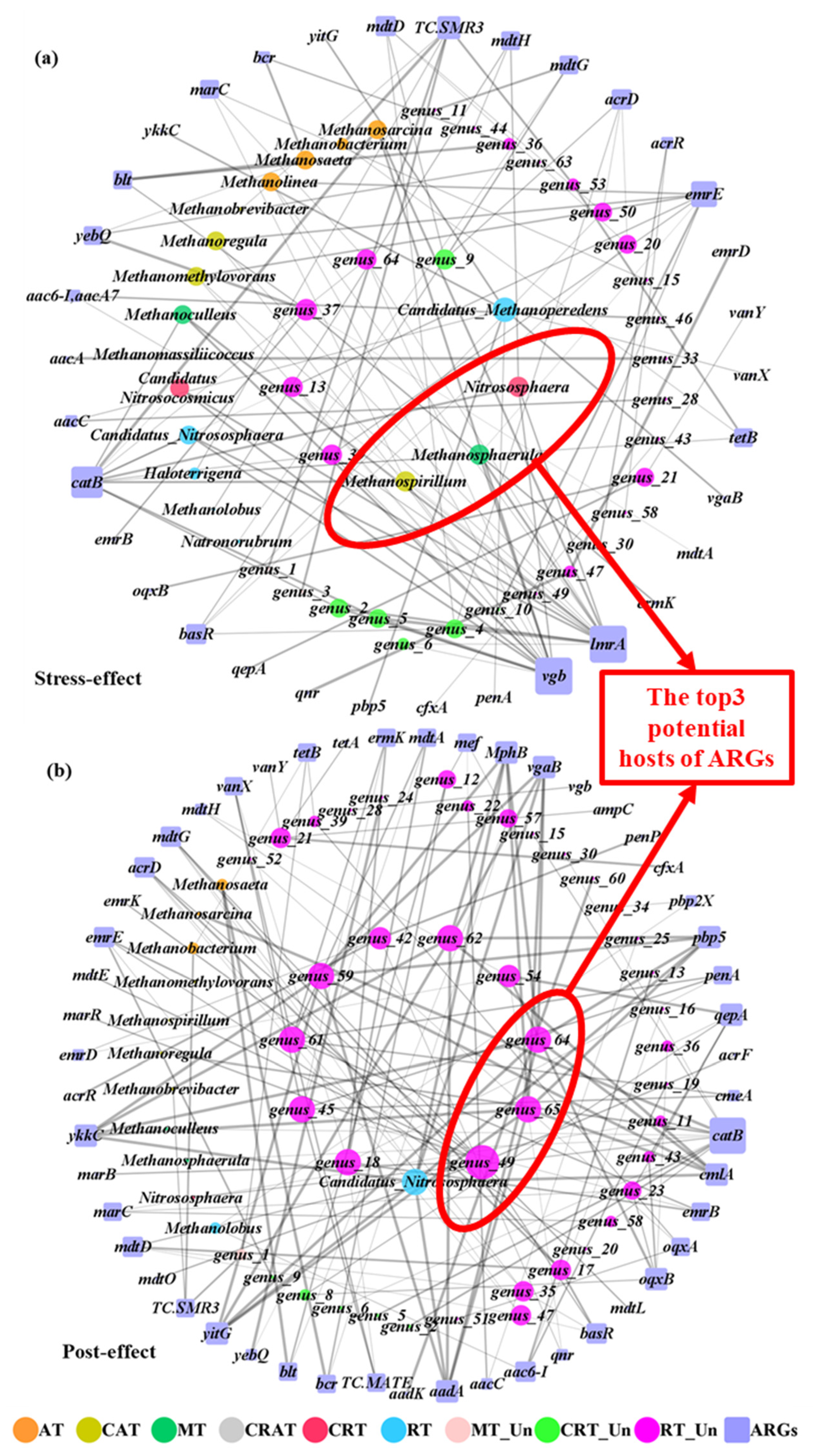
2.4. Network Analysis of Known Archaea, Unknown Archaea, and ARGs
2.4.1. Interactions of Archaeal Genera
2.4.2. Potential Hosts of ARGs
3. Materials and Methods
3.1. Setup of Batch Experiments
3.2. DNA Extraction and Archaeal Sequencing Analysis
3.3. Full-Scale Classification and ARGs Analysis
3.4. Statistical Analysis and Network Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barak, H.; Fuchs, N.; Liddor-Naim, M.; Nir, I.; Sivan, A.; Kushmaro, A. Microbial dark matter sequences verification in amplicon sequencing and environmental metagenomics data. Front. Microbiol. 2023, 14, 1247119. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.G.; Steen, A.D.; Ladau, J.; Yin, J.Q.; Crosby, L. Phylogenetically Novel Uncultured Microbial Cells Dominate Earth Microbiomes. MSystems 2018, 3, e00055-18. [Google Scholar] [CrossRef] [PubMed]
- Zamkovaya, T.; Foster, J.S.; de Crécy-Lagard, V.; Conesa, A. A network approach to elucidate and prioritize microbial dark matter in microbial communities. ISME J. 2021, 15, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Sanyal, D.; Callahan, D.L.; Conlan, X.A.; Pfeffer, F.M. Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environment and human health. Environ. Pollut. 2021, 291, 118233. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Li, X.; Zhao, J.R.; Zhang, Z.X.; Fan, X.Y. Response of microbial communities based on full-scale classification and antibiotic resistance genes to azithromycin and copper combined pollution in activated sludge nitrification laboratory mesocosms at low temperature. Bioresour. Technol. 2021, 341, 125859. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alcala, I.; Soto, J.; Lahora, A. Antibiotics as emerging pollutants. Ecotoxicological risk and control in wastewater and reclaimed water. Ecosistemas 2020, 29, 2070. [Google Scholar] [CrossRef]
- Li, S.; Ma, B.; Zhao, C.; She, Z.; Yu, N.; Pan, Y.H.; Gao, M.H.; Guo, L.; Guo, L.J.; Zhao, Y. Long-term effect of different Cu(II) concentrations on the performance, microbial enzymatic activity and microbial community of sequencing batch reactor. Environ. Pollut. 2019, 255, 113216. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Li, X.; Fan, X.Y.; Zhao, J.R.; Zhang, Z.X. Wastewater treatment plants as reservoirs and sources for antibiotic resistance genes: A review on occurrence, transmission and removal. J. Water Process Eng. 2022, 46, 102539. [Google Scholar] [CrossRef]
- Qin, K.; Wei, L.; Li, J.; Lai, B.; Zhu, F.Y.; Yu, H.; Zhao, Q.L.; Wang, K. A review of ARGs in WWTPs: Sources, stressors and elimination. Chin. Chem. Lett. 2020, 31, 2603–2613. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Zhao, J.; Zhang, Z.X.; Fan, X.Y. Impacts of combined pollution under gradient increasing and gradient decreasing exposure modes on activated sludge: Microbial communities and antibiotic resistance genes. Bioresour. Technol. 2022, 345, 126568. [Google Scholar] [CrossRef]
- Gao, P.; He, S.; Huang, S.; Li, K.Z.; Liu, Z.H.; Xue, G.; Sun, W. Impacts of coexisting antibiotics, antibacterial residues, and heavy metals on the occurrence of erythromycin resistance genes in urban wastewater. Appl. Microbiol. Biotechnol. 2015, 99, 3971–3980. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.; Zhang, H.; Fan, X.Y.; Liu, Y.K. Microbial community and antibiotic resistance genes of biofilm on pipes and their interactions in domestic hot water system. Sci. Total Environ. 2021, 767, 144364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Han, P.; Zhao, M.; Yu, Y.; Sun, D.; Hou, L.; Liu, M.; Zhao, Q.; Tang, X.; Klumper, U. Biotransformation of lincomycin and fluoroquinolone antibiotics by the ammonia oxidizers AOA, AOB and comammox: A comparison of removal, pathways, and mechanisms. Water Res. 2021, 196, 117003. [Google Scholar] [CrossRef] [PubMed]
- Vaksmaa, A.; Guerrero-Cruz, S.; van Alen, T.A.; Cremers, G.; Ettwig, K.F.; Lüke, C.; Jetten, M.S.M. Enrichment of anaerobic nitrate-dependent methanotrophic ‘Candidatus Methanoperedens nitroreducens’ archaea from an Italian paddy field soil. Appl. Microbiol. Biotechnol. 2017, 101, 7075–7084. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, L.; Yang, C.; Song, S.; Xu, Y.F. Cometabolic biodegradation of antibiotics by ammonia oxidizing microorganisms during wastewater treatment processes. J. Environ. Manag. 2022, 305, 114336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.R.; Fan, X.Y.; Li, X.; Gao, Y.X.; Zhang, Z.X. Impact of ciprofloxacin and copper combined pollution on activated sludge: Abundant-rare taxa and antibiotic resistance genes. Bioresour. Technol. 2022, 349, 126882. [Google Scholar] [CrossRef] [PubMed]
- Fuchsman, C.A.; Collins, R.E.; Rocap, G.; Brazelton, W.J. Effect of the environment on horizontal gene transfer between bacteria and archaea. PeerJ 2017, 5, 3865. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Zhang, Y.; Tang, Y.; Bai, Y.; Tao, Y.L.; Huang, B.; Wen, D.H. Identifying the key taxonomic categories that characterize microbial community diversity using full-scale classification: A case study of microbial communities in the sediments of Hangzhou Bay. FEMS Microbiol. Ecol. 2016, 92, 150. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Z.; Yang, S.; Zhu, Q.Y.; Zhu, X.Z. Effects of different oxygen conditions on pollutants removal and the abundances of tetracycline resistance genes in activated sludge systems. Chemosphere 2022, 291, 132681. [Google Scholar] [CrossRef]
- Mai, D.T.; Stuckey, D.C.; Oh, S. Effect of ciprofloxacin on methane production and anaerobic microbial community. Bioresour. Technol. 2018, 261, 240–248. [Google Scholar] [CrossRef]
- Rusanowska, P.; Harnisz, M.; Zielinski, M.; Debowski, M.; Korzeniewska, E.; Kisielewska, M.; Amenda, E. Individual and Synergistic Effects of Metronidazole, Amoxicillin, and Ciprofloxacin on Methane Fermentation with Sewage Sludge. Clean-Soil Air Water 2020, 48, 1900281. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C.; Zhang, J.X. Jointly reducing antibiotic resistance genes and improving methane yield in anaerobic digestion of chicken manure by feedstock microwave pretreatment and activated carbon supplementation. Sci. Total Environ. 2019, 372, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Du, B.B.; Yang, Q.X.; Li, X.N.; Yuan, W.; Chen, Y.L.; Wang, R.F. Impacts of long-term exposure to tetracycline and sulfamethoxazole on the sludge granules in an anoxic-aerobic wastewater treatment system. Chem. Eng. J. 2019, 684, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Y.; Song, S.Q.; Liu, H.L.; Dai, X.H.; Wang, P. Responses of methane production, microbial community and antibiotic resistance genes to the mixing ratio of gentamicin mycelial residues and wheat straw in anaerobic co-digestion process. Sci. Total Environ. 2022, 806, 150488. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.J.; Guo, B.; Li, L.; Liu, Y. Role of syntrophic acetate oxidation and hydrogenotrophic methanogenesis in co-digestion of blackwater with food waste. J. Clean. Prod. 2021, 283, 125393. [Google Scholar] [CrossRef]
- Chen, S.C.; Weng, C.Y.; Lai, M.C.; Tamaki, H.; Narihiro, T. Comparative genomic analyses reveal trehalose synthase genes as the signature in genus Methanoculleus. Mar. Genomics 2019, 47, 100673. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, J.Y.; Liu, J.B.; Yu, D.; Zhong, H.; Wang, Y.W.; Chen, M.X.; Tong, J.; Wei, Y.S. Effects of chlortetracycline, Cu and their combination on the performance and microbial community dynamics in swine manure anaerobic digestion. J. Environ. Sci. 2018, 67, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Fang, P.; Zhang, J.Y.; Wei, Y.S.; Su, Y.Y.; Zhang, Y.F. Microbial community evolution and fate of antibiotic resistance genes during sludge treatment in two full-scale anaerobic digestion plants with thermal hydrolysis pretreatment. Bioresour. Technol. 2019, 288, 121575. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Bai, Y.N.; Lu, Y.Z.; Ding, J.; Zhou, D.D.; Zeng, R.J. Degradation of organic pollutants by anaerobic methane-oxidizing microorganisms using methyl orange as example. J. Hazard. Mater. 2019, 364, 264–271. [Google Scholar] [CrossRef]
- Pitcher, A.; Rychlik, N.; Hopmans, E.C.; Spieck, E.; Rijpstra, W.I.C.; Ossebaar, J.; Schouten, S.; Wagner, M.; Damsté, J.S.S. Crenarchaeol dominates the membrane lipids of Candidatus Nitrososphaera gargensis, a thermophilic Group I. 1b Archaeon. ISME J. 2010, 4, 542–552. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Song, S.L.; Jia, Y.Y.; Wu, D.; Lu, H. Stress-responses of activated sludge and anaerobic sulfate-reducing bacteria sludge under long-term ciprofloxacin exposure. Water Res. 2019, 164, 114964. [Google Scholar] [CrossRef] [PubMed]
- Slipko, K.; Marano, R.; Cytryn, E.; Merkus, V.; Wögerbauer, M.; Krampe, J.; Jurkevitch, E.; Kreuzinger, N. Effects of subinhibitory quinolone concentrations on functionality, microbial community composition, and abundance of antibiotic resistant bacteria and qnrS in activated sludge. J. Environ. Chem. Eng. 2021, 9, 104783. [Google Scholar] [CrossRef]
- Zhao, R.X.; Feng, J.; Liu, J.; Fu, W.J.; Li, X.Y.; Li, B. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res. 2019, 151, 388–402. [Google Scholar] [CrossRef]
- Gao, Y.X.; Li, X.; Fan, X.Y.; Zhao, J.R.; Zhang, Z.X. The dissimilarity of antibiotic and quorum sensing inhibitor on activated sludge nitrification system: Microbial communities and antibiotic resistance genes. Bioresour. Technol. 2022, 351, 127016. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, R.C.; Feyzioglu, A.M.; Altinok, I. Prokaryotic community and diversity in coastal surface waters along the Western Antarctic Peninsula. Polar Sci. 2022, 31, 100764. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2, High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.; Yang, Y.; Fan, X.Y. Antibiotic resistance genes in pipe wall biofilm under eight disinfection strategies in domestic hot water system: Occurrence, removal and interactions. J. Water Process Eng. 2021, 43, 102244. [Google Scholar] [CrossRef]
- Fan, X.Y.; Gao, J.F.; Pan, K.L.; Li, D.C.; Dai, H.H.; Li, X. Functional genera, potential pathogens and predicted antibiotic resistance genes in 16 full-scale wastewater treatment plants treating different types of wastewater. Bioresour. Technol. 2018, 268, 97–106. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Li, W.; Teng, H.; Hu, W.; Dong, Z.; Zhang, D.; Liu, T.; Zheng, Q. Impact of Combined Pollution of Ciprofloxacin and Copper on the Diversity of Archaeal Communities and Antibiotic-Resistance Genes. Antibiotics 2024, 13, 734. https://doi.org/10.3390/antibiotics13080734
Chen M, Li W, Teng H, Hu W, Dong Z, Zhang D, Liu T, Zheng Q. Impact of Combined Pollution of Ciprofloxacin and Copper on the Diversity of Archaeal Communities and Antibiotic-Resistance Genes. Antibiotics. 2024; 13(8):734. https://doi.org/10.3390/antibiotics13080734
Chicago/Turabian StyleChen, Meijuan, Weiying Li, Haibo Teng, Wenxin Hu, Zhiqiang Dong, Dawei Zhang, Tianyi Liu, and Quan Zheng. 2024. "Impact of Combined Pollution of Ciprofloxacin and Copper on the Diversity of Archaeal Communities and Antibiotic-Resistance Genes" Antibiotics 13, no. 8: 734. https://doi.org/10.3390/antibiotics13080734
APA StyleChen, M., Li, W., Teng, H., Hu, W., Dong, Z., Zhang, D., Liu, T., & Zheng, Q. (2024). Impact of Combined Pollution of Ciprofloxacin and Copper on the Diversity of Archaeal Communities and Antibiotic-Resistance Genes. Antibiotics, 13(8), 734. https://doi.org/10.3390/antibiotics13080734






