Accordance of Registered Drug Packages with Guideline-Recommended Treatment Durations for Community-Acquired Pneumonia—A New Antibiotic Stewardship Target?
Abstract
1. Introduction
2. Results
3. Discussion
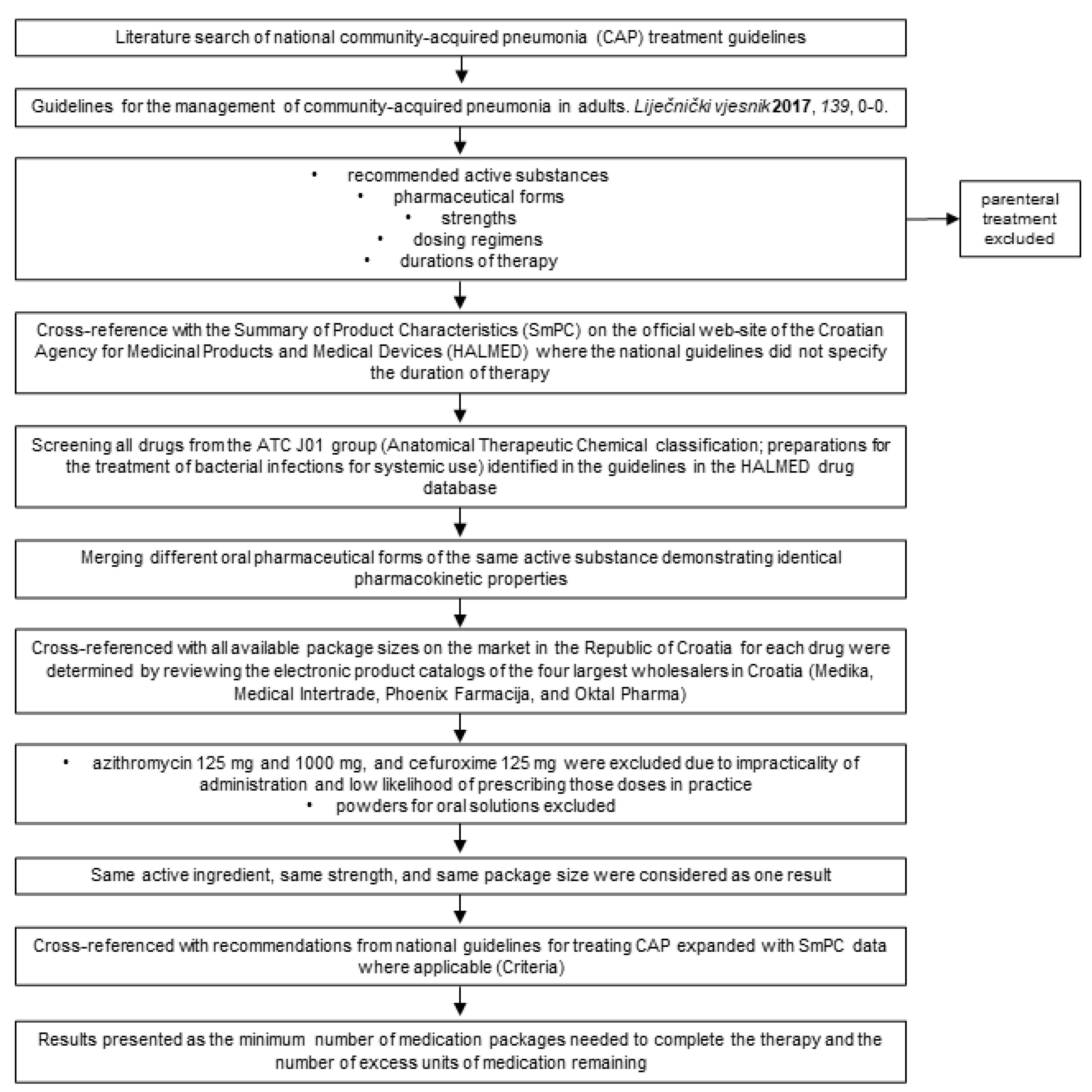
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. A European One Health Action Plan against Antimicrobial Resistance (AMR). Available online: https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf (accessed on 4 January 2024).
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland: WHO Document Production Services. 2015. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 11 January 2024).
- International Pharmaceutical Federation (FIP). Fighting Antimicrobial Resistance: The Contribution of Pharmacists. The Hague: International Pharmaceutical Federation. 2015. Available online: https://www.fip.org/file/163 (accessed on 21 January 2024).
- Meeker, D.; Linder, J.A.; Fox, C.R.; Friedberg, M.W.; Persell, S.D.; Goldstein, N.J.; Knight, T.K.; Hay, J.W.; Doctor, J.N. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA 2016, 315, 562–570. [Google Scholar] [CrossRef]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 11 January 2024).
- Kuzman, I.; Rakušić, N.; Čivljak, R.; Puljiz, I.; Kutleša, M.; Topić, A.; Mažuranić, I.; Korušić, A.; Adžić, Z.O.; Baršić, B.; et al. Guidelines for the management of community-acquired pneumonia in adults. Liječnički Vjesn. 2017, 139, 177–191. [Google Scholar]
- Boras, Z.; Marunica, E.; Trkeš, V. Treatment of Community-Acquired Pneumonia. Medicus 2016, 25, 39–45. [Google Scholar]
- Tsoumani, E.; Carter, J.A.; Salomonsson, S.; Stephens, J.M.; Bencina, G. Clinical, economic, and humanistic burden of community acquired pneumonia in Europe: A systematic literature review. Expert. Rev. Vaccines 2023, 22, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, A.; Reid, J.N.; Shariff, S.Z.; Povitz, M.; Silverman, M.; Patrick, D.M.; Morris, A.M.; McCormack, J.; Haverkate, M.R.; Marra, F. Retrospective cohort analysis of outpatient antibiotic prescribing for community-acquired pneumonia in Canadian older adults. PLoS ONE 2023, 18, e0292899. [Google Scholar] [CrossRef] [PubMed]
- Zodrow, R.; Olson, A.; Willis, S.; Grauer, D.; Klatt, M. Characterization of antibiotic overuse for common infectious disease states at hospital discharge. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e229. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, B.; Rueda-Benito, A.; O’Regan, M.; Finan, K. An audit of community-acquired pneumonia antimicrobial compliance using an intervention bundle in an Irish hospital. J. Glob. Antimicrob. Resist. 2020, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Monday, L.M.; Yazdanpaneh, O.; Sokolowski, C.; Chi, J.; Kuhn, R.; Bazzy, K.; Dhar, S. A Physician-Driven Quality Improvement Stewardship Intervention Using Lean Six Sigma Improves Patient Care for Community-Acquired Pneumonia. Glob. J. Qual. Saf. Healthc. 2021, 4, 109–116. [Google Scholar] [CrossRef]
- Rossin, S.; Barbieri, E.; Cantarutti, A.; Martinolli, F.; Giaquinto, C.; Da Dalt, L.; Dona, D. Multistep antimicrobial stewardship intervention on antibiotic prescriptions and treatment duration in children with pneumonia. PLoS ONE 2021, 16, e0257993. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Ratz, D.; Greene, M.T.; Flanders, S.A.; Gandhi, T.N.; Petty, L.A.; Huls, S.; Feng, X.; White, A.T.; Hersh, A.L. Antibiotic Stewardship Strategies and Their Association with Antibiotic Overuse After Hospital Discharge: An Analysis of the Reducing Overuse of Antibiotics at Discharge (Road) Home Framework. Clin. Infect. Dis. 2022, 75, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Galimam, S.; Panozzo, B.; Muir, K.; Chavada, R. “Antibiotic hardstop” on electronic prescribing: Impact on antimicrobial stewardship initiatives in patients with community acquired pneumonia (CAP) and infective exacerbations of chronic obstructive pulmonary disease (IECOPD). BMC Infect. Dis. 2022, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Saha, N. Correlation of Recommendations of Treatment Guidelines and Frequently Prescribed Antibiotics: Evaluation of Their Pharmaceutical Pack Size. Basic Clin. Pharmacol. Toxicol. 2018, 122, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Jukic, I.; Rusic, D.; Vukovic, J.; Zivkovic, P.M.; Bukic, J.; Leskur, D.; Seselja Perisin, A.; Luksic, M.; Modun, D. Correlation of registered drug packs with Maastricht V/Florence Consensus Report and national treatment guidelines for management of Helicobacter pylori infection. Basic Clin. Pharmacol. Toxicol. 2020, 126, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Abelenda-Alonso, G.; Rombauts, A.; Gudiol, C.; Garcia-Lerma, E.; Pallares, N.; Ardanuy, C.; Calatayud, L.; Niubo, J.; Tebe, C.; Carratala, J. Effect of positive microbiological testing on antibiotic de-escalation and outcomes in community-acquired pneumonia: A propensity score analysis. Clin. Microbiol. Infect. 2022, 28, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, I.; Seselja Perisin, A.; Bukic, J.; Leskur, D.; Bozic, J.; Modun, D.; Vukovic, J.; Rusic, D. Registered Drug Packs of Antimicrobials and Treatment Guidelines for Prostatitis: Are They in Accordance? Healthcare 2022, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Rusic, D.; Bozic, J.; Bukic, J.; Seselja Perisin, A.; Leskur, D.; Modun, D.; Tomic, S. Evaluation of accordance of antibiotics package size with recommended treatment duration of guidelines for sore throat and urinary tract infections. Antimicrob. Resist. Infect. Control 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Barancheshme, F.; Munir, M. Strategies to Combat Antibiotic Resistance in the Wastewater Treatment Plants. Front. Microbiol. 2017, 8, 2603. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, N.M.A.; Bowes, J.; Viel-Theriault, I.; Thampi, N.; Blackburn, J.; Buba, M.; Harrison, M.A.; Barrowman, N. Combined influence of practice guidelines and prospective audit and feedback stewardship on antimicrobial treatment of community-acquired pneumonia and empyema in children: 2012 to 2016. Paediatr. Child Health 2021, 26, 234–241. [Google Scholar] [CrossRef]
- Magill, S.S.; O’Leary, E.; Ray, S.M.; Kainer, M.A.; Evans, C.; Bamberg, W.M.; Johnston, H.; Janelle, S.J.; Oyewumi, T.; Lynfield, R.; et al. Assessment of the Appropriateness of Antimicrobial Use in US Hospitals. JAMA Netw. Open 2021, 4, e212007. [Google Scholar] [CrossRef]
- Powell, N.; Stephens, J.; Rule, R.; Phillips, R.; Morphew, M.; Garry, E.; Askaroff, N.; Hiley, D.; Strachan, C.; Sheehan, M.; et al. Potential to reduce antibiotic use in secondary care: Single-centre process audit of prescription duration using NICE guidance for common infections. Clin. Med. 2021, 21, e39–e44. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, G.; Davidson, A.; Chow, I.; Chiu, A. Antibiotics Utilization for Community Acquired Pneumonia in a Community Hospital Emergency Department. J. Pharm. Pract. 2022, 35, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Schaub, C.; Barnsteiner, S.; Schonenberg, L.; Bloch, N.; Drager, S.; Albrich, W.C.; Conen, A.; Osthoff, M. Antibiotic treatment durations for common infectious diseases in Switzerland: Comparison between real-life and local and international guideline recommendations. J. Glob. Antimicrob. Resist. 2023, 32, 11–17. [Google Scholar] [CrossRef]
- Brower, K.I.; Hecke, A.; Mangino, J.E.; Gerlach, A.T.; Goff, D.A. Duration of Antibiotic Therapy for General Medicine and General Surgery Patients Throughout Transitions of Care: An Antibiotic Stewardship Opportunity for Noninfectious Disease Pharmacists. Hosp. Pharm. 2021, 56, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Conner, M.; Harris, W.H.; Bomkamp, J.P. ADD It Up: An Evaluation of Antibiotic Duration at Hospital Discharge at a Community Hospital. Open Forum Infect. Dis. 2021, 8, ofab399. [Google Scholar] [CrossRef] [PubMed]
- Waagsbo, B.; Tranung, M.; Damas, J.K.; Heggelund, L. Antimicrobial therapy of community-acquired pneumonia during stewardship efforts and a coronavirus pandemic: An observational study. BMC Pulm. Med. 2022, 22, 379. [Google Scholar] [CrossRef] [PubMed]
- McGuire, T.M.; Smith, J.; Del Mar, C. The match between common antibiotics packaging and guidelines for their use in Australia. Aust. N. Z. J. Public Health 2015, 39, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Machowska, A.; Stalsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.R.; Chew, L. Turning waste medicines to cost savings: A pilot study on the feasibility of medication recycling as a solution to drug wastage. Palliat. Med. 2017, 31, 35–41. [Google Scholar] [CrossRef]
- McNulty, C.A.; Boyle, P.; Nichols, T.; Clappison, P.; Davey, P. The public’s attitudes to and compliance with antibiotics. J. Antimicrob. Chemother. 2007, 60 (Suppl. 1), i63–i68. [Google Scholar] [CrossRef]
- Treibich, C.; Lescher, S.; Sagaon-Teyssier, L.; Ventelou, B. The expected and unexpected benefits of dispensing the exact number of pills. PLoS ONE 2017, 12, e0184420. [Google Scholar] [CrossRef] [PubMed]
- Kardas, P.; Pechere, J.C.; Hughes, D.A.; Cornaglia, G. A global survey of antibiotic leftovers in the outpatient setting. Int. J. Antimicrob. Agents 2007, 30, 530–536. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and The Council of the European Union. Official Journal of the European Union. European Commission. Council Directive 2011/62/EU Amending Directive 2001/83/EC of 1 July 2011 on the Community Code Relating to Medicinal Products for Human Use, as Regards the Prevention of the Entry into the Legal Supply Chain of Falsified Medicinal Products. (L 174/74). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32011L0062 (accessed on 11 January 2024).
- The European Commission. Official Journal of the European Union. Commission Delegated Regulation (EU) 2016/161 of 2 October 2015 Supplementing Directive 2001/83/EC of the European Parliament and of the Council by Laying Down Detailed Rules for the Safety Features Appearing on the Packaging of Medicinal Products for Human Use. (L32/1). Available online: https://eur-lex.europa.eu/eli/reg_del/2016/161/oj (accessed on 11 January 2024).
- Llewelyn, M.J.; Fitzpatrick, J.M.; Darwin, E.; SarahTonkin, C.; Gorton, C.; Paul, J.; Peto, T.E.A.; Yardley, L.; Hopkins, S.; Walker, A.S. The antibiotic course has had its day. BMJ 2017, 358, j3418. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B. The New Antibiotic Mantra-“Shorter Is Better”. JAMA Intern. Med. 2016, 176, 1254–1255. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections—Full version. Clin. Microbiol. Infect. 2011, 17 (Suppl. 6), E1–E59. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Baudouin, S.V.; George, R.C.; Hill, A.T.; Jamieson, C.; Le Jeune, I.; Macfarlane, J.T.; Read, R.C.; Roberts, H.J.; Levy, M.L.; et al. BTS guidelines for the management of community acquired pneumonia in adults: Update 2009. Thorax 2009, 64 (Suppl. 3), iii1–iii55. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. NICE Guidelines. Pneumonia (Community Acquired): Antimicrobial Prescribing [NG138]. 2019. Available online: https://www.nice.org.uk/guidance/ng138 (accessed on 10 April 2021).
- Smith, R.; Coast, J. The true cost of antimicrobial resistance. BMJ 2013, 346, f1493. [Google Scholar] [CrossRef]
- van den Bergh, D.; Messina, A.P.; Goff, D.A.; van Jaarsveld, A.; Coetzee, R.; de Wet, Y.; Bronkhorst, E.; Brink, A.; Mendelson, M.; Richards, G.A.; et al. A pharmacist-led prospective antibiotic stewardship intervention improves compliance to community-acquired pneumonia guidelines in 39 public and private hospitals across South Africa. Int. J. Antimicrob. Agents 2020, 56, 106189. [Google Scholar] [CrossRef]
- Halcomb, S.M.; Johnson, A.; Kang-Birken, S.L. Impact of a pharmacy department-wide transitions-of-care program on inappropriate oral antibiotic prescribing at hospital discharge. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e185. [Google Scholar] [CrossRef]
- Thomas, A.A.; Korienek, P.J.; Reid, S.A.; Dierkhising, R.A.; Dababneh, A.S.; Lessard, S.R. Effect of Pharmacist Audit on Antibiotic Duration for Pneumonia and Urinary Tract Infection. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Iovino, G.; Nadeau, L. Effect of Pharmacist-Initiated Interventions on Duration of Antibiotic Therapy for Acute Exacerbation of Chronic Obstructive Pulmonary Disease and Community-Acquired Pneumonia. Can. J. Hosp. Pharm. 2023, 76, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Stoll, K.; Feltz, E.; Ebert, S. Pharmacist-Driven Implementation of Outpatient Antibiotic Prescribing Algorithms Improves Guideline Adherence in the Emergency Department. J. Pharm. Pract. 2021, 34, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Rusic, D.; Bukic, J.; Seselja Perisin, A.; Leskur, D.; Modun, D.; Petric, A.; Vilovic, M.; Bozic, J. Are We Making the Most of Community Pharmacies? Implementation of Antimicrobial Stewardship Measures in Community Pharmacies: A Narrative Review. Antibiotics 2021, 10, 63. [Google Scholar] [CrossRef]
- Diaz, M.C.G.; Handy, L.K.; Crutchfield, J.H., Jr.; Cadilla, A.; Hossain, J.; Werk, L.N. Impact of a Personalized Audit and Feedback Intervention on Antibiotic Prescribing Practices for Outpatient Pediatric Community-Acquired Pneumonia. Clin. Pediatr. 2020, 59, 988–994. [Google Scholar] [CrossRef]
- The Agency for Medicinal Products and Medical Devices (HALMED). Medicinal Products Database. Available online: https://www.halmed.hr/Lijekovi/Baza-lijekova (accessed on 13 April 2021).
| Active Substance | Pharmaceutical Form | Strength | Number of Doses per Package |
|---|---|---|---|
| Azithromycin | Film-coated tablets, tablets for oral suspension | 500 mg | 3 |
| Azithromycin | Film-coated tablets, hard capsules | 250 mg | 6 |
| Amoxicillin | Film-coated tablets, tablets for oral suspension, hard capsules | 500 mg | 16 |
| Amoxicillin | Film-coated tablets, tablets for oral suspension | 1000 mg | 16 |
| Amoxicillin with clavulanic acid | Film-coated tablets, tablets for oral suspension/disintegrating oral tablets | 875 mg + 125 mg | 14 |
| Amoxicillin with clavulanic acid | Film-coated tablets | 875 mg + 125 mg | 12 |
| Cefuroxime axetil | Film-coated tablets | 250 mg | 10 |
| Cefuroxime axetil | Film-coated tablets | 500 mg | 10 |
| Cefuroxime axetil | Film-coated tablets | 500 mg | 14 |
| Cefuroxime axetil | Film-coated tablets | 500 mg | 16 |
| Doxycycline | Hard capsules | 100 mg | 25 |
| Moxifloxacin | Film-coated tablets | 400 mg | 5 |
| Moxifloxacin | Film-coated tablets | 400 mg | 7 |
| Levofloxacin | Film-coated tablets | 500 mg | 10 |
| Cefpodoxime | Film-coated tablets | 100 mg | 10 |
| Cefpodoxime | Film-coated tablets | 100 mg | 20 |
| Cefpodoxime | Film-coated tablets | 200 mg | 10 |
| Cefpodoxime | Film-coated tablets | 200 mg | 20 |
| Clarithromycin | Extended-release tablets | 500 mg | 7 |
| Clarithromycin | Extended-release tablets | 500 mg | 14 |
| Clarithromycin | Film-coated tablets | 250 mg | 14 |
| Clarithromycin | Film-coated tablets | 500 mg | 14 |
| Active Substance | Package Size and Strength | Dosing Interval and Duration of Therapy | Number of Packages Needed to Complete the Therapy | Number of Excess Units | Accordance |
|---|---|---|---|---|---|
| Amoxicillin | 16 × 500 mg | 3 × 500 mg, 7 days | 2 | 11 | no |
| 16 × 500 mg | 3 × 500 mg, 10 days | 2 | 2 | no | |
| 16 × 500 mg | 3 × 1000 mg, 7 days | 3 | 6 | no | |
| 16 × 500 mg | 3 × 1000 mg, 10 days | 4 | 4 | no | |
| 16 × 1000 mg | 3 × 1000 mg, 7 days | 2 | 11 | no | |
| 16 × 1000 mg | 3 × 1000 mg, 10 days | 2 | 2 | no | |
| Cefuroxime axetil | 10 × 250 mg | 2 × 500 mg, 7 days | 3 | 2 | no |
| 10 × 500 mg | 2 × 500 mg, 7 days | 2 | 6 | no | |
| 14 × 500 mg | 2 × 500 mg, 7 days | 1 | 0 | yes | |
| 16 × 500 mg | 2 × 500 mg, 7 days | 1 | 2 | no | |
| Amoxicillin with clavulanic acid | 12 × 1 g | 2 × 1 g, 7 days | 2 | 10 | no |
| 12 × 1 g | 2 × 1 g, 10 days | 2 | 4 | no | |
| 14 × 1 g | 2 × 1 g, 7 days | 1 | 0 | yes | |
| 14 × 1 g | 2 × 1 g, 10 days | 2 | 8 | no | |
| Cefpodoxime | 10 × 100 mg | 2 × 200 mg, 7 days | 3 | 2 | no |
| 20 × 100 mg | 2 × 200 mg, 7 days | 2 | 12 | no | |
| 10 × 200 mg | 2 × 200 mg, 7 days | 2 | 6 | no | |
| 20 × 200 mg | 2 × 200 mg, 7 days | 1 | 6 | no | |
| Levofloxacin | 10 × 500 mg | 1 × 500 mg, 7 days | 1 | 3 | no |
| 10 × 500 mg | 1 × 500 mg, 14 days | 2 | 6 | no | |
| 10 × 500 mg | 2 × 500 mg, 7 days | 2 | 6 | no | |
| 10 × 500 mg | 2 × 500 mg, 14 days | 3 | 2 | no | |
| Moxifloxacin | 5 × 400 mg | 1 × 400 mg, 10 days | 2 | 0 | yes |
| 7 × 400 mg | 1 × 400 mg, 10 days | 2 | 4 | no | |
| Azithromycin | 6 × 250 mg | 1 × 500 mg, 3 days | 1 | 0 | yes |
| 3 × 500 mg | 1 × 500 mg, 3 days | 1 | 0 | yes | |
| Clarithromycin | 14 × 250 mg, film-coated tablets | 2 × 500 mg, 6 days | 2 | 4 | no |
| 14 × 250 mg, film-coated tablets | 2 × 500 mg, 14 days | 4 | 0 | yes | |
| 14 × 500 mg, film-coated tablets | 2 × 500 mg, 6 days | 1 | 2 | no | |
| 14 × 500 mg, film-coated tablets | 2 × 500 mg, 14 days | 2 | 0 | yes | |
| 7 × 500 mg, extended-release tablets | 1 × 500 mg, 6 days | 1 | 1 | no | |
| 7 × 500 mg, extended-release tablets | 1 × 500 mg, 14 days | 2 | 0 | yes | |
| 14 × 500 mg, extended-release tablets | 1 × 500 mg, 6 days | 1 | 8 | no | |
| 14 × 500 mg, extended-release tablets | 1 × 500 mg, 14 days | 1 | 0 | yes | |
| 7 × 500 mg, extended-release tablets | 1 × 1000 mg, 6 days | 2 | 2 | no | |
| 7 × 500 mg, extended-release tablets | 1 × 1000 mg, 14 days | 4 | 0 | yes | |
| 14 × 500 mg, extended-release tablets | 1 × 1000 mg, 6 days | 1 | 2 | no | |
| 14 × 500 mg, extended-release tablets | 1 × 1000 mg, 14 days | 2 | 0 | yes | |
| Doxycycline | 25 × 100 mg | 2 × 100 mg, 10 days | 1 | 5 | no |
| Antibiotic | Matching * |
|---|---|
| Amoxicillin | 0/6 |
| Cefuroxime axetil | 1/4 |
| Amoxicillin with clavulanic acid | 1/4 |
| Cefpodoxime | 0/4 |
| Levofloxacin | 0/4 |
| Moxifloxacin | 1/2 |
| Azithromycin | 2/2 |
| Clarithromycin | 6/12 |
| Doxycycline | 0/1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prusac, M.; Ortner Hadziabdic, M.; Rusic, D.; Modun, D. Accordance of Registered Drug Packages with Guideline-Recommended Treatment Durations for Community-Acquired Pneumonia—A New Antibiotic Stewardship Target? Antibiotics 2024, 13, 546. https://doi.org/10.3390/antibiotics13060546
Prusac M, Ortner Hadziabdic M, Rusic D, Modun D. Accordance of Registered Drug Packages with Guideline-Recommended Treatment Durations for Community-Acquired Pneumonia—A New Antibiotic Stewardship Target? Antibiotics. 2024; 13(6):546. https://doi.org/10.3390/antibiotics13060546
Chicago/Turabian StylePrusac, Martina, Maja Ortner Hadziabdic, Doris Rusic, and Darko Modun. 2024. "Accordance of Registered Drug Packages with Guideline-Recommended Treatment Durations for Community-Acquired Pneumonia—A New Antibiotic Stewardship Target?" Antibiotics 13, no. 6: 546. https://doi.org/10.3390/antibiotics13060546
APA StylePrusac, M., Ortner Hadziabdic, M., Rusic, D., & Modun, D. (2024). Accordance of Registered Drug Packages with Guideline-Recommended Treatment Durations for Community-Acquired Pneumonia—A New Antibiotic Stewardship Target? Antibiotics, 13(6), 546. https://doi.org/10.3390/antibiotics13060546







