Prediction of Concomitant Nosocomial Infection in Patients Previously Colonized Colorectally by Multidrug-Resistant Bacteria in an SDD Setting
Abstract
1. Introduction
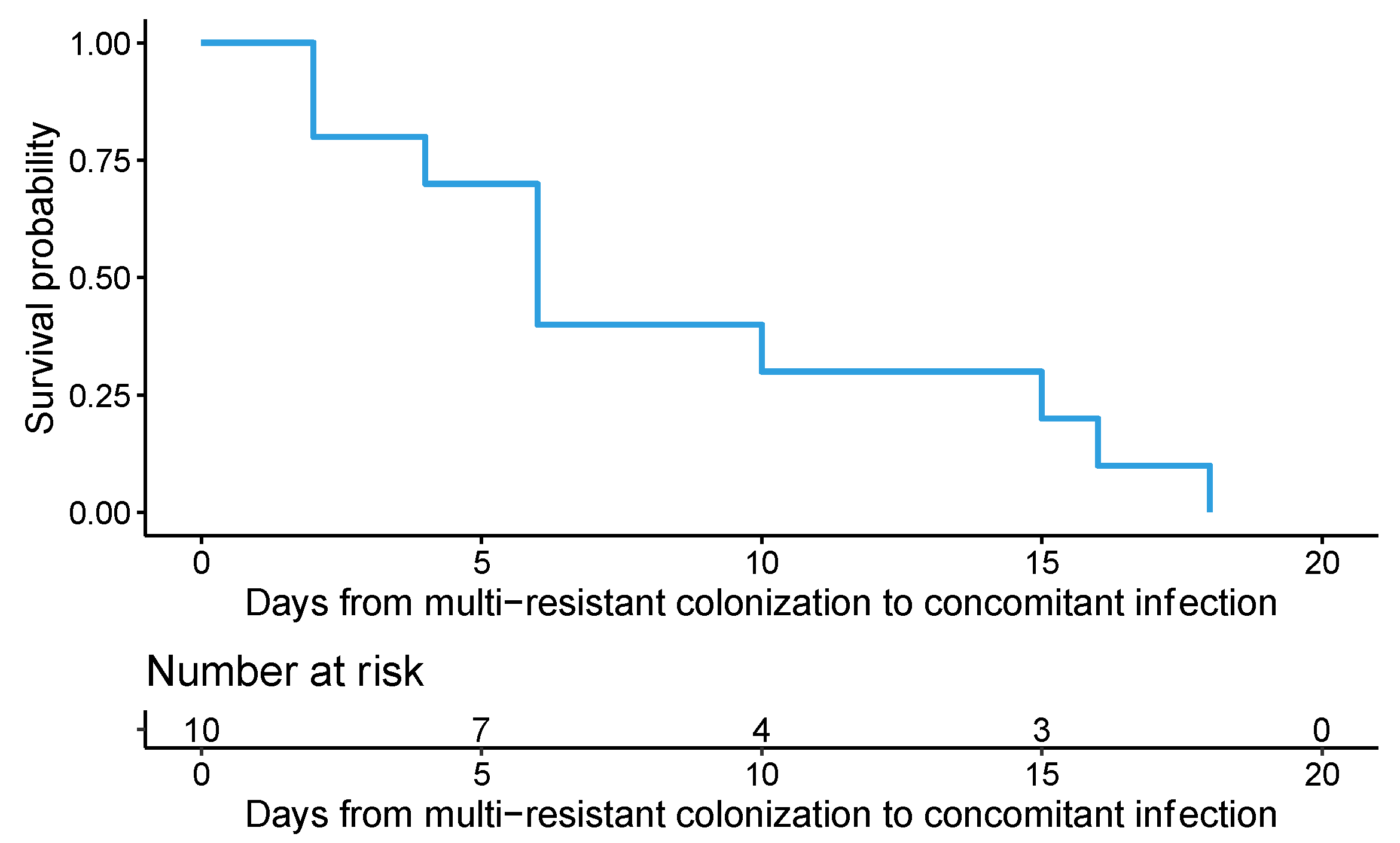
2. Results
3. Discussion
4. Material and Methods
4.1. Design and Population
4.2. Study Procedures and Definitions
- Enterobacteriaceae spp. resistant with extended-spectrum β-lactamase (ESBL) refers to all cases in which the patient showed colonization or infection by Enterobacterales producing extended-spectrum beta-lactamases. In general, Enterobacterales that are resistant to third-generation cephalosporins are considered to be beta-lactamase producers and would fall under this definition.
- MDR-Pseudomonas spp. refers to all cases in which the patient showed colonization or infection by Pseudomonas spp. resistant to three or more families of antibiotics (carbapenems, cephalosporins, piperacillin-tazobactam, quinolones, or aminoglycosides) [15].
- Carbapenem-resistant Enterobacteriaceae (CRE) refers to all cases in which the patient showed colonization or infection by Gram-negative bacilli producing carbapenemases (which confer resistance to carbapenems) before or during their stay in the ICU. Excluded from this group are OXA-48-producing Enterobacteriaceae and metallo-β-lactamase, which were separately analyzed.
- Oxacillinase-48 (OXA-48)-producing Enterobacteriaceae.
- Metallo-β-lactamase-producing Enterobacteriaceae.
- Gram-negative multidrug-resistant bacteria (MDR-GNB) refer to all cases in which the patient showed colonization or infection by Gram-negative bacilli resistant to three or more families of antibiotics. This includes other Gram-negative bacilli meeting this condition and not included in previous categories.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Any strain of Acinetobacter spp. resistant to carbapenems.
- Clostridioides difficile refers to all cases where the patient had C. difficile infection, determined using standard microbiological methods and requiring isolation and treatment.
- Vancomycin-resistant Enterococcus spp. (VRE).
- Nosocomial pneumonia, whether associated or not with mechanical ventilation;
- Urinary tract infection;
- Tracheobronchitis, whether associated or not with mechanical ventilation;
- Primary or secondary bacteremia.
4.2.1. SDD Protocol
4.2.2. Other Definitions to Consider
4.3. Variables Collected
4.4. Microbiological Methods
4.5. Statistical Analysis
4.5.1. Univariate Statistical Analysis
4.5.2. Time to the Concomitant Infection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, M.P. The legacy of penicillin’s first patient. Pharmacol. Res. 2023, 192, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; De Waele, J.J.; Eggimann, P.; Garnacho-Montero, J.; Kahlmeter, G.; Menichetti, F.; Nicolau, D.P.; Paiva, J.A.; Tumbarello, M.; Welte, T.; et al. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensiv. Care Med. 2015, 41, 776–795. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Shorr, A.F.; Bassetti, M.; Timsit, J.-F.; Micek, S.T.; Michelson, A.P.; Garnacho-Montero, J. Timing of antibiotic therapy in the ICU. Crit. Care 2021, 25, 360. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chibale, K. Strategies to Combat Multi-Drug Resistance in Tuberculosis. Acc. Chem. Res. 2021, 54, 2361–2376. [Google Scholar] [CrossRef]
- De Bus, L.; Arvaniti, K.; Sjövall, F. Empirical antimicrobials in the intensive care unit. Intensive Care Med. 2024, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Lipman, J.; Bonten, M.J.M. In 2035, will all bacteria be multidrug-resistant? No. Intensive Care Med. 2016, 42, 2017–2020. [Google Scholar] [CrossRef]
- Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias (SEMICYUC). Grupo de Trabajo de Enfermedades Infecciosas y Sepsis de SEMICYUC. Estudio Nacional de Vigilancia de la Infección Nosocomial (ENVIN-HELICS): Manual de Definiciones y Términos. 2023. Available online: https://hws.vhebron.net/envin-helics/Help/Manual_2023.pdf (accessed on 17 January 2024).
- Sánchez-Ramírez, C.; Hípola-Escalada, S.; Cabrera-Santana, M.; Hernández-Viera, M.A.; Caipe-Balcázar, L.; Saavedra, P.; Artiles-Campelo, F.; Sangil-Monroy, N.; Lübbe-Vázquez, C.F.; Ruiz-Santana, S. Long-term use of selective digestive decontamination in an ICU highly endemic for bacterial resistance. Crit. Care 2018, 22, 141. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, C. Impacto de la Aplicación de Descontaminación Digestiva Selectiva en una Unidad de Medicina Intensiva de un Hospital Terciario y Universitario Durante un año. Doctoral Dissertation, Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain, 2015. Available online: http://hdl.handle.net/10553/16952 (accessed on 17 January 2024).
- Ruiz-Santana, S.; Mora-Quintero, M.-L.; Saavedra, P.; Montiel-González, R.; Sánchez-Ramírez, C.; Pérez-Acosta, G.; Martín-Velasco, M.; Rodríguez-Mata, C.; Lorenzo-García, J.-M.; Parrilla-Toribio, D.; et al. COVID-19 Secondary Infections in ICU Patients and Prevention Control Measures: A Preliminary Prospective Multicenter Study. Antibiotics 2022, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.; Horhat, F.-G.; Bădițoiu, L.; Dumitrașcu, V.; Bagiu, I.-C.; Horhat, D.-I.; Coșniță, D.A.; Krasta, A.; Dugăeşescu, D.; Licker, M. Multidrug-Resistant Gram-Negative Bacilli: A Retrospective Study of Trends in a Tertiary Healthcare Unit. Medicina 2018, 54, 92. [Google Scholar] [CrossRef]
- Prado, V.; Hernández-Tejero, M.; Mücke, M.M.; Marco, F.; Gu, W.; Amoros, A.; Toapanta, D.; Reverter, E.; de la Peña-Ramirez, C.; Altenpeter, L.; et al. Rectal colonization by resistant bacteria increases the risk of infection by the colonizing strain in critically ill patients with cirrhosis. J. Hepatol. 2022, 76, 1079–1089. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Alessandri, F.; Moretti, S.; Borsetti, A.; Maggiorella, M.T.; Fabris, S.; Russo, A.; Ruberto, F.; De Meo, D.; Ciccozzi, M.; et al. Clinical impact of colonization with carbapenem-resistant Gram-Negative bacteria in critically ill patients admitted for severe trauma. Pathogens 2022, 11, 1295. [Google Scholar] [CrossRef]
- Cohen, R.; Babushkin, F.; Cohen, S.; Afraimov, M.; Shapiro, M.; Uda, M.; Khabra, E.; Adler, A.; Ben Ami, R.; Paikin, S. A prospective survey of MDR-Pseudomonas spp. aeruginosa colonization and infection in the intensive care unit. Antimicrob. Resist. Infect. Control 2017, 6, 7. [Google Scholar] [CrossRef]
- Gómez-Zorrilla, S.; Camoez, M.; Tubau, F.; Cañizares, R.; Periche, E.; Dominguez, M.A.; Ariza, J.; Peña, C. Prospective observational study of prior rectal colonization status as a predictor for subsequent development of MDR-Pseudomonas spp. aeruginosa clinical infections. Antimicrob. Agents Chemother. 2015, 59, 5213–5219. [Google Scholar] [CrossRef]
- Lázaro-Perona, F.; Rodríguez-Tejedor, M.; Ruiz-Carrascoso, G.; Díaz-Pollán, B.; Loeches, B.; Ramos-Ramos, J.C.; Mingorance, J. Intestinal loads of OXA-48-producing Klebsiella pneumoniae in colonized patients determined from surveillance rectal swabs. Clin. Microbiol. Infect. 2021, 27, 1169.e7–1169.e12. [Google Scholar] [CrossRef]
- McConville, T.H.; Sullivan, S.B.; Gomez-Simmonds, A.; Whittier, S.; Uhlemann, A.-C. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS ONE 2017, 12, e0186195. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Galfo, V.; Giordano, C.; Leonildi, A.; Marciano, E.; De Simone, P.; Biancofiore, G.; Boggi, U.; Barnini, S.; et al. Bloodstream infections in patients with rectal colonization by Klebsiella pneumoniae producing different type of carbapenemases: A prospective, cohort study (CHIMERA study). Clin. Microbiol. Infect. 2022, 28, 298.e1–298.e7. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Sarwar, S.; Fowler, R.A.; Cuthbertson, B.H. Effect of selective decontamination on antimicrobial resistance in intensive care units: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, S.; Pifferi, S.; Brazzi, L.; Pecoraro, V.; Montrucchio, G.; D’Amico, R. Topical antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving mechanical ventilation. Cochrane Database Syst. Rev. 2021, 2021, CD000022. [Google Scholar] [CrossRef]
- Del Rosario-Quintana, C.; Tosco-Núñez, T.; Lorenzo, L.; Martín-Sánchez, A.M.; Molina-Cabrillana, J. Prevalencia y factores asociados a la colonización de microorganismos multirresistentes en centros de larga estancia de Gran Canaria. Rev. Esp. Geriatr. Gerontol. 2015, 50, 232–236. [Google Scholar] [CrossRef]
- Fernández-Verdugo, A.; Fernández-Fernández, J.; Dolores-Escudero, A.; Cofiño-Castañeda, L.A.; Forcelledo, L.; Telenti, M.; Garcia-Prieto, E.; Rodriguez-Garcia, R.; Alvarez-Garcia, L.; Perez-Garcia, A.; et al. Epidemiological surveillance for multidrug-resistant microorganisms in a general ICU. Rev. Esp. Quimioter. 2017, 30, 201–206. Available online: https://www.seq.es/seq/0214-3429/30/3/fernandez05apr2017.pdf (accessed on 12 April 2024).
- Tejerina-Álvarez, E.E.; de la Cal-López, M.Á. Selective decontamination of the digestive tract: Concept and application. Med. Intensiv. 2023, 47, 603–615. [Google Scholar] [CrossRef]
- Papafotiou, C.; Roussos, S.; Sypsa, V.; Bampali, S.; Spyridopoulou, K.; Karapanou, A.; Moussouli, A.; Samarkos, M.; Daikos, G.L.; Psichogiou, M. Predictive score for patients with carbapenemase-producing enterobacterales colonization upon admission in a tertiary care hospital in an endemic area. J. Antimicrob. Chemother. 2022, 77, 3331–3339. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Seppelt, I.M.; Goodman, F.; Billot, L.; Correa, M.; Davis, J.S.; Gordon, A.C.; Hammond, N.E.; Iredell, J.; Li, Q.; et al. Effect of Selective Decontamination of the Digestive Tract on Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation: A Randomized Clinical Trial. JAMA 2022, 328, 1911–1921. [Google Scholar] [PubMed]
- Tian, Y.; Yao, Y.; Zhou, J.; Diao, X.; Chen, H.; Cai, K.; Ma, X.; Wang, S. Dynamic APACHE II Score to Predict the Outcome of Intensive Care Unit Patients. Front. Med. 2022, 8, 744907. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Oteo, J.; Bou, G.; Chaves, F.; Oliver, A. Métodos microbiológicos para la vigilancia del estado de portador de bacterias multirresistentes. Enfermedades Infecc. Microbiol. Clin. 2017, 35, 667–675. [Google Scholar] [CrossRef]
- Bou Arevalo, G.; Chaves Sánchez, F.; Oliver Palomo, A.; Oteo Iglesias, J. Métodos Microbiológicos para la Vigilancia del estado de Portador de Bacterias Multirresistentes. 55. Oteo Iglesias, J. (Coordinador). Procedimientos en Microbiología Clínica. Cercenado Mansilla, E., Cantón Moreno, R. (Editores). Recomendaciones de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica. 2015. Available online: https://seimc.org/contenidos/documentoscientificos/procedimientosmicrobiologia/seimc-procedimientomicrobiologia55.pdf (accessed on 1 July 2024).
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023; ISBN 978-1-68440-170-3 [Print]; ISBN 978-1-68440-171-0 [Electronic]. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.1. 2023. Available online: http://www.eucast.org (accessed on 1 July 2024).
- Giske, C.G.; Martinez-Martinez, L.; Cantón, R.; Stefani, S.; Skov, R.; Glupczynski, Y.; Nordmann, P.; Wootton, M.; Miriagou, V.; Simonsen, G.S.; et al. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. The European Committee on Antimicrobial Susceptibility Testing. 2017. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 1 July 2024).
- Conover, W.J.; Iman, R.L. On Multiple-Comparisons Procedures; Technical Report LA-7677-MS; Los Alamos Scientific Laboratory: Los Alamos, NM, USA, 1979. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 7 April 2024).
| Multidrug-Resistant Infections | |||||
|---|---|---|---|---|---|
| Overall N = 130 | No N = 98 | Non-Concomitant N = 22 | Concomitant N = 10 | p-Value | |
| Age (years) | 64 (53; 73) | 62 (53; 73) | 69 (56; 75) | 70 (52; 74) | 0.484 |
| Sex male | 90 (69.2) | 70 (71.4) | 13 (59.1) | 7 (70.0) | 0.505 |
| APACHE-II score | 16 (11; 20) | 14 (9.2; 19) a | 21 (18; 25) b | 22 (16; 26.8) b | <0.001 |
| Charlson index score | 3 (2; 5.8) | 3 (2; 5) | 4 (2.2; 6) | 3.5(2.2; 4.8) | 0.601 |
| Glasgow coma score | 12 (3; 15) | 12 (3; 15) | 10 (4.5; 15) | 13.5(10; 15) | 0.783 |
| ICU death | 12 (9.2) | 7 (7.1) | 3 (13.6) | 2 (20.0) | 0.176 |
| Urgent surgery | 18 (13.8) | 13 (13.3) | 2 (9.1) | 3 (30.0) | 0.259 |
| Immunosuppressed patients | 9 (6.9) | 7 (7.1) | 2 (9.1) | 0 | 0.843 |
| Neutropenic patients | 3 (2.3) | 2 (2.0) | 1 (4.5) | 0 | 0.575 |
| Parenteral nutrition | 11 (8.5) | 3 (3.1) a | 5 (22.7) b | 3 (30.0) b | <0.001 |
| Traumatic patients | 6 (4.6) | 6 (6.1) | 0 | 0 | 0.749 |
| C.A.D. patients | 25 (19.2) | 23 (23.5) | 1 (4.5) | 1 (10.0) | 0.085 |
| ATB 48 h | 92 (70.8) | 64 (65.3) a | 21 (95.5) b | 7 (70.0) a | 0.009 |
| Surgery (last 30 days) | 5 (3.9) | 3 (3.1) | 2 (9.1) | 0 | 0.359 |
| Clostridioides difficile | 2 (1.5) | 1 (1.0) | 1 (4.5) | 0 | 0.433 |
| Renal replacement therapy | 11 (8.5) | 3 (3.1) a | 3 (13.6) a,b | 5 (50.0) b | <0.001 |
| Renal failure | 18 (13.8) | 12 (12.2) | 5 (22.7) | 1 (10.0) | 0.407 |
| COPD | 13 (10.0) | 10 (10.2) | 3 (13.6) | 0 | 0.591 |
| Cirrhosis | 3 (2.3) | 3 (3.1) | 0 | 0 | 1 |
| Neoplasm | 5 (3.9) | 4 (4.1) | 1 (4.5) | 0 | 1 |
| Diabetes mellitus | 40 (30.8) | 30 (30.6) | 7 (31.8) | 3 (30.0) | 1 |
| COVID-19 patients | 7 (5.4) | 5 (5.1) | 2 (9.1) | 0 | 0.781 |
| Impella® device | 1 (0.8) | 0 | 0 | 1 (10.0) | 0.077 |
| ECMO | 9 (7.0) | 6 (6.1) | 1 (4.8) | 2 (20.0) | 0.225 |
| Ventricular assistance | 1 (0.8) | 1 (1.0) | 0 | 0 | 1 |
| Aortic counter pulse balloon | 7 (5.4) | 5 (5.1) | 1 (4.8) | 1 (10.0) | 0.779 |
| Transplant types | 0.81 | ||||
| No | 122 (93.8) | 91 (92.9) | 21 (95.5) | 10 (100.0) | |
| Liver | 1 (0.8) | 1 (1.0) | 0 | 0 | |
| Heart | 4 (3.1) | 4 (4.1) | 0 | 0 | |
| HSC | 3 (2.3) | 2 (2.0) | 1 (4.5) | 0 | |
| Colonized | Concomitant Infection | Incidence (95% CI) * | |
|---|---|---|---|
| MDR-GNB | 84 | 0 | - |
| CRE | 12 | 0 | - |
| OXA-48 | 37 | 7 | 18.9 (7.96–35.2) |
| MDR-Pseudomonas spp. | 9 | 3 | 44.4 (13.7–78.8) |
| Metallo-β-lactamase | 3 | 0 | - |
| ESBL | 6 | 0 | - |
| Factor * | p-Value | Hazard Ratio (95% CI) |
|---|---|---|
| OXA-48 | 0.004 | 10.1 (2.084; 49.2) |
| Renal replacement therapy | 0.005 | 6.3 (1.77; 22.5) |
| Rectal colonization by MDR-Pseudomonas spp. | 0.007 | 8.68 (1.8; 41.8) |
| NP N = 4 | UTI N = 1 | TCB N = 4 | BAC N = 1 | |
|---|---|---|---|---|
| MDR-GNB | - | - | - | - |
| CRE | - | - | - | - |
| OXA-48 | 2 (50) | 1 (100) | 3 (75) | 1 (100) |
| MDR-Pseudomonas spp. | 2 (50) | - | 1 (25) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Santana, S.; Dearriba-Reyes, J.; Saavedra, P.; Iglesias-Llorente, L.; Alonso-Acero, L.; Hernández-Socorro, C.-R.; Sánchez-Ramírez, C. Prediction of Concomitant Nosocomial Infection in Patients Previously Colonized Colorectally by Multidrug-Resistant Bacteria in an SDD Setting. Antibiotics 2024, 13, 717. https://doi.org/10.3390/antibiotics13080717
Ruiz-Santana S, Dearriba-Reyes J, Saavedra P, Iglesias-Llorente L, Alonso-Acero L, Hernández-Socorro C-R, Sánchez-Ramírez C. Prediction of Concomitant Nosocomial Infection in Patients Previously Colonized Colorectally by Multidrug-Resistant Bacteria in an SDD Setting. Antibiotics. 2024; 13(8):717. https://doi.org/10.3390/antibiotics13080717
Chicago/Turabian StyleRuiz-Santana, Sergio, José Dearriba-Reyes, Pedro Saavedra, Laura Iglesias-Llorente, Laura Alonso-Acero, Carmen-Rosa Hernández-Socorro, and Catalina Sánchez-Ramírez. 2024. "Prediction of Concomitant Nosocomial Infection in Patients Previously Colonized Colorectally by Multidrug-Resistant Bacteria in an SDD Setting" Antibiotics 13, no. 8: 717. https://doi.org/10.3390/antibiotics13080717
APA StyleRuiz-Santana, S., Dearriba-Reyes, J., Saavedra, P., Iglesias-Llorente, L., Alonso-Acero, L., Hernández-Socorro, C.-R., & Sánchez-Ramírez, C. (2024). Prediction of Concomitant Nosocomial Infection in Patients Previously Colonized Colorectally by Multidrug-Resistant Bacteria in an SDD Setting. Antibiotics, 13(8), 717. https://doi.org/10.3390/antibiotics13080717







