Bioactive Bioxanthracene and Cyclodepsipeptides from the Entomopathogenic Fungus Blackwellomyces roseostromatus BCC56290
Abstract
1. Introduction
2. Results and Discussion
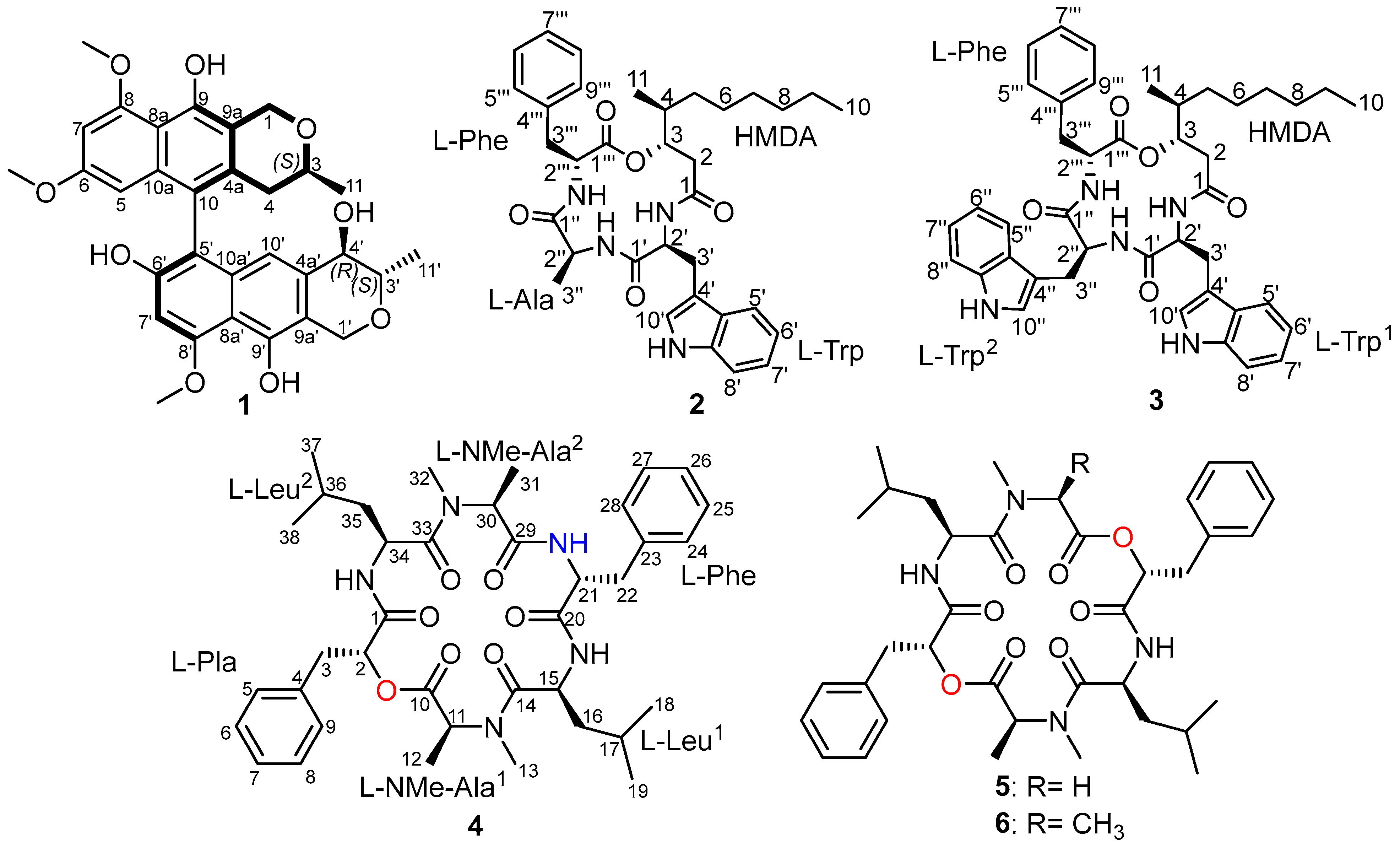
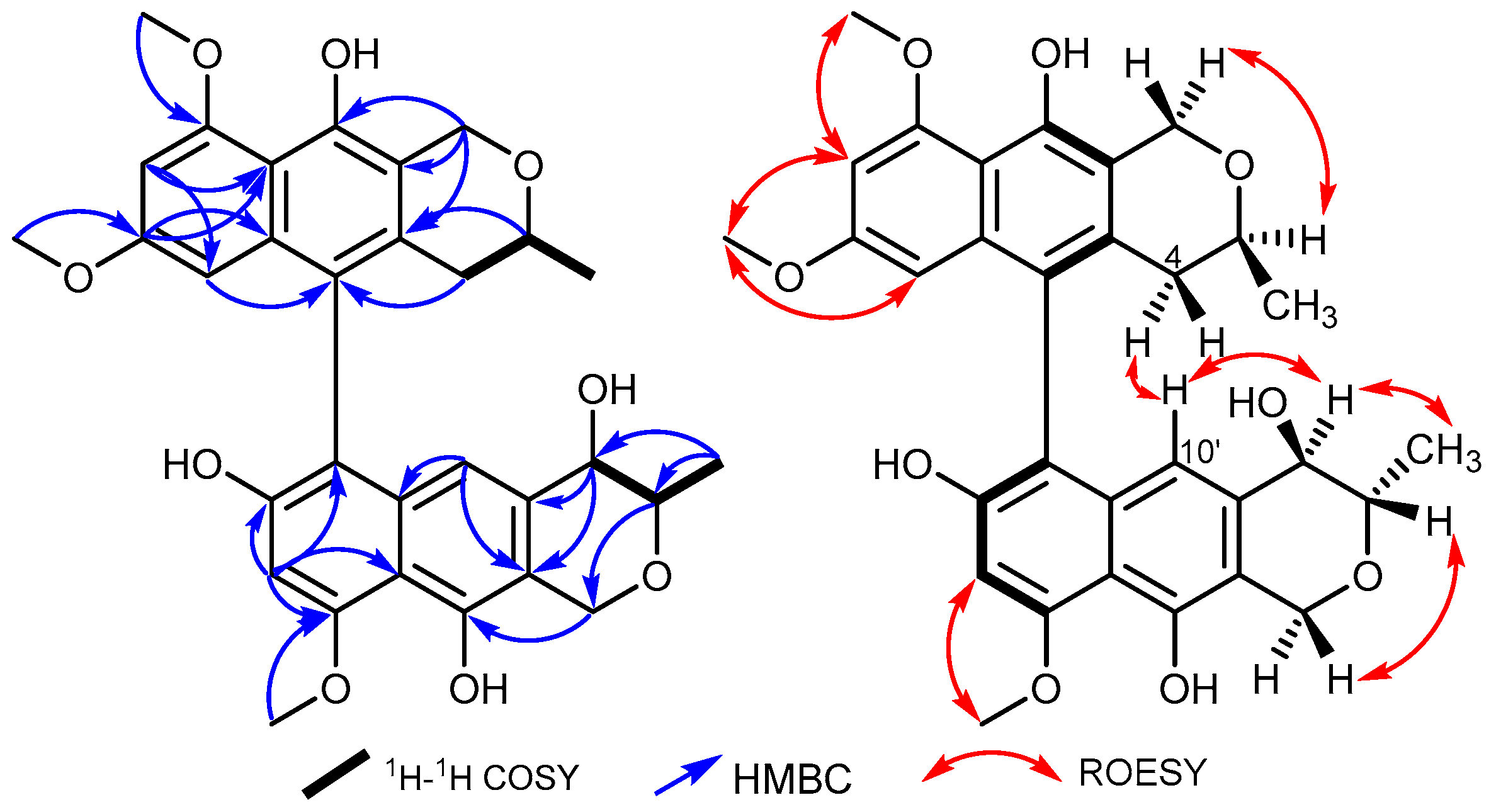
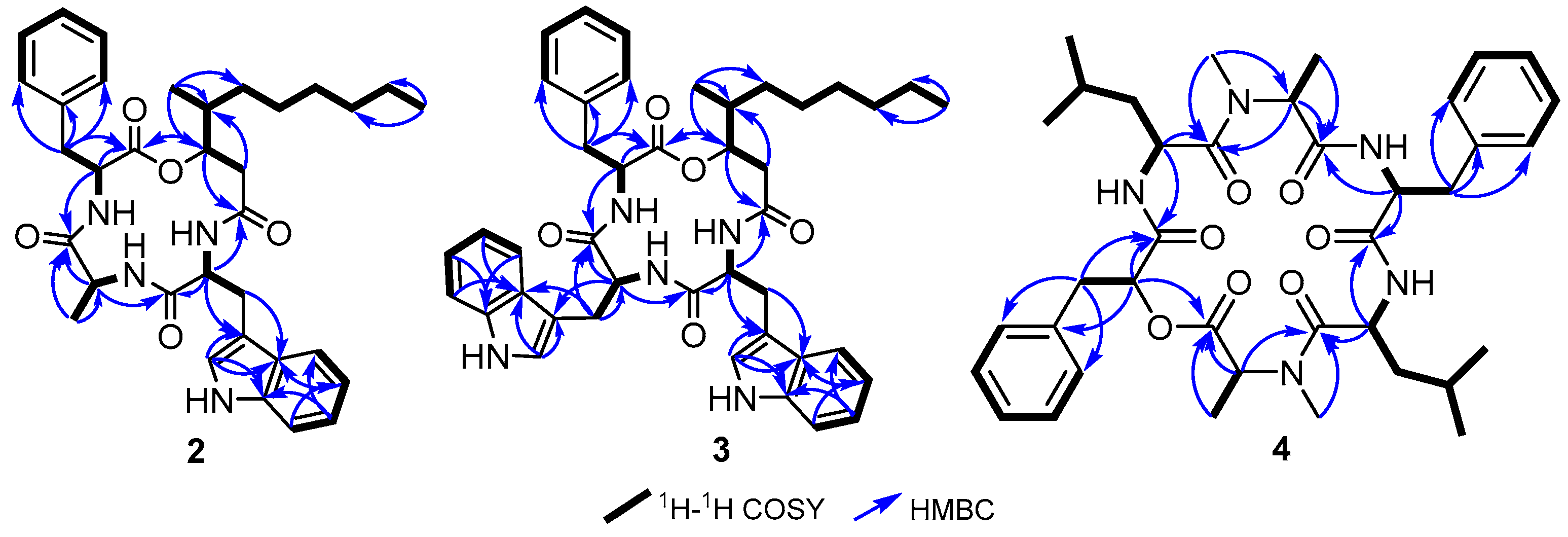
2.1. Isolation and Identification of 1–6
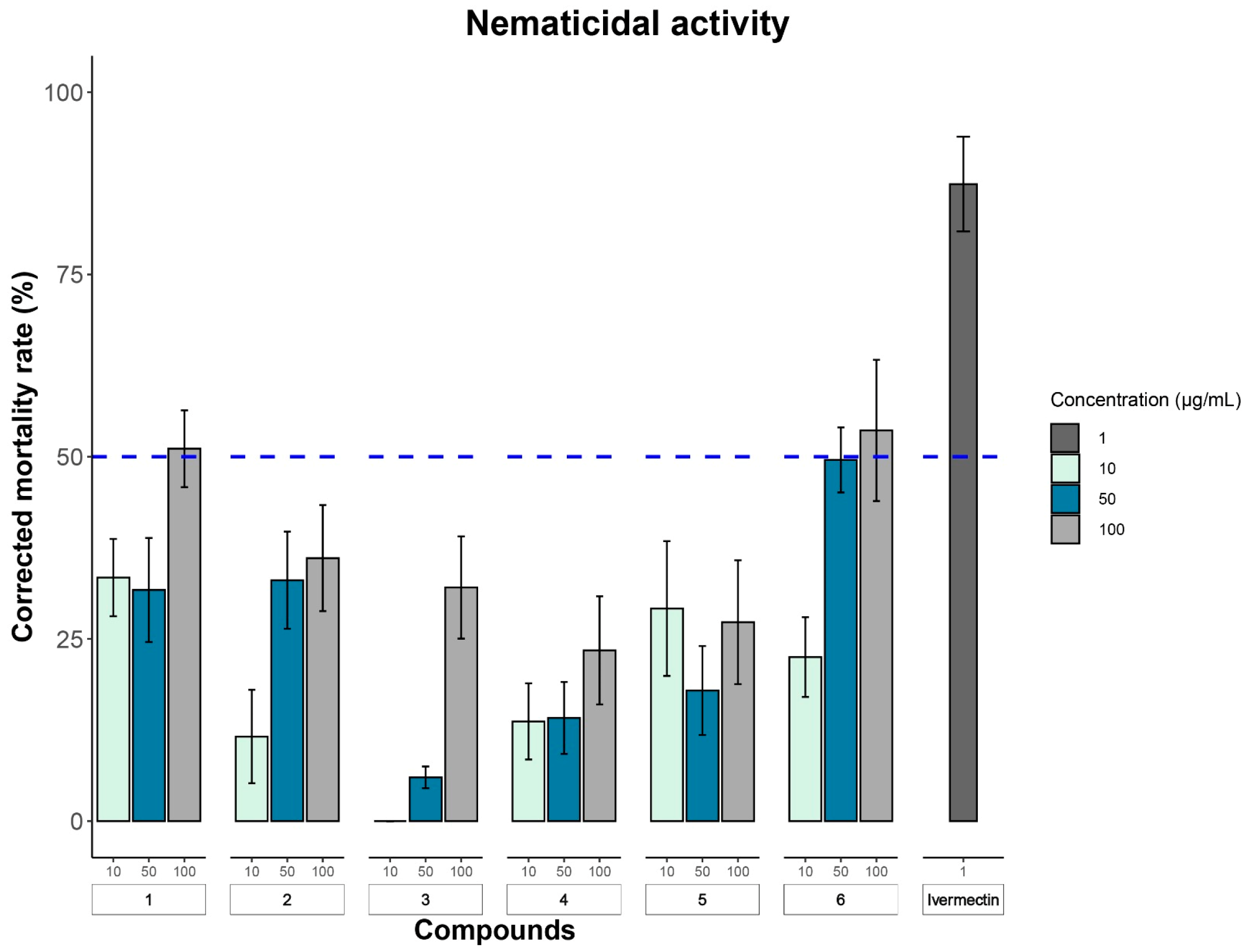
2.2. Biological Assays
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
3.4. Derivatization with Marfey’s Reagent and Elucidation of Amino Acid Configurations
3.5. Cytotoxicity Assay
3.6. Antimicrobial Assay
3.7. Nematicidal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkatesh, G.; Sakthi Priya, P.; Anithaa, V.; Dinesh, G.K.; Velmurugan, S.; Abinaya, S.; Karthika, P.; Sivasakthivelan, P.; Soni, R.; Thennarasi, A. Role of entomopathogenic fungi in biocontrol of insect pests. In Plant Protection; Soni, R., Suyal, D.C., Goel, R., Eds.; De Gruyter: Berlin, Germany, 2022; pp. 505–548. [Google Scholar]
- Zhang, L.; Fasoyin, O.E.; Molnár, I.; Xu, Y. Secondary metabolites from hypocrealean entomopathogenic fungi: Novel bioactive compounds. Nat. Prod. Rep. 2020, 37, 1181–1206. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Gibson, D.M.; Donzelli, B.G.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Gibson, D.M.; Krasnoff, S.B. Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat. Prod. Rep. 2010, 27, 1241–1275. [Google Scholar] [CrossRef] [PubMed]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Noisripoom, W.; Tasanathai, K.; Khonsanit, A.; Thanakitpipattana, D.; Himaman, W.; Kobmoo, N.; Luangsa-ard, J.J. Molecular phylogeny and morphology reveal cryptic species in Blackwellomyces and Cordyceps (Cordycipitaceae) from Thailand. Mycol. Prog. 2020, 19, 957–983. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.C.; Wu, L.X.; Wang, Y.; Xu, A.; Lin, W.F. Blackwellomyces kaihuaensis and Metarhizium putuoense (Hypocreales), two new entomogenous fungi from subtropical forests in Zhejiang Province, Eastern China. Forests 2023, 14, 2333. [Google Scholar] [CrossRef]
- Umeyama, A.; Takahashi, K.; Grundniewska, A.; Shimizu, M.; Hayashi, S.; Kato, M.; Okamoto, Y.; Suenaga, M.; Ban, S.; Kumada, T.; et al. In vitro antitrypanosomal activity of the cyclodepsipeptides, cardinalisamides A–C, from the insect pathogenic fungus Cordyceps cardinalis NBRC 103832. J. Antibiot. 2014, 67, 163–166, Erratum in J. Antibiot. 2014, 67, 487. [Google Scholar] [CrossRef]
- Feng, P.; Shang, Y.; Cen, K.; Wang, C. Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 11365–11370. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nguyen, N.-H.; Yoshinari, T.; Hachisu, M.; Nguyen, P.-T.; Shimizu, K. Identification of the oosporein biosynthesis gene cluster in an entomopathogenic fungus Blackwellomyces cardinalis. Mycoscience 2024, 65, 96–104. [Google Scholar] [CrossRef]
- Toki, S.; Ando, K.; Yoshida, M.; Kawamoto, I.; Sano, H.; Matsuda, Y. ES-242-1, a novel compound from Verticillium sp., binds to a site on N-methyl-D-aspartate receptor that is coupled to the channel domain. J. Antibiot. 1992, 45, 88–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toki, S.; Ando, K.; Kawamoto, I.; Sano, H.; Yoshida, M.; Matsuda, Y. ES-242-2, -3, -4, -5, -6, -7, and -8, novel bioxanthracenes produced by Verticillium sp., which act on the N-methyl-D-aspartate receptor. J. Antibiot. 1992, 45, 1047–1054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Isaka, M.; Kongsaeree, P.; Thebtaranonth, Y. Bioxanthracenes from the insect pathogenic fungus Cordyceps pseudomilitaris BCC 1620. J. Antibiot. 2001, 54, 36–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Isaka, M.; Srisanoh, U.; Lartpornmatulee, N.; Boonruangprapa, T. ES-242 derivatives and cycloheptapeptides from Cordyceps sp. Strains BCC 16173 and BCC 16176. J. Nat. Prod. 2007, 70, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Toki, S.; Tsukuda, E.; Nozawa, M.; Nonaka, H.; Yoshida, M.; Matsuda, Y. The ES-242s, novel N-methyl-D-aspartate antagonists of microbial origin, interact with both the neurotransmitter recognition site and the ion channel domain. J. Biol. Chem. 1992, 267, 14884–14892. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, J.F.; Grove, J.F. Cyclodepsipeptides from Beauveria bassiana Bals. Part 1. Beauverolides H and I. J. Chem. Soc. Perkin 1. 1977, 3, 270–273. [Google Scholar] [CrossRef]
- Jegorov, A.; Sedmera, P.; Matha, V.; Simek, P.; Zahradnickova, H.; Landa, Z.; Eyal, J. Beauverolides L and La from Beauveria tenella and Paecilomyces fumosoroseus. Phytochemistry 1994, 37, 1301–1303. [Google Scholar] [CrossRef]
- Matsuda, D.; Namatame, I.; Tomoda, H.; Kobayashi, S.; Zocher, R.; Kleinkauf, H.; Omura, S. New beauveriolides produced by amino acid-supplemented fermentation of Beauveria sp. FO-6979. J. Antibiot. 2004, 57, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.L.; Zhang, M.L.; Zhang, H.D.; Huang, J.Z.; Li, L. Genome mining and biosynthesis of the Acyl-CoA: Cholesterol acyltransferase inhibitor beauveriolide I and III in Cordyceps militaris. J. Biotechnol. 2020, 309, 85–91. [Google Scholar] [CrossRef]
- Helaly, S.E.; Kuephadungphan, W.; Phainuphong, P.; Ibrahim, M.A.A.; Tasanathai, K.; Mongkolsamrit, S.; Luangsa-ard, J.J.; Phongpaichit, S.; Rukachaisirikul, V.; Stadler, M. Pigmentosins from Gibellula sp. as antibiofilm agents and a new glycosylated asperfuran from Cordyceps javanica. Beilstein J. Org. Chem. 2019, 15, 2968–2981. [Google Scholar] [CrossRef]
- Jegorov, A.; Paizs, B.; Kuzma, M.; Zabka, M.; Landa, Z.; Sulc, M.; Barrow, M.P.; Havlicek, V. Extraribosomal cyclic tetradepsipeptides beauverolides: Profiling and modeling the fragmentation pathways. J. Mass Spectrom. 2004, 39, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Madariaga-Mazon, A.; Gonzalez-Andrade, M.; Toriello, C.; Navarro-Barranco, H.; Mata, R. Potent anti-calmodulin activity of cyclotetradepsipeptides isolated from Isaria fumosorosea using a newly designed biosensor. Nat. Prod. Commun. 2015, 10, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Hu, K.; Wang, F.; Tang, J.W.; Zhang, L.; Sun, H.D.; Cai, X.H.; Puno, P.T. 3-Hydroxy-4-methyldecanoic acid-containing cyclotetradepsipeptides from an endolichenic Beauveria sp. J. Nat. Prod. 2021, 84, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Viehrig, K.; Surup, F.; Harmrolfs, K.; Jansen, R.; Kunze, B.; Müller, R. Concerted action of P450 plus helper protein to form the amino-hydroxy-piperidone moiety of the potent protease inhibitor crocapeptin. J. Am. Chem. Soc. 2013, 135, 16885–16894. [Google Scholar] [CrossRef]
- Sum, W.C.; Mitschke, N.; Schrey, H.; Wittstein, K.; Kellner, H.; Stadler, M.; Matasyoh, J.C. Antimicrobial and cytotoxic cyathane-xylosides from cultures of the basidiomycete Dentipellis fragilis. Antibiotics 2022, 11, 1072. [Google Scholar] [CrossRef]
- Sum, W.C.; Ebada, S.S.; Kirchenwitz, M.; Wanga, L.; Decock, C.; Stradal, T.E.B.; Matasyoh, J.C.; Mándi, A.; Kurtán, T.; Stadler, M. Neurite outgrowth-inducing drimane-type sesquiterpenoids isolated from cultures of the polypore Abundisporus violaceus MUCL 56355. J. Nat. Prod. 2023, 86, 2457–2467. [Google Scholar] [CrossRef]
- Wennrich, J.-P.; Ebada, S.S.; Sepanian, E.; Holzenkamp, C.; Khalid, S.J.; Schrey, H.; Maier, W.; Kurtán, T.; Ashrafi, S.; Stadler, M. Omnipolyphilins A and B: Novel chlorinated cyclotetrapeptides and naphtho-α-pyranones from two plant nematode-derived fungi Polyphilus sieberi. J. Agric. Food Chem. 2024, 72, 6998–7009. [Google Scholar] [CrossRef] [PubMed]
- Phutthacharoen, K.; Toshe, R.; Khalid, S.J.; Llanos-López, N.A.; Wennrich, J.-P.; Schrey, H.; Ebada, S.S.; Hyde, K.D.; Stadler, M. Lachnuoic Acids A–F: Ambuic acid congeners from a saprotrophic Lachnum species. Chem. Biodiver. 2024, 21, e202400385. [Google Scholar] [CrossRef]
- Schneider-Orelli, O. Entomologisches Praktikum. Einführung in Die Land-Und Forstwirtschaftliche Insektenkunde, 2nd ed.; H. R. Sauerländer: Aarau, Switzerland, 1947. [Google Scholar]
| Pos. | δC, a,c Type | δH b Multi (J [Hz]) | Pos. | δC, a,c Type | δH b Multi (J [Hz]) |
|---|---|---|---|---|---|
| 1 | 64.3, CH2 | α 4.66 d (15.4) β 5.00 d (15.4) | 1′ | 64.1, CH2 | α 4.59 d (15.2) β 4.82 d (15.2) |
| 3 | 69.9, CH | 3.64 m | 3′ | 74.9, CH | 3.31 overlapped |
| 4 | 34.2, CH2 | α 1.96 dd (16.7, 10.9) β 2.20 dd (16.7, 3.0) | 4′ | 69.6, CH | 3.99 t (8.4) |
| 4a | 134.1, C | 4a′ | 139.2, C | ||
| 5 | 98.3, CH | 6.04 d (2.2) | 5′ | 110.2, C | |
| 6 | 156.7, C | 6′ | 152.0, C | ||
| 7 | 96.3, CH | 6.56 d (2.2) | 7′ | 97.3, CH | 6.73 s |
| 8 | 157.3, C | 8′ | 156.2, C | ||
| 8a | 108.8, C | 8a′ | 108.1, C | ||
| 9 | 148.1, C | 9′ | 148.3, C | ||
| 9a | 114.3, C | 9a′ | 113.8, C | ||
| 10 | 122.0, C | 10′ | 111.9, CH | 6.57 d (1.2) | |
| 10a | 134.9, C | 10a′ | 135.0, C | ||
| 11 | 21.6, CH3 | 1.06 d (6.2) | 11′ | 18.6, CH3 | 1.18 d (6.0) |
| 6-OCH3 | 54.9, CH3 | 3.46 s | 4′-OH | - | 5.19 d (8.0) |
| 8-OCH3 | 56.5, CH3 | 4.04 s | 6′-OH | - | 9.20 br s |
| 9-OH | - | 9.49 s | 8′-OCH3 | 56.1, CH3 | 4.05 s |
| 9′-OH | - | 9.46 s |
| Pos. | 2 | 3 | ||
|---|---|---|---|---|
| δC, a,c Type | δH b Multi (J [Hz]) | δC, a,c Type | δH b Multi (J [Hz]) | |
| HMDA | ||||
| 1 | 170.3, CO | 170.3, CO | ||
| 2 | 35.8, CH2 | α 2.34 dd (13.8, 9.2) β 2.46 dd (13.8, 4.2) | 35.6, CH2 | α 2.37 dd (14.5, 8.3) β 2.41 dd (14.5, 4.7) |
| 3 | 76.1, CH | 4.83 ddd (9.7, 5.8, 4.2) | 76.1, CH | 4.85 td (7.4, 4.9) |
| 4 | 35.2, CH | 1.91 ddd (9.8, 5.9, 2.4) | 34.9, CH | 2.01 m |
| 5 | 31.0, CH2 | α 0.96 m; β 1.34 m | 31.1, CH2 | α 0.95 m; β 1.36 m |
| 6 | 26.5, CH2 | α 1.12 overlapped β 1.28 overlapped | 26.4, CH2 | α 1.12 overlapped β 1.29 overlapped |
| 7 | 31.3, CH2 | 1.23 m | 31.3, CH2 | 1.23 m |
| 8 | 29.0, CH2 | 1.23 m | 29.0, CH2 | 1.23 m |
| 9 | 22.1, CH2 | 1.25 m | 22.2, CH2 | 1.25 m |
| 10 | 14.0, CH3 | 0.85 t (6.9) | 14.0, CH3 | 0.84 t (6.8) |
| 11 | 15.3, CH3 | 0.75 d (6.9) | 15.4, CH3 | 0.75 d (6.9) |
| Trp/Trp1 | ||||
| 1′ | 171.4, CO | 170.2, CO | ||
| 2′ | 55.9, CH | 4.21 q (7.6) | 56.5, CH | 4.18 overlapped |
| 3′ | 25.6, CH2 | α 3.02 dd (14.5, 7.9) β 3.12 dd (14.5, 7.6) | 25.6, CH2 | α 3.07 dd (15.3, 7.1) β 3.11 overlapped |
| 4′ | 109.8, C | 110.0, C | ||
| 4a′ | 127.0, C | 127.1, C | ||
| 5′ | 118.0, CH | 7.51 d (8.0) | 118.2, CH | 7.48 dd (8.0) |
| 6′ | 118.1, CH | 6.97 ddd (8.0, 6.9, 1.0) | 118.4, CH | 6.98 overlapped |
| 7′ | 120.8, CH | 7.06 ddd (8.0, 6.9, 1.2) | 121.0, CH | 7.07 m |
| 8′ | 111.2, CH | 7.32 d (8.0) | 111.5, CH | 7.35 d (8.1) |
| 8a′ | 136.0, C | 136.1, C | ||
| 9′-NH | - | 10.86 d (2.4) | - | 10.83 d (2.5) |
| 10′ | 123.3, CH | 7.11 d (2.4) | 123.6, CH | 7.10 d (2.5) |
| NH | - | 8.51 d (7.4) | - | 8.43 d (7.7) |
| Ala/Trp2 | ||||
| 1″ | 170.7, CO | 171.9, CO | ||
| 2″ | 48.6, CH | 3.82 p (6.9) | 54.2, CH | 4.15 overlapped |
| 3″ | 15.4, CH3 | 1.11 d (6.9) | 25.0, CH2 | α 2.94 dd (14.8, 7.1) β 3.25 dd (14.7, 7.1) |
| 4″ | 111.0, C | |||
| 4a″ | 127.4, C | |||
| 5″ | 118.3, CH | 7.47 d (7.9) | ||
| 6″ | 118.2, CH | 6.95 overlapped | ||
| 7″ | 120.8, CH | 7.04 m | ||
| 8″ | 111.3, CH | 7.32 d (8.2) | ||
| 8a″ | 136.0, C | |||
| 9″-NH | - | 10.69 d (2.4) | ||
| 10″ | 123.0, CH | 6.81 d (2.4) | ||
| NH | - | 8.47 d (7.3) | - | 8.30 d (7.5) |
| Phe | ||||
| 1‴ | 168.7, CO | 169.1, CO | ||
| 2‴ | 54.8, CH | 4.62 dd (9.1, 7.8) | 55.0, CH | 4.60 q (8.0) |
| 3‴ | 37.6, CH2 | α 2.86 dd (13.9, 7.5) β 2.92 dd (13.9, 8.0) | 37.3, CH2 | 2.90 q (6.5) |
| 4‴ | 136.7, C | 136.8, C | ||
| 5‴, 9‴ | 128.6, CH | 7.19 overlapped | 128.8, CH | 7.18 overlapped |
| 6‴, 8‴ | 126.5, CH | 7.21 overlapped | 126.7, CH | 7.21 overlapped |
| 7‴ | 128.1, CH | 7.26 t (7.1) | 128.3, CH | 7.24 t (7.1) |
| NH | - | 7.31 d (8.8) | - | 7.77 d (8.7) |
| Pos. | δC, a,c Type | δH b Multi (J [Hz]) | Pos. | δC, a,c Type | δH b Multi (J [Hz]) |
|---|---|---|---|---|---|
| L-Pla | L-Phe | ||||
| 1 | 168.0, CO | 20 | 169.8, CO | ||
| 2 | 74.0, CH | 5.05 dd (10.5, 3.9) | 21 | 55.8, CH | 4.03 ddd (11.7, 8.0, 3.4) |
| 3 | 37.4, CH2 | α 2.90 dd (14.1, 10.5) β 3.15 overlapped | 22 | 34.2, CH2 | α 3.19 overlapped β 3.34 overlapped |
| 4 | 137.2, C | 23 | 139.5, C | ||
| 5, 9 | 129.1, CH | 7.15 m (2H) | 24, 28 | 129.2, CH | 7.28 m (2H) |
| 6, 8 | 128.0, CH | 7.27 m (2H) | 25, 27 | 126.3, CH | 7.20 m (2H) |
| 7 | 125.9, CH | 7.18 m (1H) | 26 | 128.8, CH | 7.21 m (1H) |
| L-NMe-Ala1 | 21-NH | - | 7.92 d (7.9) | ||
| 10 | 170.0, CO | L-NMe-Ala2 | |||
| 11 | 58.0, CH | 3.48 q (7.0) | 29 | 170.2, CO | |
| 12 | 13.2, CH3 | 1.19 d (7.0) | 30 | 60.8, CH | 3.48 q (7.0) |
| 13 | 36.5, CH3 | 3.16 s | 31 | 12.8, CH3 | 1.32 d (7.0) |
| L-Leu1 | 32 | 37.6, CH3 | 3.12 s | ||
| 14 | 171.2, CO | L-Leu2 | |||
| 15 | 45.8, CH | 4.93 dt (9.5, 7.0) | 33 | 171.8, CO | |
| 16 | 40.4, CH2 | α 1.57 overlapped β 1.64 overlapped | 34 | 46.1, CH | 4.81 td (8.2, 6.3) |
| 17 | 23.5, CH | 1.58 overlapped | 35 | 39.6, CH2 | α 1.47 overlapped β 1.53 overlapped |
| 18 | 21.5–23.0, CH3 | 0.84–0.89 overlapped | 36 | 24.0, CH | 1.45 overlapped |
| 19 | 21.5–23.0, CH3 | 0.84–0.89 overlapped | 37 | 21.5–23.0, CH3 | 0.84–0.89 overlapped |
| 15-NH | - | 7.85 d (9.6) | 38 | 21.5–23.0, CH3 | 0.84–0.89 overlapped |
| 34-NH | - | 7.42 d (8.9) |
| IC50 (µM) | Positive Control | ||||||
|---|---|---|---|---|---|---|---|
| Test Cell Line | 1 | 2 | 3 | 4 | 5 | 6 | Epothilone B (nM) |
| L929 (murine) | ** | * | * | ** | ** | 26.0 | 0.65 |
| KB3.1 (cervix) | 29.2 | * | * | 36.2 | 2.2 | 8.4 | 0.17 |
| PC-3 (prostate) | n.t. | n.t. | n.t. | n.t. | 2.5 | 7.5 | 0.09 |
| MCF-7 (breast) | n.t. | n.t. | n.t. | n.t. | 6.9 | 13.9 | 0.07 |
| SKOV-3 (ovary) | n.t. | n.t. | n.t. | n.t. | 25.0 | 12.3 | 0.09 |
| A431 (skin) | n.t. | n.t. | n.t. | n.t. | 4.3 | 8.5 | 0.06 |
| A549 (lung) | n.t. | n.t. | n.t. | n.t. | 11.5 | 11.1 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phutthacharoen, K.; Llanos-López, N.A.; Toshe, R.; Noisripoom, W.; Khonsanit, A.; Luangsa-ard, J.J.; Hyde, K.D.; Ebada, S.S.; Stadler, M. Bioactive Bioxanthracene and Cyclodepsipeptides from the Entomopathogenic Fungus Blackwellomyces roseostromatus BCC56290. Antibiotics 2024, 13, 585. https://doi.org/10.3390/antibiotics13070585
Phutthacharoen K, Llanos-López NA, Toshe R, Noisripoom W, Khonsanit A, Luangsa-ard JJ, Hyde KD, Ebada SS, Stadler M. Bioactive Bioxanthracene and Cyclodepsipeptides from the Entomopathogenic Fungus Blackwellomyces roseostromatus BCC56290. Antibiotics. 2024; 13(7):585. https://doi.org/10.3390/antibiotics13070585
Chicago/Turabian StylePhutthacharoen, Kunthida, Natalia A. Llanos-López, Rita Toshe, Wasana Noisripoom, Artit Khonsanit, Janet Jennifer Luangsa-ard, Kevin D. Hyde, Sherif S. Ebada, and Marc Stadler. 2024. "Bioactive Bioxanthracene and Cyclodepsipeptides from the Entomopathogenic Fungus Blackwellomyces roseostromatus BCC56290" Antibiotics 13, no. 7: 585. https://doi.org/10.3390/antibiotics13070585
APA StylePhutthacharoen, K., Llanos-López, N. A., Toshe, R., Noisripoom, W., Khonsanit, A., Luangsa-ard, J. J., Hyde, K. D., Ebada, S. S., & Stadler, M. (2024). Bioactive Bioxanthracene and Cyclodepsipeptides from the Entomopathogenic Fungus Blackwellomyces roseostromatus BCC56290. Antibiotics, 13(7), 585. https://doi.org/10.3390/antibiotics13070585









