Antimicrobial Activity of Water-Soluble Silver Complexes Bearing C-Scorpionate Ligands
Abstract
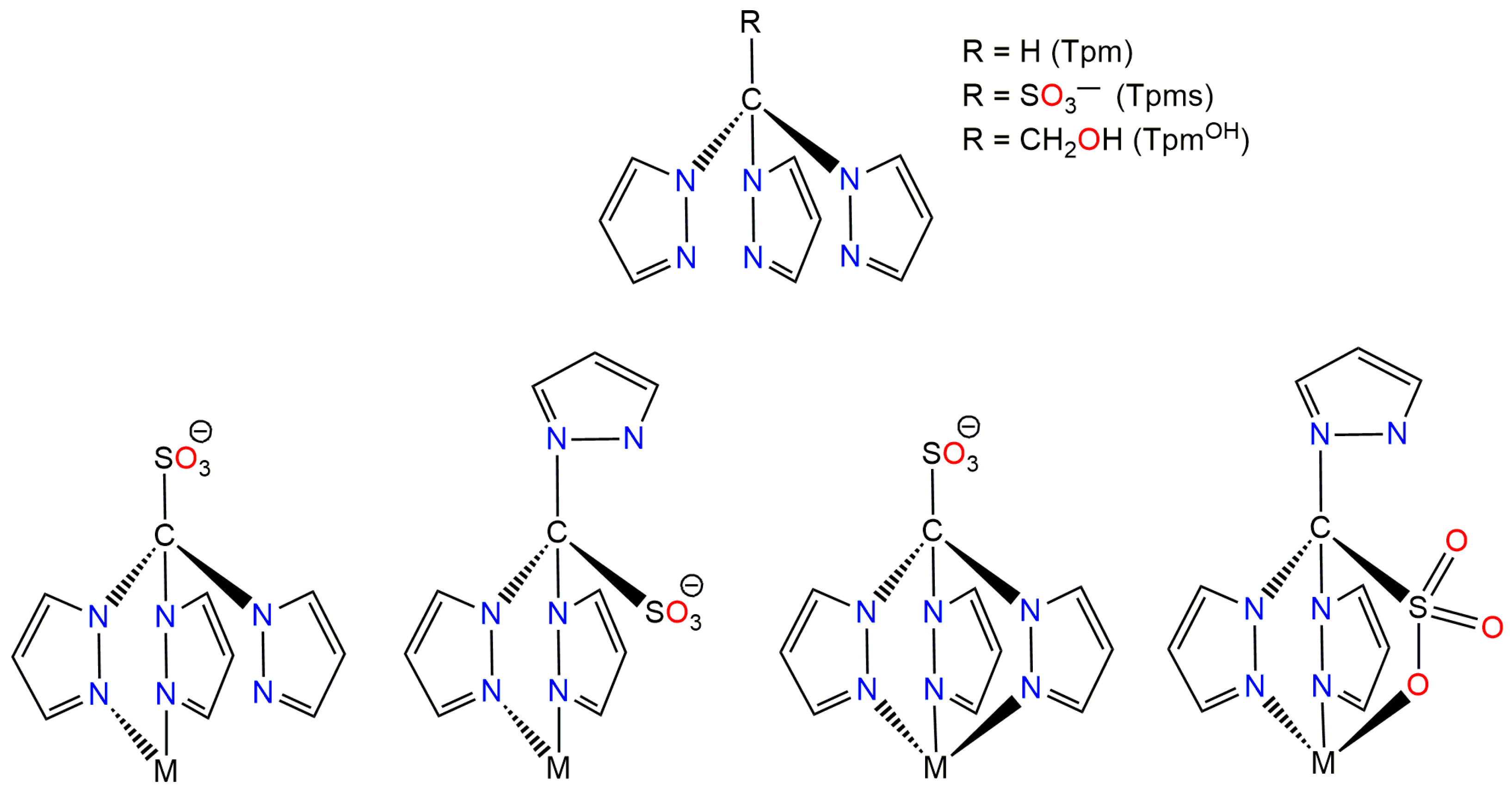
1. Introduction
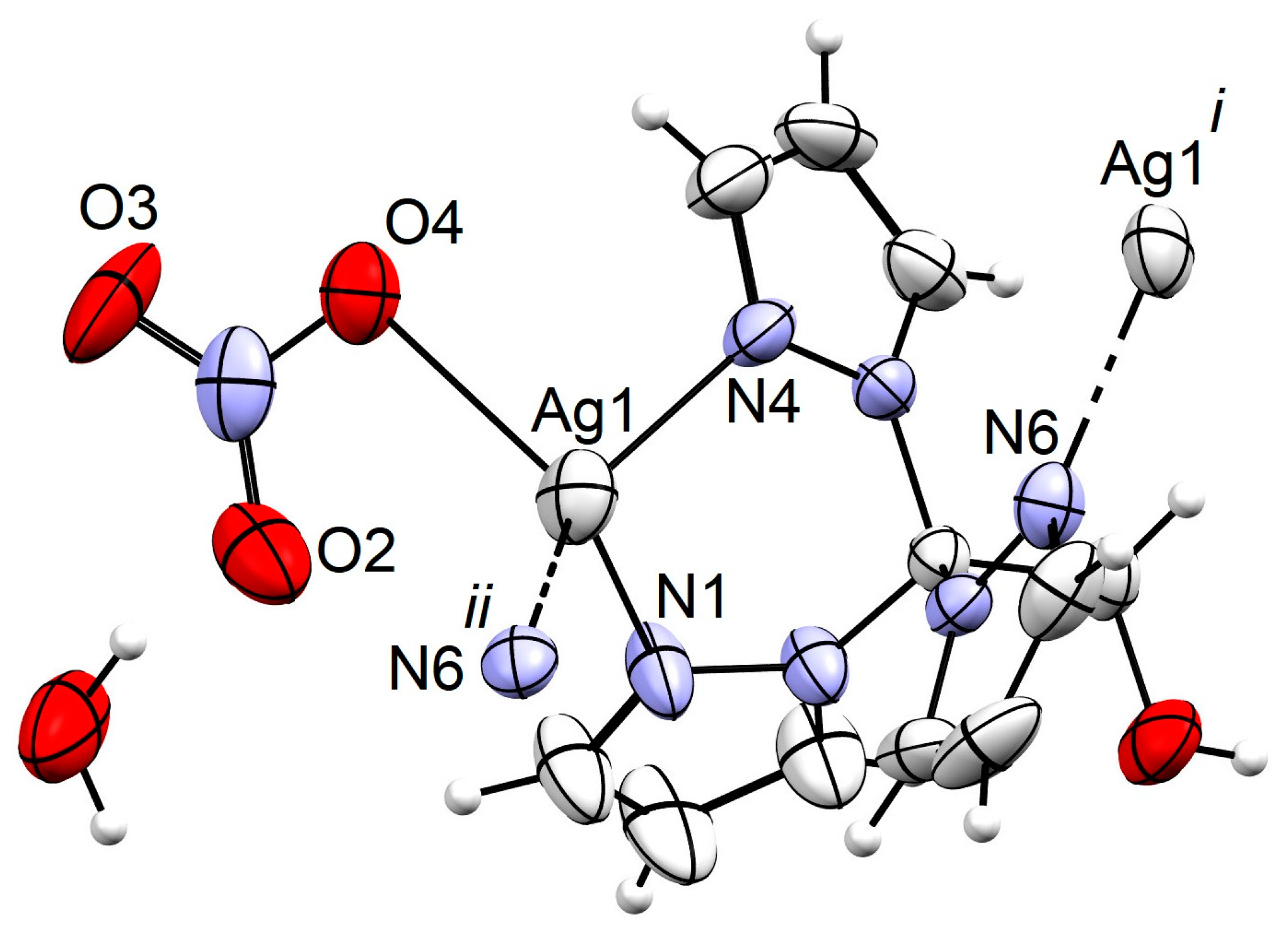
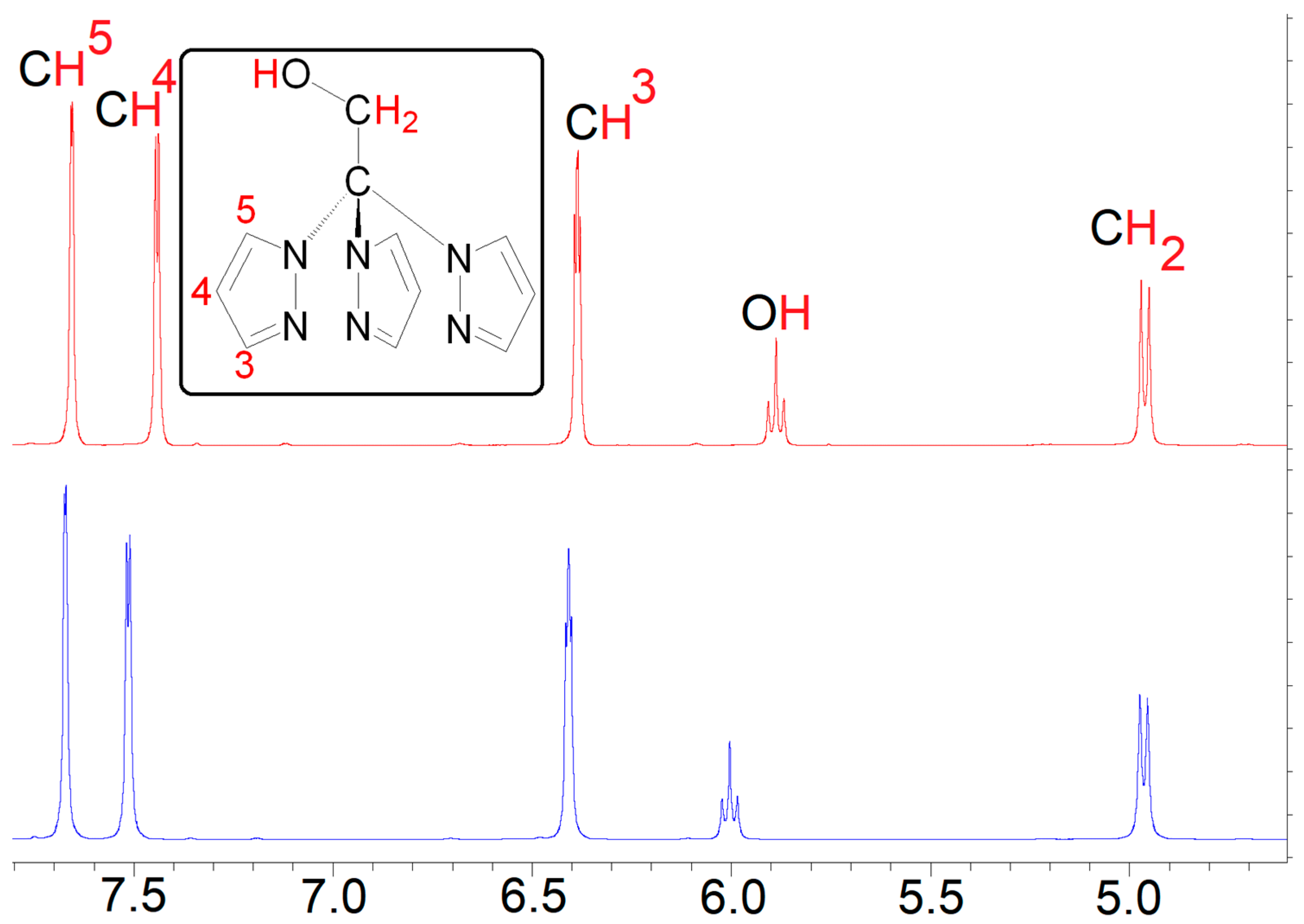
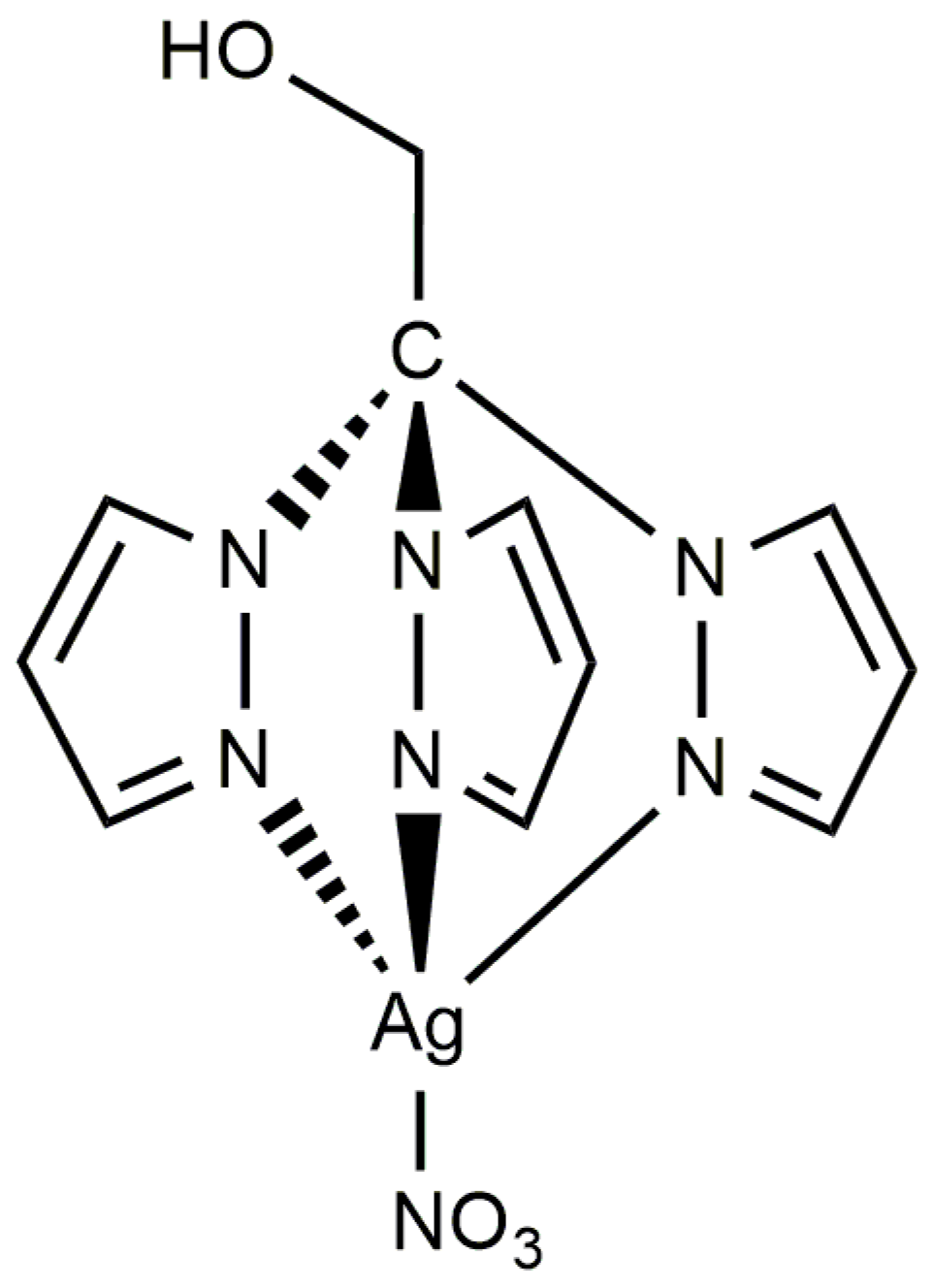
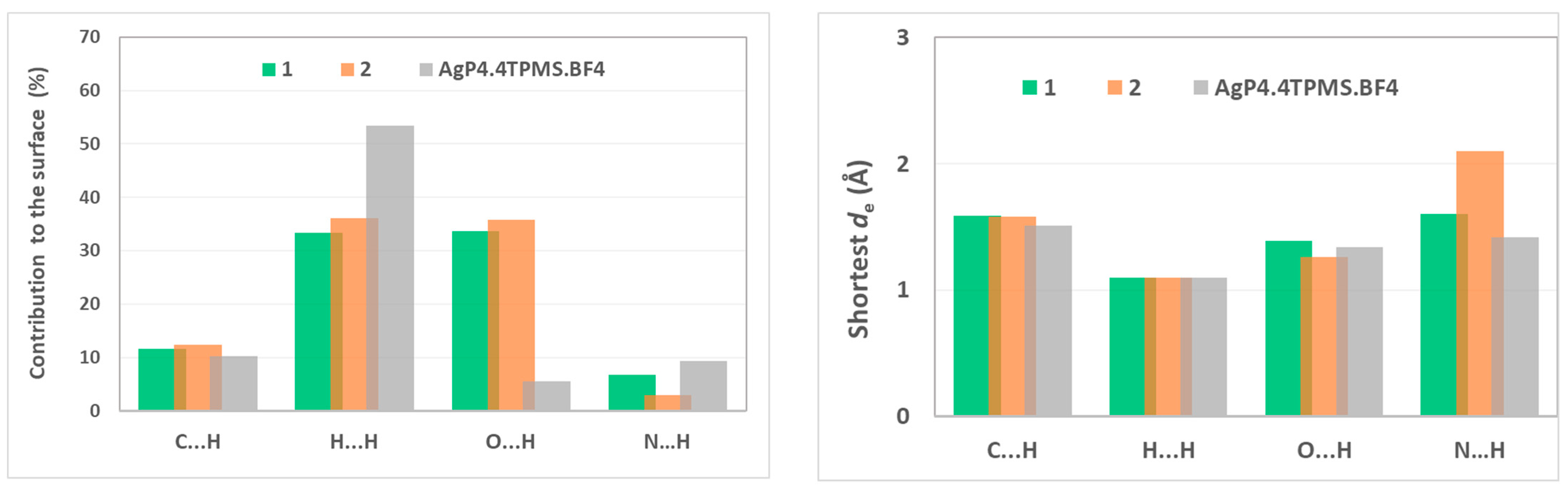
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of [Ag(NO3)(μ-1κN;2κN′,N″-TPMOH)]n (1)
3.3. X-ray Structure Determination
3.4. Bacterial and Fungal Strains and Determination of Antimicrobial Activity
3.5. Toxicity of Complexes to the Nematode Caenorhabditis Elegans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theuretzbacher, U. Future Antibiotics Scenarios: Is the Tide Starting to Turn? Int. J. Antimicrob. Agents 2009, 34, 15–20. [Google Scholar] [CrossRef] [PubMed]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 7 February 2024).
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef]
- Singh, P.A.; Desai, S.D.; Singh, J. A Review on Plant Antimicrobials of Past Decade. Curr. Top. Med. Chem. 2018, 18, 812–833. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J. The Good, the Bad, and the Ugly of Metals as Antimicrobials. BioMetals 2023, 2023, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, L.; You, G.; Li, X.; Kang, J. Metallic Elements Combine with Herbal Compounds Upload in Microneedles to Promote Wound Healing: A Review. Front. Bioeng. Biotechnol. 2023, 11, 1283771. [Google Scholar] [CrossRef]
- Żyro, D.; Sikora, J.; Szynkowska-Jóźwik, M.I.; Ochocki, J. Silver, Its Salts and Application in Medicine and Pharmacy. Int. J. Mol. Sci. 2023, 24, 15723. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Huo, P.P.; Chandran, K.; Manickam Dakshinamoorthi, B.; Yun, S.I.; Liu, B. A Review on the Toxicity of Silver Nanoparticles against Different Biosystems. Chemosphere 2022, 292, 133397. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic Chemistry—From Teaching Paradigms to Medicinal Applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Silver, S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luan, S.; Yin, Z.; He, M.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. Recent Advances in the Medical Use of Silver Complex. Eur. J. Med. Chem. 2018, 157, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) N-Heterocyclic Carbene Complexes: A Winning and Broad Spectrum of Antimicrobial Properties. Int. J. Mol. Sci. 2021, 22, 2497. [Google Scholar] [CrossRef] [PubMed]
- Kaloğlu, M.; Kaloğlu, N.; Günal, S.; Özdemir, İ. Synthesis of N-Heterocyclic Carbene-Based Silver Complexes and Their Antimicrobial Properties against Bacteria and Fungi. J. Coord. Chem. 2022, 74, 3031–3047. [Google Scholar] [CrossRef]
- Karci, H.; Dündar, M.; Nawaz, Z.; Özdemir, İ.; Gürbüz, N.; Koç, A.; Özdemir, İ.; Mansour, L.; Hamdi, N. Sythesis, Characterisation, Anticancer and Antimicrobial Activity of Ag-N-Heterocyclic Carbene Complexes Containing Benzimidazole Derivatives. Inorg. Chim. Acta 2024, 565, 121992. [Google Scholar] [CrossRef]
- Carvalho, M.A.; De Paiva, R.E.F.; Bergamini, F.R.G.; Gomes, A.F.; Gozzo, F.C.; Lustri, W.R.; Formiga, A.L.B.; Shishido, S.M.; Ferreira, C.V.; Corbi, P.P. A Silver Complex with Tryptophan: Synthesis, Structural Characterization, DFT Studies and Antibacterial and Antitumor Assays in Vitro. J. Mol. Struct. 2013, 1031, 125–131. [Google Scholar] [CrossRef]
- Stathopoulou, M.E.K.; Banti, C.N.; Kourkoumelis, N.; Hatzidimitriou, A.G.; Kalampounias, A.G.; Hadjikakou, S.K. Silver Complex of Salicylic Acid and Its Hydrogel-Cream in Wound Healing Chemotherapy. J. Inorg. Biochem. 2018, 181, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.P.; Chai, J.W.; Fan, C.; Ouyang, J.H.; Duan, W.J.; Sun, B.; Chen, J.; Yuan, L.X.; Xu, X.Q.; Chen, J.X. Water-Stable Silver-Based Metal-Organic Frameworks of Quaternized Carboxylates and Their Antimicrobial Activity. ACS Appl. Bio Mater. 2020, 3, 8525–8531. [Google Scholar] [CrossRef] [PubMed]
- Kuchar, J.; Rust, J.; Lehmann, C.W.; Mohr, F. Silver(I) Complexes with Camphorsulfonato and Phosphine Ligands: Structural Diversity and Antibacterial Activity. Inorg. Chem. 2020, 59, 10557–10568. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Król, J.; Bazanów, B.; Poradowski, D.; Chrószcz, A.; Nesterov, D.S.; Kirillov, A.M.; Smoleński, P. Antiviral, Antibacterial, Antifungal, and Cytotoxic Silver(I) BioMOF Assembled from 1,3,5-Triaza-7-Phoshaadamantane and Pyromellitic Acid. Molecules 2020, 25, 2119. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Florek, M.; Bażanów, B.; Panek, J.; Krogul-Sobczak, A.; Oliveira, M.C.; Król, J.; Śliwińska-Hill, U.; Nesterov, D.S.; Kirillov, A.M.; et al. Silver Coordination Polymers Driven by Adamantoid Blocks for Advanced Antiviral and Antibacterial Biomaterials. ACS Appl. Mater. Interfaces 2024, 16, 13411–13421. [Google Scholar] [CrossRef]
- Pandurangan, K.; Gallagher, S.; Morgan, G.G.; Müller-Bunz, H.; Paradisi, F. Structure and Antibacterial Activity of the Silver(i) Complex of 2-Aminophenoxazine-3-One. Metallomics 2010, 2, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Stenger-Smith, J.; Chakraborty, I.; Sameera, W.M.C.; Mascharak, P.K. Antimicrobial Silver (I) Complexes Derived from Aryl-Benzothiazoles as Turn-on Sensors: Syntheses, Properties and Density Functional Studies. Inorg. Chim. Acta 2018, 471, 326–335. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Petrelli, D.; Vitali, L.A.; Guedes Da Silva, M.F.C.; Martins, L.M.D.R.S.; Smoleński, P.; et al. Synthesis, Antimicrobial and Antiproliferative Activity of Novel Silver(I) Tris(Pyrazolyl)Methanesulfonate and 1,3,5-Triaza-7-Phosphadamantane Complexes. Inorg. Chem. 2011, 50, 11173–11183. [Google Scholar] [CrossRef]
- Smoleński, P.; Pettinari, C.; Marchetti, F.; Guedes Da Silva, M.F.C.; Lupidi, G.; Badillo Patzmay, G.V.; Petrelli, D.; Vitali, L.A.; Pombeiro, A.J.L. Syntheses, Structures, and Antimicrobial Activity of New Remarkably Light-Stable and Water-Soluble Tris(Pyrazolyl)Methanesulfonate Silver(I) Derivatives of N-Methyl-1,3,5-Triaza-7-Phosphaadamantane Salt—[MPTA]BF4. Inorg. Chem. 2015, 54, 434–440. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(Pyrazol-1-Yl)Methane Metal Complexes for Catalytic Mild Oxidative Functionalizations of Alkanes, Alkenes and Ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Alkorta, I.; Claramunt, R.M.; Díez-Barra, E.; Elguero, J.; de la Hoz, A.; López, C. The Organic Chemistry of Poly(1H-Pyrazol-1-Yl)Methanes. Coord. Chem. Rev. 2017, 339, 153–182. [Google Scholar] [CrossRef]
- Silva, F.; Fernandes, C.; Campello, M.P.C.; Paulo, A. Metal Complexes of Tridentate Tripod Ligands in Medical Imaging and Therapy. Polyhedron 2017, 125, 186–205. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Copper Complexes Bearing C-Scorpionate Ligands: Synthesis, Characterization and Catalytic Activity for Azide-Alkyne Cycloaddition in Aqueous Medium. Inorg. Chim. Acta 2018, 483, 371–378. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-Soluble C-Scorpionate Complexes—Catalytic and Biological Applications. Eur. J. Inorg. Chem. 2016, 2016, 2236–2252. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Hydrosoluble Complexes Bearing Tris(Pyrazolyl)Methane Sulfonate Ligand: Synthesis, Characterization and Catalytic Activity for Henry Reaction. Catalysts 2019, 9, 611. [Google Scholar] [CrossRef]
- Almeida, J.; Roma-Rodrigues, C.; Mahmoud, A.G.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L.; Martins, L.M.D.R.S.; Baptista, P.V.; Fernandes, A.R. Structural Characterization and Biological Properties of Silver(I) Tris(Pyrazolyl)Methane Sulfonate. J. Inorg. Biochem. 2019, 199, 110789. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.G.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. A New Amido-Phosphane as Ligand for Copper and Silver Complexes. Synthesis, Characterization and Catalytic Application for Azide–Alkyne Cycloaddition in Glycerol. Dalton Trans. 2021, 50, 6109–6125. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Martins, L.M.D.R.S.; Guedes Da Silva, M.F.C.; Fernandes, A.R.; Silva, A.; Borralho, P.M.; Santos, S.; Rodrigues, C.M.P.; Pombeiro, A.J.L. Cobalt Complexes Bearing Scorpionate Ligands: Synthesis, Characterization, Cytotoxicity and DNA Cleavage. Dalton Trans. 2012, 41, 12888–12897. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, L.; Wang, C.; Shi, C.; Li, Y.; Peng, Y.; Chen, H.; Zhang, J.; Cheng, B.; Chen, C.; et al. Review of the toxicity and potential molecular mechanisms of parental or successive exposure to environmental pollutants in the model organism Caenorhabditis elegans. Environ. Pollut. 2022, 311, 119927. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Kläui, W.; Berghahn, M.; Rheinwald, G.; Lang, H. Tris(Pyrazolyl)Methanesulfonates: A Novel Class of Water-Soluble Ligands. Angew. Chemie Int. Ed. 2000, 39, 2464–2466. [Google Scholar] [CrossRef]
- Reger, D.L.; Grattan, T.C.; Brown, K.J.; Little, C.A.; Lamba, J.J.S.; Rheingold, A.L.; Sommer, R.D. Syntheses of Tris(Pyrazolyl)Methane Ligands and {[Tris(Pyrazolyl)Methane]Mn(CO)3}SO3CF3 Complexes: Comparison of Ligand Donor Properties. J. Organomet. Chem. 2000, 607, 120–128. [Google Scholar] [CrossRef]
- SMART & SAINT Software Reference Manuals, Version 6.22; Bruker AXS Analytic X-Ray Systems, Inc.: Madison, WI, USA, 2000.
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction; University of Gottingen: Gottingen, Germany, 2000. [Google Scholar]
- Sheldrick, G.M. SHELXTL V5.1, Software Reference Manual; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. MIC Determination of Non-Fastidious and Fastidious Organisms. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 1 July 2024).
- The European Committee on Antimicrobial Susceptibility Testing. Susceptibility Testing of Yeasts. Available online: https://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_yeasts (accessed on 1 July 2024).
- Lambert, R.J.W.; Pearson, J. Susceptibility Testing: Accurate and Reproducible Minimum Inhibitory Concentration (MIC) and Non-inhibitory Concentration (NIC) Values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.P.; Pinheiro, T.; Martins, M.S.; Carvalho, M.F.N.N.; Feliciano, J.R.; Leitão, J.H.; Silva, R.A.L.; Guerreiro, J.F.; Alves, L.M.C.; Custódio, I.; et al. Tuning the Biological Activity of Camphorimine Complexes through Metal Selection. Antibiotics 2022, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.P.; Sousa, S.A.; Galvão, A.M.; Mata, J.M.; Leitão, J.H.; Carvalho, M.F.N.N. Key Parameters on the Antibacterial Activity of Silver Camphor Complexes. Antibiotics 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.F.N.N.; Leite, S.; Costa, J.P.; Galvão, A.M.; Leitão, J.H. Ag(I) Camphor Complexes: Antimicrobial Activity by Design. J. Inorg. Biochem. 2019, 199, 110791. [Google Scholar] [CrossRef]
- Leitão, J.H.; Sousa, S.A.; Leite, S.A.; Carvalho, M.F.N.N. Silver Camphor Imine Complexes: Novel Antibacterial Compounds from Old Medicines. Antibiotics 2018, 7, 65. [Google Scholar] [CrossRef]


| MIC (μg/mL) | |||
|---|---|---|---|
| 1 | 2 | TPMOH | |
| E. coli ATCC25922 | 7.7 ± 0.2 | 14.5 ± 4.3 | >250 |
| P. aeruginosa 477 | 2.0 ± 0.1 | 19.5 ± 0.9 | >250 |
| B. contaminans IST408 | 5.5 ± 1.3 | 19.6 ± 4.0 | >250 |
| S. aureus Newman | 17.9 ± 3.2 | 51.4 ± 8.7 | >250 |
| C. albicans SC5134 | 15.4 ± 0.4 | 6.3 ± 1.0 | >125 |
| C. glabrata CBS138 | 31.5 ± 0.1 | 5.7 ± 0.2 | >125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.G.; Sousa, S.A.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Leitão, J.H. Antimicrobial Activity of Water-Soluble Silver Complexes Bearing C-Scorpionate Ligands. Antibiotics 2024, 13, 647. https://doi.org/10.3390/antibiotics13070647
Mahmoud AG, Sousa SA, Guedes da Silva MFC, Martins LMDRS, Leitão JH. Antimicrobial Activity of Water-Soluble Silver Complexes Bearing C-Scorpionate Ligands. Antibiotics. 2024; 13(7):647. https://doi.org/10.3390/antibiotics13070647
Chicago/Turabian StyleMahmoud, Abdallah G., Sílvia A. Sousa, M. Fátima C. Guedes da Silva, Luísa M. D. R. S. Martins, and Jorge H. Leitão. 2024. "Antimicrobial Activity of Water-Soluble Silver Complexes Bearing C-Scorpionate Ligands" Antibiotics 13, no. 7: 647. https://doi.org/10.3390/antibiotics13070647
APA StyleMahmoud, A. G., Sousa, S. A., Guedes da Silva, M. F. C., Martins, L. M. D. R. S., & Leitão, J. H. (2024). Antimicrobial Activity of Water-Soluble Silver Complexes Bearing C-Scorpionate Ligands. Antibiotics, 13(7), 647. https://doi.org/10.3390/antibiotics13070647











