Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics
Abstract
1. Introduction
2. Antimicrobial and Antifungal Activity of Caucasian Medicinal Plants
3. Major Groups of Antimicrobial Compounds of Caucasian Medicinal Plants
3.1. Phenolic Compounds
3.1.1. Phenolic Acids
3.1.2. Flavonoids
3.1.3. Tannins
3.2. Terpenes
3.3. Saponins
3.4. Alkaloids
3.5. Sulfur-Containing Compounds of Allium Species
| Family | Plant Species | Plant Part | Extract Type | Class of Bioactive Compound | Bioactive Compound | Susceptible Microorganisms | References |
|---|---|---|---|---|---|---|---|
| Alliaceae | A. atroviolaceum | Bulb | essential oil | Sulfur-containing compounds | methyl methyl thiomethyl disulfide (61%) dimethyl trisulfide (15%) methyl allyl disulfide (4%) | Gram-positive | [158] |
| A. sativum | aqueous extract | Simple phenolic compounds (phenolic acids) | gentisic acid 38 μg/g FW chlorogenic acid 36 μg/g FW 4-hydroxybenzoic acid 16 μg/g FW p-coumaric acid 26 μg/g FW | Gram-positive, Gram-negative, fungi | [25,156,157] | ||
| Sulfur-containing compounds | alliin 1580 38 μg/g FW allicin 280 μg/g FW ajoene diallyl sulfide diallyl disulfide | ||||||
| A. ursinum | aqueous extract | Simple phenolic compounds (phenolic acids) | chlorogenic acid 40 μg/g FW p-coumaric acid 102 μg/g FW | Gram-positive, Gram-negative, fungi | [25] | ||
| Sulfur-containing compounds | alliin 260 μg/g FW allicin 130 μg/g FW | ||||||
| Amaryllidaceae | G. transcaucasicus | Bulb | 99% methanol extract | Polyphenols (flavonoids) | naringin kaempferol rutin | Gram-positive, Gram-negative, fungi | [28] |
| Simple phenolic compounds (phenolic acids) | gallic acid 439.5 μg/g DW syringic acid 117.7 μg/g DW ferulic acid 244.2 μg/g DW | ||||||
| Bulb | 96% ethanol extract | Alkaloids Sterols Cardiac glycosides | Gram-positive, Gram-negative, fungi | [29] | |||
| Flower | 99% methanol extract | Polyphenols (flavonoids) | naringin 72.6 μg/g DW quercetin 915.5 μg/g DW apigenin 67.1 μg/g DW genistein 131.5 μg/g DW | Gram-positive, Gram-negative | [28] | ||
| Simple phenolic compounds (phenolic acids) | gallic acid 112.1 μg/g DW syringic acid 926.2 μg/g DW | ||||||
| Shoot | 99% methanol extract | Polyphenols (flavonoids) | naringin 112.9 μg/g DW quercetin 259.3 μg/g DW kaempferol 411.5 μg/g DW genistein 202 μg/g DW | Gram-positive, Gram-negative | [28] | ||
| Simple phenolic compounds (phenolic acids) | gallic acid 345.8 μg/g DW syringic acid 705.5 μg/g DW ferulic acid 412 μg/g DW | ||||||
| Apiaceae | E. caucasicum | Roots | essential oil | Fatty Acid Esters | hexyl isovalerate hexyl valerate | Gram-positive, Gram-negative | [30] |
| Terpenes | trans-pinocarvyl acetate | ||||||
| Aerial part | Fatty Acid Esters | hexyl isovalerate | [31] | ||||
| Terpenes | trans-pinocarvyl acetate caryophyllene oxide | ||||||
| Shoots | 80% methanol extract | Simple phenolic compounds (phenolic acids) | rosmarinic acid chicoric acid (Scheme 1) | [159] | |||
| Asteraceae | A. fragrans | Aerial part | essential oil | Terpenes | α-thujone β-thujone 1,8-cineole davanone d camphor cadinol verbenene ortho-oci-men | Gram-positive, Gram-negative | [32] |
| Leaves | essential oil | Terpenes | Chrysanthenon 23.8% 1,8-cineole 23.7% β-caryophyllene 9.6% p-cymene 7.7% filifolide-A 5.7% filifolone 5.7% camphor terpinene-4-ol artemisyl acetate camphene | Gram-positive, Gram-negative | [33,34] | ||
| Roots | essential oil | Terpenes | camphor 67% camphene 16.9% | Gram-positive, Gram-negative | [34] | ||
| Stem | essential oil | Terpenes | camphor 1,8-cineole borneol artedouglasia oxide a chrysanthenyl acetate | Gram-positive, Gram-negative | [33] | ||
| Flowers | essential oil | Terpenes | camphor 1,8-cineole terpinene-4-ol borneol carvacrol | Gram-positive, Gram-negative | [33] | ||
| Hypericaceae | H. alpestre | Aerial part | 99% methanol extract | Saponins Steroids Polyphenols | Flavonoids Coumarins | Gram-positive, Gram-negative, fungi | [99] |
| Juglandaceae | J. regia | Leaves | 80% methanol extract | Polyphenols (flavonoids) | - | Gram-positive, Gram-negative | [38] |
| 99% methanol extract | Polyphenols (tannins) Antioxidants | alpha-tocopherol | Gram-positive, Gram-negative | [92] | |||
| Simple phenolic compounds (phenolic acids) | caffeic acid p-coumaric acid ellagic acid malic acid chlorogenic acid | ||||||
| Bark | 99% methanol extract | Simple phenolic compounds (phenolic acids) | chlorogenic acid caffeic acid ferulic acid sinapic acid gallic acid ellagic acid vanillic acid | Gram-positive, Gram-negative, fungi | [41] | ||
| Green husks | 99% methanol extract | Simple phenolic compounds (phenolic acids) | coumaric acid ellagic acid chlorogenic acid | Gram-positive, Gram-negative, fungi | [41] | ||
| P. fraxinifolia | Stem | essential oil | Terpenes Fatty Acids | 2,4-heptadienal hexanol 2-pyrrolidinone menthone menthol thymol vinylguajacol hexadecanoic acid | Gram-positive, Gram-negative, fungi | [42] | |
| Lamiaceae | C. nepeta | Aerial part | essential oil | Terpenes | piperitenone oxide 47.8% limonene 18.6% piperitone oxide 13.6% 6-hydroxycarvotanacetone 5.1% | Gram-positive, Gram-negative, fungi | [43,44] |
| M. pulegium | Flowering aerial part | essential oil | Terpenes | piperitone 38% piperitenone 33% α-terpineol 4.7% 1,8-cineole menthone 4% borneol 3% pulegone 0.6% | Gram-positive, Gram-negative, fungi | [46,47] | |
| T. caucasicus | Aerial part | essential oil | Terpenes | 1,8-cineol 21.5% thymol 12.6% β-fenchyl alcohol 8.7% nerolidol 7.8% terpinolene 7.2% α-pinene 7% myrcene 6.8% | Gram-positive, Gram-negative, fungi | [48] | |
| Lauraceae | L. nobilis | Leaves | essential oil | Simple phenolic compounds (phenolic acids) | chlorogenic acid 48.1 μg/g DW caffeic acid 789.3 μg/g DW p-coumaric acid 375. 9 μg/g DW sinapic acid 1513.9 μg/g DW ferulic acid 70.4 μg/g DW cinnamic acid 513.4 μg/g DW | Gram-positive, Gram-negative, fungi | [49,50] |
| Simple phenolic compounds (phenolic acids) | protocatechuic acid 68.6 μg/g DW salicylic acid 29. 4 μg/g DW syringic acid 789.1 μg/g DW | ||||||
| Polyphenols (flavonoids) | myricetin 47.2 μg/g DW quercetin 44.9 μg/g DW kaempferol 688.1 μg/g DW luteolin 839.1 μg/g DW apigenin 262.7 μg/g DW hesperetin 31.2 μg/g DW | ||||||
| Terpenes | 1,8-cineole 31.9% a-terpinyl acetate 5.9% β-pinene 2.5% sabinene 8.8% β-linalool 4.9% piperitenone isomenthone pulegone | [50,52,160] | |||||
| Malvaceae | A. rosea | Whole plant | 98% ethyl acetate extract | Saponins Phenolic compounds (tannins and phlobtannins) Terpenoids Alkaloids Cardiac glycosides | - | Gram-positive, Gram-negative | [53] |
| Polygonaceae | R. obtusifolius | Seeds | 99% methanol extract | Saponins Terpenoids Phenolic compounds Coumarins | - | Gram-positive, Gram-negative, fungi | [99] |
| 99.8% acetone extract | Polyphenols (tannins and flavonoids) | - | |||||
| Primulacea | C. coum | Aerial part | 99% methanol extract | Proteins Phenolic compounds Saponins Cardiac glycosides Steroids | - | Gram-positive, fungi | [55] |
| P. macrocalyx | Whole plant | 99% methanol extract | Polyphenols (flavonoids) | 3′-methoxyflavone 2′-methoxyflavone 2′,5′-dimethoxyflavone 2′-methoxy-5′-hydroxyflavone 3′-hydroxy-4′,5′-dimethoxyflavone 5,6,2′,6′-tetramethoxyflavone 5,6,2′,3′,6′-pentamethoxyflavone 3′-hydroxyflavone 2′-hydroxyflavone 5,6,8,2′,6′-pentamethoxyflavone 5,6,2′-trimethoxyflavone | Fungi | [56] | |
| Rosaceae | A. eupatoria | Whole plant | 99% methanol extract | Saponins Steroids Polyphenols (tannins) | luteolin apigenin | Gram-positive, Gram-negative, fungi | [99] |
| 99.8% acetone extract | Steroids Phenolic compounds | - | |||||
| F. ulmaria | Middle leaves | 60% ethanol extract | Simple phenolic compounds (phenolic acids Polyphenols (flavonoids) | gallic acid 0.8 mg/g extract caftaric acid 0.6 mg/g extract chlorogenic acid 1.3 mg/g extract p-coumaric acid 0.2 mg/g extract catechin 4.1 mg/g extract rutin 4.8 mg/g extract isoquercitrin 2.6 mg/g extract ellagic acid 0.4 mg/g extract | Gram-positive, Gram-negative | [60] | |
| Middle stem | 60% ethanol extract | Simple phenolic compounds (phenolic acids) Polyphenols (flavonoids) | gallic acid 1.3 mg/g extract caftaric acid 0.05 mg/g extract chlorogenic acid 0.2 mg/g extract ellagic acid 0.4 mg/g extract isoquercitrin 0.07 mg/g extract | Gram-positive, Gram-negative | [60] | ||
| Flowers | 60% ethanol extract | Simple phenolic compounds (phenolic acids) Polyphenols (flavonoids) | gallic acid 5.8 mg/g extract caftaric acid 2.9 mg/g extract chlorogenic acid 0.3 mg/g extract caffeic acid 0.1 mg/g extract p-coumaric acid 0.04 mg/g extract ellagic acid 5.8 mg/g extract rutin 4.2 mg/g extract isoquercitrin 2.4 mg/g extract spiraeoside 20.4 mg/g extract cymaroside 0.09 mg/g extract | Gram-positive, Gram-negative | [60] | ||
| Fruits | 60% ethanol extract | Simple phenolic compounds (phenolic acids) Polyphenols (flavonoids) | gallic acid 4.3 mg/g extract caftaric acid 1.5 mg/g extract chlorogenic acid 0.6 mg/g extract p-coumaric acid 0.7 mg/g extract caffeic acid 0.07 mg/g extract ellagic acid 3.4 mg/g extract rutin 2.1 mg/g extract spiaeoside 2.4 mg/g extract isoquercitrin 0.5 mg/g extract | Gram-positive, Gram-negative | [60] | ||
| Aerial part | 99% methanol extract | Simple phenolic compounds (phenolic acids) Polyphenols (flavonoids) | gallic acid 7.02 mg/g extract ellagic acid 8.9 mg/g extract rutin 6.2 mg/g extract quercetin 15. 5 mg/g extract catechin 11.3 mg/g extract | Gram-positive, Gram-negative, fungi | [61] | ||
| Roots | 60% ethanol extract | Simple phenolic compounds (phenolic acids) Polyphenols (flavonoids) | gallic acid 0.1 mg/g extract salicylic acid 0.6 mg/g extract ellagic acid 1.2 mg/g extract catechin 8.0 mg/g extract rutin 0.7 mg/g extract isoquercitrin 0.05 mg/g extract | Gram-positive, Gram-negative, fungi | [60] | ||
| Roots | 99% methanol extract | Polyphenols (flavonoids) | catechin 17.2 mg/g extract epicatechin 3.1 mg/g extract | Gram-positive, Gram-negative, fungi | [61] | ||
| R. canina | Fruits | hexane/acetone/ethanol (2:1:1), and 0.05% (w/v) butylated hydroxytoluene extract | Carotenes | carotene lycopene | Gram-positive, Gram-negative, fungi | [63] | |
| Acetone/water (80:20 v/v) extract | Simple phenolic compounds (phenolic acids) Polyphenols (flavonoids) | vanilic acid 260 μg/kg DW cafeic acid 2 μg/kg DW syringic acid 110 μg/kg DW gallic 298 μg/kg DW ellagic acid 80 μg/kg DW procatechuic acid 210 μg/kg DW myricetin 5.4 μg/kg DW rutin 22 μg/kg DW catechin 11.9 μg/kg DW quercetin 1.5 μg/kg DW | [100] | ||||
| S. officinalis | Aerial part | 99% methanol extract | Phenolic compounds (tannins, flavonoids and coumarins) Steroids Glycosides Quinones | - | Gram-positive, Gram-negative, fungi | [99] | |
| petroleum ether extract, followed by extraction with 80% methanol | Simple phenolic compounds (phenolic acids) | caffeic acid p-coumaric acid syringic acid vannilic acid ferulic acid | [89] | ||||
| Roots | 70% ethanol extract | Polyphenols (tannins) | 2,3-hexahydroxydiphenoyl-glucose 15.3 mg/g DW sanguiin H-10 derivative 9 mg/g DW punicalagin gallate 3.8 mg/g DW sanguiin H-1 7.7 mg/g DW galoyl-bis-hexahydroxydiphenyl-glucoside, isomer 2.8 mg/g DW ellagic acid 2.3 mg/g DW | Gram-positive, Gram-negative, fungi | [64] | ||
| Polyphenols (flavonoids) | c-type (epi)catechin trimer 10.5 mg/g DW b-type (epi)catechin dimer catechin 17.2 mg/g DW | ||||||
| Simple phenolic compounds (phenolic acids) | 3-caffeoylquinic acid caffeic acid-glucoside chlorogenic acid p-coumaroylquinic acid ellagic acid | ||||||
| petroleum ether extract, followed by extraction with 80% methanol | Simple phenolic compounds (phenolic acids) | gallic acid protocatechuic acid | [89] | ||||
| Leaves | 70% ethanol extract | Polyphenols (tannins) Polyphenols (flavonoids) Simple phenolic compounds (phenolic acids) | 2,3-hexahydroxydiphenoyl-glucose 6.9 mg/g DW sanguiin H-10 derivative 1.9 mg/g DW punicalagin gallate 9.9 mg/g DW sanguiin H-1 8.3 mg/g DW b-type (epi)catechin dimer 6.1 mg/g DW catechin 7.4 mg/g DW quercetin-galloyl-glucoside 2.9 mg/g DW quercetin-glucoside 18.6 mg/g DW kaempferol-glucuronide 7.6 mg/g DW 3-caffeoylquinic acid 1 mg/g DW caffeic acid-glucoside 2.4 mg/g DW chlorogenic acid 1.6 mg/g DW p-coumaroylquinic acid 2.8 mg/g DW | Gram-positive, Gram-negative | [64] | ||
| Flowers | 70% ethanol extract | Polyphenols (tannins) Polyphenols (flavonoids) Simple phenolic compounds (phenolic acids) | 2,3-hexahydroxydiphenoyl-glucose 12.6 mg/g DW sanguiin H-10 derivative 8.3 mg/g DW punicalagin gallate 11.1 mg/g DW sanguiin 19.7 mg/g DW ellagic acid pentoside 2.6 mg/g DW cyanidin-glucoside 0.5 mg/g DW b-type (epi)catechin dimer 5 mg/g DW catechin 2.3 mg/g DW 3-caffeoylquinic acid 2.1 mg/g DW caffeic acid-glucoside 5.4 mg/g DW chlorogenic acid 2.2 mg/g DW p-coumaroylquinic acid 3.1 mg/g DW | Gram-positive, Gram-negative | [64] | ||
| Sapindaceae | A. cappadocicum | Whole plant | 99% methanol extract | Alkaloids Saponins Flavone glycosides Quinones | Gram-positive, Gram-negative, fungi | [65] | |
| Zygophyllaceae | P. harmala | Whole plant | 99% methanol extract | Alkaloids Saponins Flavone glycosides | peganine harmaline | Gram-positive, Gram-negative, fungi | [66,140] |
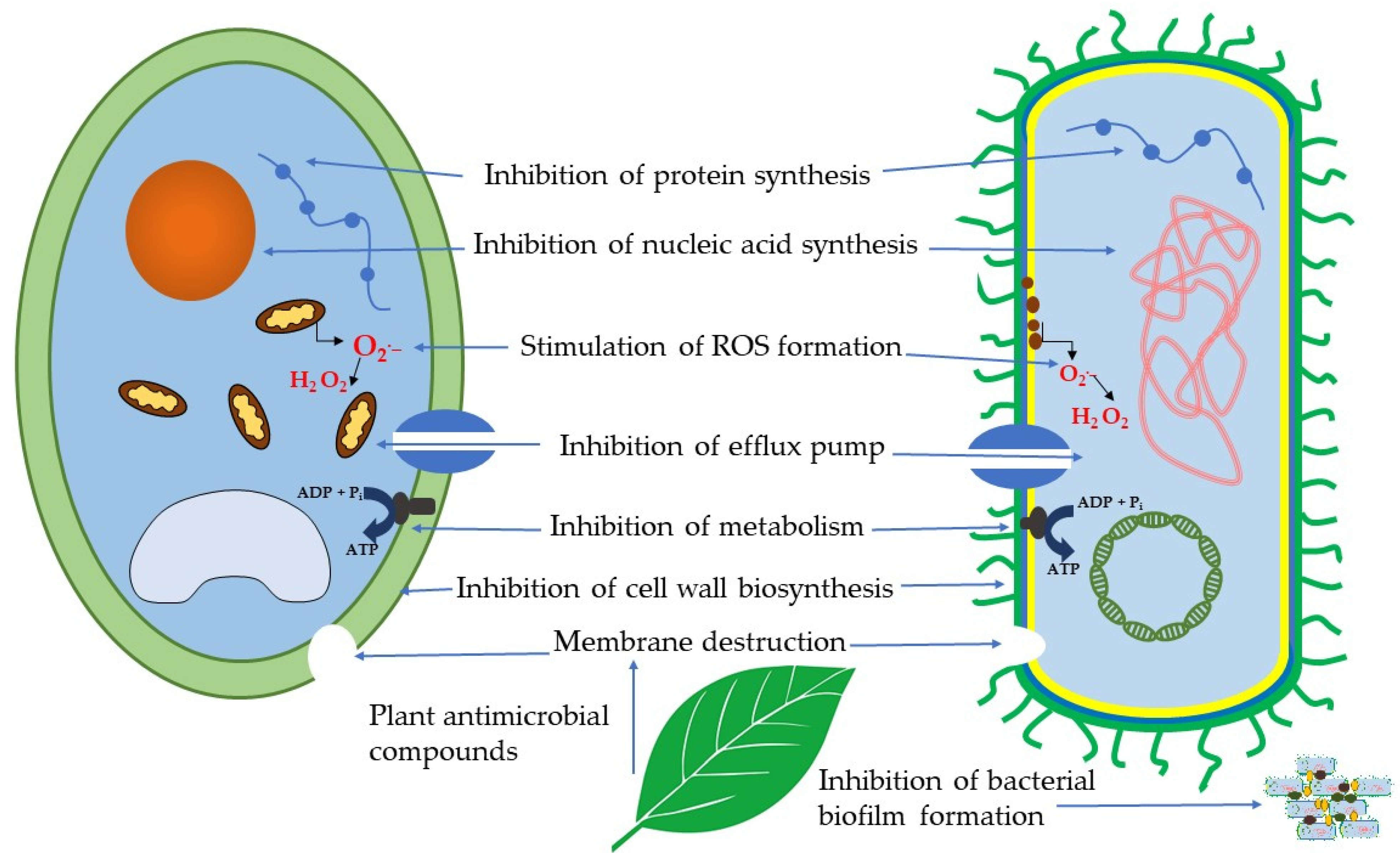
4. Mechanisms of Action of Antibacterial and Antifungal Plant Compounds
4.1. Membrane Destruction
4.2. Inhibition of Cell Wall Biosynthesis
4.3. Inhibition of Biofilm Formation
4.4. Inhibition of Nucleic Acid and Protein Synthesis
4.5. Inhibition of Metabolism
4.6. Stimulation of ROS Generation
4.7. Inhibition of Efflux Pumps
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DW | dry weight |
| FW | fresh weight |
| Bacterial species | |
| A. alternata | Alternaria alternata |
| A. baumannii | Acinetobacter baumannii |
| A. flavus | Aspergillus flavus |
| A. niger | Aspergillus niger |
| A. terreus | Aspergillus terreus |
| A. viscosus | Actinomyces viscosus |
| A. versicolor | Aspergillus versicolor |
| B. anthracis | Bacillus anthracis |
| B. cereus | Bacillus cereus |
| B. subtilis | Bacillus subtilis |
| B.thermosphacta | Brochothrix thermosphacta |
| C. albicans | Candida albicans |
| C. auris | Candida auris |
| C. difficile | Clostridium difficile |
| C. dubliniensis | Candida dubliniensis |
| C. glabrata | Candida glabrata |
| C. guilliermondii | Candida guilliermondii |
| C. krusei | Candida krusei |
| C. lunatus | Cochliobolus lunatus |
| C. neoformans | Cryptococcus neoformans |
| C. parapsilosis | Candida parapsilosis |
| C. perfringens | Clostridium perfringens |
| C. rugosa | Candida rugosa |
| C. tropicalis | Candida tropicalis |
| D.stemonitis | Doratomycesstemonitis |
| E. aerogenes | Enterobacter aerogenes |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| E. faecium | Enterococcus faecium |
| F. fujikuroi | Fusarium fujikuroi |
| F. nucleatum | Fusobacterium nucleatum |
| F. oxysporum | Fusarium oxysporum |
| F. solani | Fusarium solani |
| H. pylori | Helicobacter pylori |
| K. oxytoca | Klebsiella oxytoca |
| K. pneumoniae | Klebsiella pneumoniae |
| L. monocytogenes | Listeria monocytogenes |
| M. canis | Microsporum canis |
| M. gypseum | Microsporum gypseum |
| M. haemolytica | Mannheimia haemolytica |
| P. aeruginosa | Pseudomonas aeruginosa |
| P.canescens | Penicilliumcanescens |
| P. chrysogenum | Penicillium chrysogenum |
| P. citrinum | Penicillium citrinum |
| P.cyclopium | Penicilliumcyclopium |
| P. digitatum | Penicillium digitatum |
| P. expansum | Penicillium expansum |
| P. fastigiate | Phialophora fastigiate |
| P. fluorescens | Pseudomonas fluorescens |
| P. gingivalis | Porphyromonas gingivalis |
| P. guilliermondii | Pichia guilliermondii |
| P. italicum | Penicillium italicum |
| P. intermedia | Prevotella intermedia |
| P. mirabilis | Proteus mirabilis |
| P. multocida | Pasteurella multocida |
| P. rettgeri | Providencia rettgeri |
| P. ultimum | Pythium ultimum |
| P. vulgaris | Proteus vulgaris |
| R. solanacearum | Ralstonia solanacearum |
| R. oryzae | Rhizopus oryzae |
| S. abony | Salmonella abony |
| S. aureus | Staphylococcus aureus |
| S. cerevisiae | Saccharomyces cerevisiae |
| S. dysenteriae | Shigella dysenteriae |
| S. enterica | Salmonella enterica |
| S. enteritidis | Salmonella enteritidis |
| S. epidermidis | Staphylococcus epidermidis |
| S. flexneri | Shigella flexneri |
| S. mitis | Streptococcus mitis |
| S. mutans | Streptococcus mutans |
| S. paratyphi | Salmonella paratyphi |
| S. pneumoniae | Streptococcus pneumoniae |
| S. pyogenes | Streptococcus pyogenes |
| S. salivarius | Streptococcus salivarius |
| S. sanguinis | Streptococcus sanguinis |
| S. saprophyticus | Staphylococcus saprophyticus |
| S. typhi | Salmonella typhi |
| S. typhimurium | Salmonella typhimurium |
| T.harzianum | Trichodermaharzianum |
| T.longibrachiatum | Trichodermalongibrachiatum |
| V. cholera | Vibrio cholera |
| Plant species | |
| Agrimonia eupatoria L. | A. eupatoria |
| Acer cappadocicum Gled. | A. cappadocicum |
| Aconitum carmichaeli var. carmichaeli | A. carmichaeli |
| Alcea rosea L. | A. rosea |
| Allium atroviolaceum Hornem. Ex Steud. | A. atroviolaceum |
| Allium sativum L. | A. sativum |
| Allium ursinum L. | A. ursinum |
| Artemisia fragrans Willd. | A. fragrans |
| Carum copticum (L.) Benth. & Hook.f. ex Hiern | C. copticum |
| Clinopodium nepeta (L.) Kuntze | C. nepeta |
| Cyclamen coum Mill. | C. coum |
| Eryngium caucasicum Trautv. | E. caucasicum |
| Filipendula ulmaria (L.) Maxim. | F. ulmaria |
| Galanthus transcaucasicus Fomin | G. transcaucasicus |
| Hypericum alpestre Steven. | H. alpestre |
| Juglans regia L. | J. regia |
| Laurus nobilis L. | L. nobilis |
| Lilium monadelphum subsp. armenum (Miscz. ex Grossh.) Kudrjasch. | L. armenum |
| Mentha pulegium L. | M. pulegium |
| Peganum harmala L. | P. harmala |
| Primula macrocalyx Bunge | P. macrocalyx |
| Pterocarya fraxinifolia (Poir.) Spach | P. fraxinifolia |
| Rosa canina L. | R. canina |
| Rumex obtusifolius L. | R. obtusifolius |
| Sanguisorba officinalis L. | S. officinalis |
| Thymus caucasicus Willd. ex Benth. | T. caucasicus |
References
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Antimicrobial resistance collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the global burden of disease study 2019. Lancet Lond. Engl. 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, V.; Roviello, G.N. The potential role of vaccines in preventing antimicrobial resistance (amr): An update and future perspectives. Vaccines 2023, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Baym, M.; Stone, L.K.; Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science 2016, 351, aad3292. [Google Scholar] [CrossRef]
- Autiero, I.; Roviello, G.N. Interaction of Laurusides 1 and 2 with the 3C-like protease (Mpro) from wild-type and omicron variant of SARS-CoV-2: A Molecular Dynamics Study. Int. J. Mol. Sci. 2023, 24, 5511. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti-COVID-19 utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In Silico Investigation on the interaction of chiral phytochemicals from opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. [Google Scholar] [CrossRef]
- Baker, S.; Gilhen-Baker, M.; Roviello, G.N. The Role of nutrition and forest-bathing in the physical rehabilitation of physically inactive patients: From the molecular aspects to new nature-inspired techniques. Int. J. Environ. Res. Public. Health 2022, 20, 793. [Google Scholar] [CrossRef]
- Ricci, A.; Roviello, G.N. Exploring the Protective effect of food drugs against viral diseases: Interaction of functional food ingredients and SARS-CoV-2, influenza virus, and HSV. Life 2023, 13, 402. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Keita, K.; Darkoh, C.; Okafor, F. Secondary plant metabolites as potent drug candidates against antimicrobial-resistant pathogens. SN Appl. Sci. 2022, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.R.; Rangarajan, R.; Sarada, D.V.L.; Kumar, C.S. Evaluation of antibacterial activity and phytochemical screening of Wrightia tinctoria L. Pharmacogn. J. 2010, 2, 19–22. [Google Scholar] [CrossRef]
- Manukyan, A.; Lumlerdkij, N.; Heinrich, M. Caucasian endemic medicinal and nutraceutical plants: In-vitro antioxidant and cytotoxic activities and bioactive compounds. J. Pharm. Pharmacol. 2019, 71, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.F. Plants and humans in the near east and the Caucasus: Ancient and traditional uses of plants as food and medicine, a diachronic ethnobotanical review. Ethnobiol. Lett. 2014, 5, 22–23. [Google Scholar] [CrossRef]
- Price, M.F. Cooperation in the European Mountains. 2: The Caucasus; Environmental research series; IUCN: Gland, Switzerland, 2000; ISBN 978-2-8317-0534-7. [Google Scholar]
- Takhtadzhian, A.L.; Crovello, T.J.; Cronquist, A. Floristic Regions of the World; University of California Press: Berkeley, CA, USA, 1986; ISBN 978-0-520-04027-4. [Google Scholar]
- Grossheim, A.A. Analysis of the Flora of the Caucasus: Proceedings of the Botanical Institute of Azerbaijan; FAN USSR: Baku, Azerbaijan, 1936. [Google Scholar]
- Takhtajan, A.L. Konspekt Florii Kavkaz; Sankt Peterburg University Press: Sankt Peterburg, Russia, 2003; Volume 1, 204p. [Google Scholar]
- Tarkhnishvili, D. Historical Biogeography of the Caucasus; Nova Publ.: New York, NY, USA, 2014; 234p, ISBN 978-1-63321-910-6. [Google Scholar]
- Plant Diversity in the Central Great Caucasus: A Quantitative Assessment; Nakhutsrishvili, G.S., Abdaladze, O., Batsatsashvili, K., Spehn, E.M., Körner, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; 170p, ISBN 978-3-319-55777-9. [Google Scholar]
- Mamedov, N.; Mehdiyeva, N.P.; Craker Lyle, E. Medicinal plants used in traditional medicine of the Caucasus and North America. J. Med. Act. Plants 2015, 4, 42–66. [Google Scholar] [CrossRef]
- Eynanlou Yaghmerlou, S.; Malekzadeh, H.; Ghaznavi, D.; Zeighami, H.; Tavakolizadeh, M. Anti-bacterial effects of Allium atroviolaceum hydroalcoholic extract on oral bacteria of Streptococcus viridans groups. Jundishapur J. Nat. Pharm. Prod. 2024, 19. [Google Scholar] [CrossRef]
- Chehregani, A.; Azimishad, F.; Alizade, H.H. Study on antibacterial effect of some Allium species from Hamedan-Iran. IJAB 2007, 9, 873–876. [Google Scholar]
- Barbu, I.A.; Ciorîță, A.; Carpa, R.; Moț, A.C.; Butiuc-Keul, A.; Pârvu, M. Phytochemical characterization and antimicrobial activity of several Allium extracts. Molecules 2023, 28, 3980. [Google Scholar] [CrossRef]
- Chowdhury, A.K.; Ahsan, M.; Islam, S.N.; Ahmed, Z.U. Efficacy of aqueous extract of garlic & allicin in experimental shigellosis in rabbits. Indian J. Med. Res. 1991, 93, 33–36. [Google Scholar]
- Saha, S.K.; Saha, S.; Hossain, M.A.; Paul, S.K. In vitro assessment of antibacterial eEffect of garlic (Allium Sativum) extracts on Pseudomonas aeruginosa. Mymensingh Med. J. MMJ 2015, 24, 222–232. [Google Scholar] [PubMed]
- Karimi, E.; Mehrabanjoubani, P.; Homayouni-Tabrizi, M.; Abdolzadeh, A.; Soltani, M. Phytochemical evaluation, antioxidant properties and antibacterial activity of Iranian medicinal herb Galanthus transcaucasicus Fomin. J. Food Meas. Charact. 2018, 12, 433–440. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Yousefbeyk, F.; Amin, G.; Salehi Sormaghi, M.; Azadi, B.; Samadi, N.; Amini Moghadam Farouj, N.; Amin, M. Investigation on pharmacological and antimicrobial activities of Galanthus transcaucasicus Fomin growing in Iran. Planta Med. 2010, 76, P474. [Google Scholar] [CrossRef]
- Hamedi, A.; Pasdaran, A.; Pasdaran, A. Antimicrobial activity and analysis of the essential oils of selected endemic edible Apiaceae plants root from Caspian Hyrcanian region (North of Iran). Pharm. Sci. 2019, 25, 138–144. [Google Scholar] [CrossRef]
- Mohamadipour, S.; Hatamzadeh, A.; Bakhshi, D.; Pasdaran, A. Antimicrobial activities of Caucalis platycarpos L. and Eryngium caucasicum Trautv. essential oils. Aust. J. Crop Sci. 2018, 12, 1357–1362. [Google Scholar] [CrossRef]
- Younessi-Hamzekhanlu, M.; Sanjari, S.; Dejahang, A.; Karkaj, E.S.; Nojadeh, M.S.; Gönenç, T.M.; Ozturk, M. Evaluation of essential oil from different Artemisia fragrans Willd. populations: Chemical composition, antioxidant, and antibacterial activity. J. Essent. Oil Bear. Plants 2020, 23, 1218–1236. [Google Scholar] [CrossRef]
- Aminkhani, A.; Sharifi, S.; Hosseinzadeh, P. Chemical constituent, antimicrobial activity, and synergistic effect of the stem, leaf, and flower essential oil of the Artemisia fragrans Willd. from Khoy. Chem. Biodivers. 2021, 18, e2100241. [Google Scholar] [CrossRef] [PubMed]
- Shafaghat, A.; Noormohammadi, Y.; Zaifizadeh, M. Composition and antibacterial activity of essential oils of Artemisia fragrans Willd. leaves and roots from Iran. Nat. Prod. Commun. 2009, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Ginovyan, M.; Trchounian, A. Novel approach to combat antibiotic resistance: Evaluation of some Armenian herb crude extracts for their antibiotic modulatory and antiviral properties. J. Appl. Microbiol. 2019, 127, 472–480. [Google Scholar] [CrossRef]
- Ginovyan, M.; Petrosyan, M.; Trchounian, A. Antimicrobial activity of some plant materials used in Armenian traditional medicine. BMC Complement. Altern. Med. 2017, 17, 50. [Google Scholar] [CrossRef]
- Fathi, H.; Ebrahimzadeh, M.; Ahanjan, M. Comparison of the antimicrobial activity of Caucasian wingnut leaf extract (Pterocarya fraxinifolia) and walnut (Juglans regia L.) plants. Acta Biol. Indica 2015, 4, 67–74. [Google Scholar]
- Chaleshtori, R.S.; Chaleshtori, F.S.; Rafieian, M. Biological characterization of Iranian walnut (Juglans regia) leaves. Turk. J. Biol. 2011, 35, 635–639. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Hajlaoui, H.; Valentin, E.; Bakhrouf, A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Moori Bakhtiari, N.; Khalafi, E. Antibacterial activity of the hydro-alcoholic extract of Juglans regia L. stem bark on human bacterial infection. Int. Arch. Health Sci. 2015, 2, 139–143. [Google Scholar]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Azirian, M.; Hadjiakhoondi, A.; Vatankhah, E.; Hosseinizadeh, S.; Tavakoli, S.; Akhbari, M. Variation in chemical components and biological activity of Pterocarya fraxinifolia Lam. stems at different developmental stages. Res. J. Pharmacogn. 2017, 4, 41–50. [Google Scholar]
- Debbabi, H.; El Mokni, R.; Chaieb, I.; Nardoni, S.; Maggi, F.; Caprioli, G.; Hammami, S. Chemical composition, antifungal and insecticidal activities of the essential oils from Tunisian Clinopodium nepeta subsp. nepeta and Clinopodium nepeta subsp. glandulosum. Molecules 2020, 25, 2137. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, G.; Yilmaz, G.; Ekşi, G.; DemiRci, B. Chemical composition and antibacterial activity of Clinopodium nepeta subsp. glandulosum (Req.) Govaerts essential oil. Nat. Volatiles Essent. Oils 2021, 8, 75–80. [Google Scholar] [CrossRef]
- Atakishiyeva, Y.; Ghasemi, M. Investigation of the antibacterial effect of native Peganum harmala, Mentha pulegium and Alcea rosea hydro-alcoholic extracts on antibiotic resistant Streptococcus pneumoniae and Klebsiella pneumonia isolated from Baku, Azerbaijan. IEM 2016, 2, 12–14. [Google Scholar]
- Mahboubi, M.; Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 2008, 119, 325–327. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F. Screening of the potential bioactivities of pennyroyal (Mentha pulegium L.) essential oil. Antibiotics 2021, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Asbaghian, S.; Shafaghat, A.; Zarea, K.; Kasimov, F.; Salimi, F. Comparison of volatile constituents, and antioxidant and antibacterial activities of the essential oils of Thymus caucasicus, T. kotschyanus and T. vulgaris. Nat. Prod. Commun. 2011, 6, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D. Comparative study on the chemical composition of laurel (Laurus nobilis L.) leaves from Greece and Georgia and the antibacterial activity of their essential oil. Heliyon 2020, 6, e05491. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Nazzaro, F.; Souza, L.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Sakran, K.A.; Raharjo, D.; Mertaniasih, N.M. Antimicrobial activities of Laurus nobilis leaves ethanol extract on Staphylococcus aureus, Salmonellae typhi, and Escherichia coli. Indones. J. Trop. Infect. Dis. 2021, 9, 119. [Google Scholar] [CrossRef]
- Rizwana, H.; Kubaisi, N.A.; Al-Meghailaith, N.N.; Moubayed, N.M.; Albasher, G. Evaluation of chemical composition, antibacterial, antifungal, and cytotoxic activity of Laurus nobilis L. grown in Saudi Arabia. J. Pure Appl. Microbiol. 2019, 13, 2073–2085. [Google Scholar] [CrossRef]
- Nazir, S.; Ahmad, M.K.; Ali, F.; Ganie, S.A.; Nazir, Z.U. Phytochemical analysis and antibacterial potential of Onosma hispidium and Alcea rosea. Biomedicine 2022, 42, 47–52. [Google Scholar] [CrossRef]
- Özcan, F.; Semerci, A.B.; Tunç, K. A study on antimicrobial and antioxidant activities of Cyclamen coum, Colchicum turcicum and Colchicum bornmuelleri species. Curr. Pers. MAPs 2020, 3, 121–127. [Google Scholar]
- Amin Jaradat, N.; Al-Masri, M.; Hussen, F.; Zaid, A.N.; Ali, I.; Tammam, A.; Mostafa Odeh, D.; Hussein Shakarneh, O.; Rajabi, A. Preliminary Phytochemical and biological screening of Cyclamen coum a member of Palestinian flora. Pharm. Sci. 2017, 23, 231–237. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Li, C.; Khutsishvili, M.; Fayvush, G.; Atha, D.; Zhang, Y.; Borris, R.P. Unusual flavones from Primula macrocalyx as inhibitors of OAT1 and OAT3 and as antifungal agents against Candida rugosa. Sci. Rep. 2019, 9, 9230. [Google Scholar] [CrossRef]
- Copland, A.; Nahar, L.; Tomlinson, C.T.M.; Hamilton, V.; Middleton, M.; Kumarasamy, Y.; Sarker, S.D. Antibacterial and free radical scavenging activity of the seeds of Agrimonia eupatoria. Fitoterapia 2003, 74, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Muruzović, M.Ž.; Mladenović, K.G.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. Extracts of Agrimonia eupatoria L. as sources of biologically active compounds and evaluation of their antioxidant, antimicrobial, and antibiofilm activities. J. Food Drug Anal. 2016, 24, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Watkins, F.; Pendry, B.; Sanchez-Medina, A.; Corcoran, O. Antimicrobial assays of three native British plants used in Anglo-Saxon medicine for wound healing formulations in 10th century England. J. Ethnopharmacol. 2012, 144, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Savina, T.; Lisun, V.; Feduraev, P.; Skrypnik, L. Variation in phenolic compounds, antioxidant and antibacterial activities of extracts from different plant organs of meadowsweet (Filipendula ulmaria (L.) Maxim.). Molecules 2023, 28, 3512. [Google Scholar] [CrossRef] [PubMed]
- Katanić, J.; Boroja, T.; Stanković, N.; Mihailović, V.; Mladenović, M.; Kreft, S.; Vrvić, M.M. Bioactivity, stability and phenolic characterization of Filipendula ulmaria (L.) Maxim. Food Funct. 2015, 6, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.Ł.; Kačániová, M. Characterization of Rosa canina fruits collected in urban areas of Slovakia. Genome size, iPBS profiles and antioxidant and antimicrobial activities. Molecules 2020, 25, 1888. [Google Scholar] [CrossRef] [PubMed]
- Miljković, V.M.; Nikolić, L.; Mrmošanin, J.; Gajić, I.; Mihajilov-Krstev, T.; Zvezdanović, J.; Miljković, M. Chemical profile and antioxidant and antimicrobial activity of Rosa canina L. dried fruit commercially available in Serbia. Int. J. Mol. Sci. 2024, 25, 2518. [Google Scholar] [CrossRef] [PubMed]
- Tocai (Moţoc), A.-C.; Ranga, F.; Teodorescu, A.G.; Pallag, A.; Vlad, A.M.; Bandici, L.; Vicas, S.I. Evaluation of polyphenolic composition and antimicrobial properties of Sanguisorba officinalis L. and Sanguisorba minor Scop. Plants 2022, 11, 3561. [Google Scholar] [CrossRef] [PubMed]
- Kausar, F.; Farooqi, M.-A.; Farooqi, H.-M.-U.; Salih, A.-R.-C.; Khalil, A.-A.-K.; Kang, C.-W.; Mahmoud, M.H.; Batiha, G.-E.-S.; Choi, K.-H.; Mumtaz, A.-S. Phytochemical investigation, antimicrobial, antioxidant and anticancer activities of Acer cappadocicum Gled. Life Basel Switz. 2021, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Fazli Bazaz, S.; Haririzadeh, G.; Abootorabi, B.Z.; Mohamadi, A.M.; Khashyarmanesh, Z. Chemical composition and antibacterial properties of Peganum harmala L. Avicenna J. Phytomedicine 2019, 9, 530. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA group preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- The Angiosperm Phylogeny Group. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- Oteng Mintah, S.; Asafo-Agyei, T.; Archer, M.-A.; Atta-Adjei Junior, P.; Boamah, D.; Kumadoh, D.; Appiah, A.; Ocloo, A.; Duah Boakye, Y.; Agyare, C. Medicinal plants for treatment of prevalent diseases. In Pharmacognosy—Medicinal Plants; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-83880-610-1. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Methods for the determination of susceptibility of bacteria to antimicrobial agents. Terminology. Clin. Microbiol. Infect. 1998, 4, 291–296. [CrossRef]
- Bhargav, H.S.; Shastri, S.D.; Poornav, S.P.; Darshan, K.M.; Nayak, M.M. Measurement of the zone of inhibition of an antibiotic. In Proceedings of the 2016 6th International Conference on Advanced Computing (IACC), Bhimavaram, India, 27–28 February 2016; pp. 409–414. [Google Scholar]
- Pirtskhalava, M.; Mittova, V.; Tsetskhladze, Z.R.; Palumbo, R.; Pastore, R.; Roviello, G.N. Georgian medicinal plants as rich natural sources of antioxidant derivatives: A review on the current knowledge and future perspectives. Curr. Med. Chem. 2024, 31. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE Pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Kumar Mishra, K.; Deep Kaur, C.; Kumar Sahu, A.; Panik, R.; Kashyap, P.; Prasad Mishra, S.; Dutta, S. Medicinal plants having antifungal properties. In Medicinal Plants—Use in Prevention and Treatment of Diseases; Abdul Rasool Hassan, B., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-887-7. [Google Scholar]
- Khalil, N.A.; ALFaris, N.A.; ALTamimi, J.Z.; Mohamed Ahmed, I.A. Anti-inflammatory effects of bay laurel (Laurus nobilis L.) towards the Gut microbiome in dextran sodium sulfate induced colitis animal models. Food Sci. Nutr. 2024, 12, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. 2020, 264, 108940. [Google Scholar] [CrossRef]
- Izadi, M.; Moosawi Jorf, S.A.; Nikkhah, M.; Moradi, S. Antifungal activity of hydrocolloid nano encapsulated Carum copticum essential oil and Peganum harmala extract on the pathogenic fungi Alternaria alternata. Physiol. Mol. Plant Pathol. 2021, 116, 101714. [Google Scholar] [CrossRef]
- Khezri, K.; Farahpour, M.R.; Mounesi Rad, S. Efficacy of Mentha pulegium essential oil encapsulated into nanostructured lipid carriers as an in vitro antibacterial and infected wound healing agent. Colloids Surf. Physicochem. Eng. Asp. 2020, 589, 124414. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Bjarnsholt, T.; Skindersoe, M.E.; Hentzer, M.; Kristoffersen, P.; Köte, M.; Nielsen, J.; Eberl, L.; Givskov, M. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 2005, 187, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Mamari, H.H. Phenolic compounds: Classification, chemistry, and updated techniques of analysis and synthesis. In Biochemistry; Badria, F.A., Ed.; IntechOpen: London, UK, 2022; Volume 26, ISBN 978-1-83969-346-5. [Google Scholar]
- Manso, T.; Lores, M.; De Miguel, T. Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial activity and action approach of the olive oil polyphenol extract against Listeria monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E. Mutual influence of polyphenols and Lactobacillus spp. bacteria in food: A Review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Z.; Lam, K.-L.; Zeng, S.; Tan, B.K.; Hu, J. Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Biernasiuk, A.; Wozniak, M.; Bogucka-Kocka, A. Determination of free and bounded phenolic acids in the rhizomes and herb of Sanguisorba officinalis L. Curr. Issues Pharm. Med. Sci. 2015, 28, 254–256. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Croitoru, A.; Ficai, D.; Craciun, L.; Ficai, A.; Andronescu, E. Evaluation and exploitation of bioactive compounds of walnut, Juglans regia. Curr. Pharm. Des. 2019, 25, 119–131. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. P-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef]
- Ma, C.-M.; Abe, T.; Komiyama, T.; Wang, W.; Hattori, M.; Daneshtalab, M. Synthesis, anti-fungal and 1,3-β-d-glucan synthase inhibitory activities of caffeic and quinic acid derivatives. Bioorg. Med. Chem. 2010, 18, 7009–7014. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.R.; Ema, T.I.; Siddiquee, M.F.-R.; Shahriar, A.; Ahmed, H.; Mosfeq-Ul-Hasan, M.; Rahman, N.; Islam, R.; Uddin, M.R.; Mizan, M.F.R. Natural flavonols: Actions, mechanisms, and potential therapeutic utility for various diseases. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 47. [Google Scholar] [CrossRef]
- Ginovyan, M.; Ayvazyan, A.; Nikoyan, A.; Tumanyan, L.; Trchounian, A. Phytochemical screening and detection of antibacterial components from crude extracts of some Armenian herbs using TLC-bioautographic technique. Curr. Microbiol. 2020, 77, 1223–1232. [Google Scholar] [CrossRef]
- Jiménez, S.; Gascón, S.; Luquin, A.; Laguna, M.; Ancin-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Rosa canina extracts have antiproliferative and antioxidant effects on Caco-2 human colon cancer. PLoS ONE 2016, 11, e0159136. [Google Scholar] [CrossRef]
- Ignasimuthu, K.; Prakash, R.; Murthy, P.S.; Subban, N. Enhanced bioaccessibility of green tea polyphenols and lipophilic activity of EGCG octaacetate on Gram-negative bacteria. LWT 2019, 105, 103–109. [Google Scholar] [CrossRef]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure–activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Li, H.; Qiu, Z.; Yu, Y. Comparative assessment of the antibacterial efficacies and mechanisms of different tea extracts. Foods 2022, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- McGillick, B.E.; Kumaran, D.; Vieni, C.; Swaminathan, S. β-hydroxyacyl-acyl carrier protein dehydratase (FabZ) from Francisella tularensis and Yersinia pestis: Structure determination, enzymatic characterization, and cross-inhibition studies. Biochemistry 2016, 55, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Flavones inhibit the hexameric replicative helicase RepA. Nucleic Acids Res. 2001, 29, 5058–5066. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as inhibitors of antibiotic resistant bacteria—Mechanisms underlying rutin interference with bacterial virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-P.; He, Y.-H.; Zhang, S.-Y.; Shi, Y.-P. Antibacterial activity and action mechanism of flavonoids against phytopathogenic bacteria. Pestic. Biochem. Physiol. 2022, 188, 105221. [Google Scholar] [CrossRef]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Silymarin exerts antifungal effects via membrane-targeted mode of action by increasing permeability and inducing oxidative stress. Biochim. Biophys. Acta BBA Biomembr. 2017, 1859, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019, 9, 1683. [Google Scholar] [CrossRef] [PubMed]
- Canonico, B.; Candiracci, M.; Citterio, B.; Curci, R.; Squarzoni, S.; Mazzoni, A.; Papa, S.; Piatti, E. Honey flavonoids inhibit Candida albicans morphogenesis by affecting DNA behavior and mitochondrial function. Future Microbiol. 2014, 9, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Yiğit, D.; Yiğit, N.; Mavi, A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) Kernels. Braz. J. Med. Biol. Res. 2009, 42, 346–352. [Google Scholar] [CrossRef]
- Serpa, R.; França, E.J.G.; Furlaneto-Maia, L.; Andrade, C.G.T.J.; Diniz, A.; Furlaneto, M.C. In vitro antifungal activity of the flavonoid baicalein against Candida species. J. Med. Microbiol. 2012, 61, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, J.A.; Steynberg, J.P.; Brandt, E.V.; Ferreira, D.; Roux, D.G. Phlobatannins, a novel class of ring-isomerized condensed tannins. J. Chem. Soc. Chem. Commun. 1985, 23, 1678. [Google Scholar] [CrossRef]
- Hill, G.D. Plant antinutritional factors|Characteristics. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4578–4587. ISBN 978-0-12-227055-0. [Google Scholar]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Boakye, Y.D. Anti-infective properties and time-kill kinetics of Phyllanthus muellerianus and its major constituent, geraniin. Med. Chem. 2016, 6, 95–104. [Google Scholar] [CrossRef]
- Trentin, D.S.; Silva, D.B.; Amaral, M.W.; Zimmer, K.R.; Silva, M.V.; Lopes, N.P.; Giordani, R.B.; Macedo, A.J. Tannins possessing bacteriostatic effect impair Pseudomonas aeruginosa adhesion and biofilm formation. PLoS ONE 2013, 8, e66257. [Google Scholar] [CrossRef]
- Olchowik-Grabarek, E.; Sękowski, S.; Kwiatek, A.; Płaczkiewicz, J.; Abdulladjanova, N.; Shlyonsky, V.; Swiecicka, I.; Zamaraeva, M. The structural changes in the membranes of Staphylococcus aureus caused by hydrolysable tannins witness their antibacterial activity. Membranes 2022, 12, 1124. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, C.; Song, L.; Li, T.; Cui, S.; Zhang, L.; Jia, Y. Antimicrobial activity and mechanism of larch bark procyanidins against Staphylococcus aureus. Acta Biochim. Biophys. Sin. 2017, 49, 1058–1066. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Latté, K.P.; Kolodziej, H. Antifungal effects of hydrolysable tannins and related compounds on dermatophytes, mould fungi and yeasts. Z. Für Naturforschung C 2000, 55, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C. Production and engineering of terpenoids in plant cell culture. Nat. Chem. Biol. 2007, 3, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Mahizan, N.A.; Yang, S.-K.; Moo, C.-L.; Song, A.A.-L.; Chong, C.-M.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.E.; Lai, K.-S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Fereydouni, N.; Moradi, H.; Karimivaselabadi, A.; Zarenezhad, E.; Osanloo, M. Nanoformulated herbal compounds: Enhanced antibacterial efficacy of camphor and thymol-loaded nanogels. BMC Complement. Med. Ther. 2024, 24, 138. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, H.; Haraguchi, A.; Mitarai, H.; Yuda, A.; Wada, H.; Shuxin, W.; Ziqing, R.; Weihao, S.; Wada, N. In vitro evaluation of the antimicrobial properties of terpinen-4-ol on apical periodontitis-associated bacteria. J. Infect. Chemother. 2024, 30, 306–314. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Kimbaris, A.C.; Plessas, S.; Mantzourani, I.; Voidarou, C.; Pagonopoulou, O.; Tsigalou, C.; Fournomiti, M.; Bontsidis, C.; Stavropoulou, E.; et al. Combined action of piperitenone epoxide and antibiotics against clinical isolates of Staphylococcus aureus and Escherichia coli. Front. Microbiol. 2019, 10, 2607. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, L.D.S.; Dias, A.D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Akseer, R.; Lim, S.-H.E.; Lai, K.-S. Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PLoS ONE 2019, 14, e0214326. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Karima, G.; Khan, M.Z.; Shin, J.H.; Kim, J.D. Therapeutic Effects of saponins for the prevention and treatment of cancer by ameliorating inflammation and angiogenesis and inducing antioxidant and apoptotic effects in human cells. Int. J. Mol. Sci. 2022, 23, 10665. [Google Scholar] [CrossRef] [PubMed]
- Boysen, R.I.; Hearn, M.T.W. High Performance liquid chromatographic separation methods. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; pp. 5–49. ISBN 978-0-08-045382-8. [Google Scholar]
- Gökkaya, İ.; Renda, G.; Subaş, T.; Özgen, U. Phytochemical, pharmacological, and toxicological studies on Peganum harmala L.: An overview of the last decade. Clin. Exp. Health Sci. 2023, 13, 664–678. [Google Scholar] [CrossRef]
- Sen, S.; Makkar, H.P.S.; Becker, K. Alfalfa saponins and their implication in animal nutrition. J. Agric. Food Chem. 1998, 46, 131–140. [Google Scholar] [CrossRef]
- Bachran, C.; Sutherland, M.; Heisler, I.; Hebestreit, P.; Melzig, M.F.; Fuchs, H. The saponin-mediated enhanced uptake of targeted saporin-based drugs is strongly dependent on the saponin structure. Exp. Biol. Med. 2006, 231, 412–420. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant alkaloids: Structures and bioactive properties. In Plant-Derived Bioactives; Swamy, M.K., Ed.; Springer: Singapore, 2020; pp. 85–117. ISBN 9789811523601. [Google Scholar]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Beuria, T.K.; Santra, M.K.; Panda, D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry 2005, 44, 16584–16593. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Liu, H.; Peng, Y.; Xiao, P. In Silico target fishing and pharmacological profiling for the isoquinoline alkaloids of Macleaya cordata (Bo Luo Hui). Chin. Med. 2015, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Mabhiza, D.; Chitemerere, T.; Mukanganyama, S. Antibacterial properties of alkaloid extracts from Callistemon citrinus and Vernonia adoensis against Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Med. Chem. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-F.; Le, Y.-J.; Sun, X.; Yang, X.-Y.; He, Q.-Y. Proteomic investigation into the action mechanism of berberine against Streptococcus pyogenes. J. Proteom. 2020, 215, 103666. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Mubarak, M.S.; Amin, S. Antifungal potential of alkaloids as an emerging therapeutic target. Curr. Drug Targets 2017, 18, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Boon, S.A.; Ijaz, M.K.; McKinney, J.; Gerba, C.P. Antifungal activity and mechanism of action of natural product derivates as potential environmental disinfectants. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad036. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Bailey, J.H. Allicin, the Antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Yamada, Y.; Azuma, K. Evaluation of the in vitro antifungal activity of allicin. Antimicrob. Agents Chemother. 1977, 11, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A Comparison of the antibacterial and antifungal activities of thiosulfinate analogues of allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.-M.; Liu, W.-H.; Yin, M.-C. Two Diallyl sulphides derived from garlic inhibit meticillin-resistant Staphylococcus aureus infection in diabetic mice. J. Med. Microbiol. 2007, 56, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Sebtosheikh, P.; Qomi, M.; Ghadami, S.; Mojab, F. Analysis of essential oil from leaves and bulb of Allium atroviolaceum. Int. Pharm. Acta 2020, 3, 3e8. [Google Scholar] [CrossRef]
- Hatami, M.; Karimi, M.; Aghaee, A.; Bovand, F.; Ghorbanpour, M. Morphological Diversity, Phenolic acids, and antioxidant properties in eryngo (Eryngium caucasicum Trautv): Selection of superior populations for agri-food industry. Food Sci. Nutr. 2022, 10, 3905–3919. [Google Scholar] [CrossRef] [PubMed]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evid. Based Complement. Alternat. Med. 2021, 2021, 1–30. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control 2006, 34, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A Critical Review on Phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Dahlem Junior, M.A.; Nguema Edzang, R.W.; Catto, A.L.; Raimundo, J.-M. Quinones as an efficient molecular scaffold in the antibacterial/antifungal or antitumoral arsenal. Int. J. Mol. Sci. 2022, 23, 14108. [Google Scholar] [CrossRef] [PubMed]
- Sanver, D.; Murray, B.S.; Sadeghpour, A.; Rappolt, M.; Nelson, A.L. Experimental modeling of flavonoid–biomembrane interactions. Langmuir 2016, 32, 13234–13243. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S. Efficacy and Mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Lee, H.; Woo, E.-R.; Lee, D.G. Apigenin induces cell shrinkage in Candida albicans by membrane perturbation. FEMS Yeast Res. 2018, 18, foy003. [Google Scholar] [CrossRef]
- Dorsaz, S.; Snäkä, T.; Favre-Godal, Q.; Maudens, P.; Boulens, N.; Furrer, P.; Ebrahimi, S.N.; Hamburger, M.; Allémann, E.; Gindro, K.; et al. Identification and mode of action of a plant natural product targeting human fungal pathogens. Antimicrob. Agents Chemother. 2017, 61, e00829-17. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the β-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter Pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-W.; Lee, J.-Y.; Kang, D.-I.; Lee, J.-U.; Shin, S.Y.; Kim, Y. Screening of flavonoids as candidate antibiotics against Enterococcus faecalis. J. Nat. Prod. 2009, 72, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, W.A.; Zhu, R.; Peng, W.; Al-Hariri, M.; Kobeissy, F.; Tran, P.; Hamood, A.N.; Hegazy, M.F.; Paré, P.W.; Mechref, Y. Multitargeted flavonoid inhibition of the pathogenic bacterium Staphylococcus aureus: A proteomic characterization. J. Proteome Res. 2017, 16, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli: Synergistic of flavonoid on E. coli. J. Appl. Microbiol. 2012, 112, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.Á.; Butassi, E.; Ribas, J.C.; Svetaz, L.A.; Cortés, J.C.G. Natural products targeting the synthesis of β(1,3)-d-glucan and chitin of the fungal cell wall. existing drugs and recent findings. Phytomedicine 2021, 88, 153556. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.M. Fungal ß(1,3)-D-Glucan Synthesis. Med. Mycol. 2001, 39, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Arita-Morioka, K.; Yamanaka, K.; Mizunoe, Y.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef] [PubMed]
- Awolola, G.; Koorbanally, N.; Chenia, H.; Shode, F.; Baijnath, H. Antibacterial and anti-biofilm activity of flavonoids and triterpenes isolated from the extracts of Ficus sansibarica Warb. subsp. sansibarica (Moraceae) extracts. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Hamilton, V.E.S.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin: Aggregation of S. aureus by galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef]
- Zhang, Q.; Lyu, Y.; Huang, J.; Zhang, X.; Yu, N.; Wen, Z.; Chen, S. Antibacterial activity and mechanism of sanguinarine against Providencia rettgeri in vitro. PeerJ 2020, 8, e9543. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, X.-D.; Hong, J.; Liu, C.; Zhang, X.-L.; Zheng, J.-P.; Xu, Y.-J.; Ou, Z.-Y.; Zheng, J.-L.; Yu, D.-J. Inhibitory effect of two traditional Chinese medicine monomers, berberine and matrine, on the quorum sensing system of antimicrobial-resistant Escherichia coli. Front. Microbiol. 2019, 10, 2584. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.A.; Da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 2015, 22, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Reece, R.J.; Maxwell, A. DNA Gyrase: Structure and function. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 335–375. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure–activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Mahalapbutr, P.; Suriya, U.; Somboon, T.; Aiebchun, T.; Shi, L.; Maitarad, P.; Rungrotmongkol, T. In silico screening of DNA gyrase B potent flavonoids for the treatment of Clostridium difficile infection from PhytoHub database. Braz. Arch. Biol. Technol. 2021, 64, e21200402. [Google Scholar] [CrossRef]
- Shadrick, W.R.; Ndjomou, J.; Kolli, R.; Mukherjee, S.; Hanson, A.M.; Frick, D.N. Discovering new medicines targeting helicases: Challenges and recent progress. SLAS Discov. 2013, 18, 761–781. [Google Scholar] [CrossRef]
- Bhosle, A.; Chandra, N. Structural Analysis of dihydrofolate reductases enables rationalization of antifolate binding affinities and suggests repurposing possibilities. FEBS J. 2016, 283, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Tamura, M.; Imai, K.; Ishigami, T.; Ochiai, K. Catechin inhibits Candida albicans dimorphism by disrupting Cek1 phosphorylation and cAMP synthesis. Microb. Pathog. 2013, 56, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, A.; Stojan, J.; Krastanova, I.; Kristan, K.; Brunskole Švegelj, M.; Lamba, D.; Lanišnik Rižner, T. Structural basis for inhibition of 17β-hydroxysteroid dehydrogenases by phytoestrogens: The case of fungal 17β-HSDcl. J. Steroid Biochem. Mol. Biol. 2017, 171, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P.; Mencherini, T.; Sansone, F.; Del Gaudio, P.; Granata, I.; Porta, A.; Aquino, R.P. Screening of a polar extract of Paeonia rockii: Composition and antioxidant and antifungal activities. J. Ethnopharmacol. 2011, 138, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Chinnam, N.; Dadi, P.K.; Sabri, S.A.; Ahmad, M.; Kabir, M.A.; Ahmad, Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 2010, 46, 478–486. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. The Tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resist. 2018, 11, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Fathima, A.; Rao, J.R. Selective toxicity of catechin—A natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]
- Kwun, M.S.; Lee, D.G. Quercetin-induced yeast apoptosis through mitochondrial dysfunction under the accumulation of magnesium in Candida albicans. Fungal Biol. 2020, 124, 83–90. [Google Scholar] [CrossRef]
- Kang, K.; Fong, W.-P.; Tsang, P.W.-K. Novel Antifungal activity of purpurin against Candida species in vitro. Med. Mycol. 2010, 48, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Waditzer, M.; Bucar, F. Flavonoids as inhibitors of bacterial efflux pumps. Molecules 2021, 26, 6904. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.J.S.D.O.; Miranda, G.M.; Bessa, J.R.; Araújo, A.C.J.D.; Freitas, P.R.; Almeida, R.S.D.; Paulo, C.L.R.; Neto, J.B.D.A.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as bacterial efflux pump inhibitors: A systematic review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Manoharlal, R.; Shukla, S.; Puri, N.; Prasad, T.; Ambudkar, S.V.; Prasad, R. Curcumin modulates efflux mediated by yeast ABC multidrug transporters and is synergistic with antifungals. Antimicrob. Agents Chemother. 2009, 53, 3256–3265. [Google Scholar] [CrossRef] [PubMed]
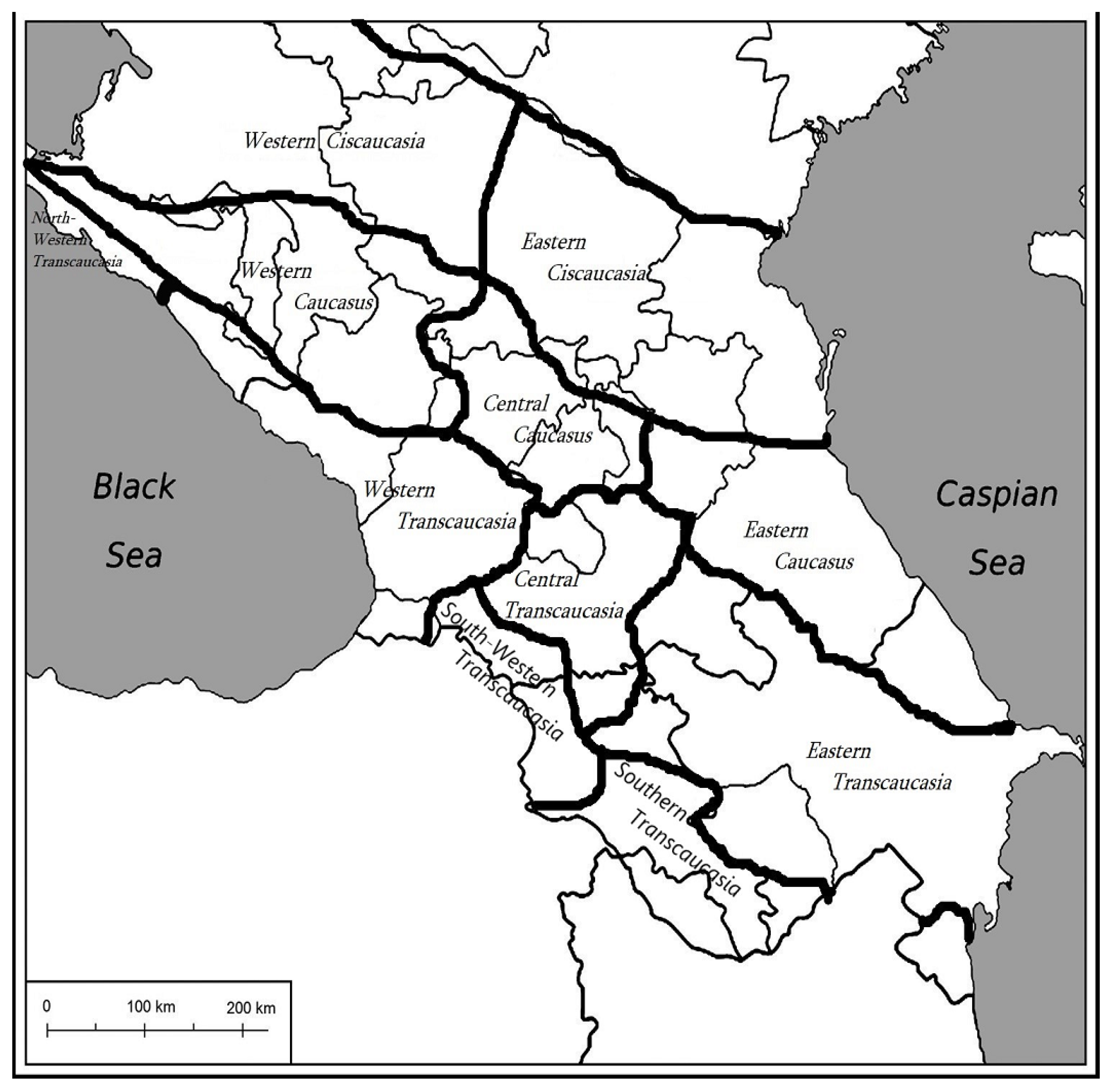
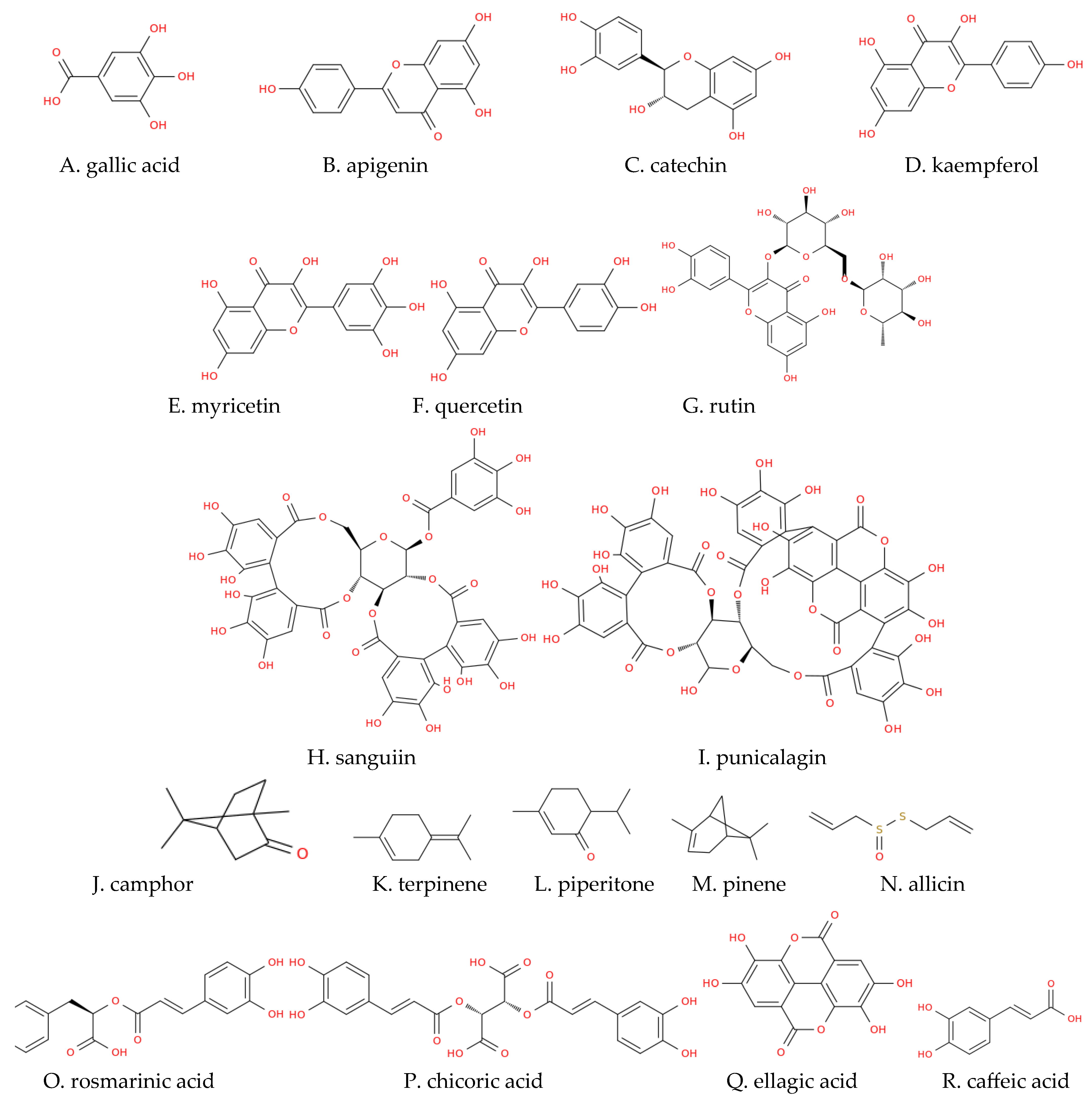

| Family | Species | Distribution | Plant Part | Extract Type | Susceptible Microorganism | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-Positive | MIC (mg/mL)/IZ, mm | Gram-Negative | MIC (mg/mL)/IZ, mm | Fungi | MIC (mg/mL)/IZ, mm | ||||||
| Alliaceae | Allium atroviolaceum Hornem. Ex Steud. | Entire Caucasus | Bulbs | 70% methanol extract | S. mitis S. mutans S. sanguinis S. salivarius | 6.25/- 6.25/- 3.12/- 6.25/- | - | - | - | - | [23] |
| Bulbs | aqueous extract | B. subtilis B. cereus S. aureus | 0.31/42.6 1.25/25 0.63/19.4 | S. flexneri K. pneumoniae E. coli | 1.25/12 2.50/8.5 2.50/23.5 | - | - | [24] | |||
| Allium sativum L. | Entire Caucasus | Bulbs | 70% ethanol extract aqueous extract | S. aureus | 12.5/15 | E. coli S. flexneri P. aeruginosa | 6.25/14 5 */- 0.6/25 | C. albicans C. parapsilosis | 12.5/27 6.25/22 | [25,26,27] | |
| Allium ursinum L. | Entire Caucasus | Bulbs | 70% ethanol extract | S. aureus | 25/16 | E. coli | 6.25/17 | C. albicans C. parapsilosis | 6.25/29 6.25/20 | [25] | |
| Amaryllidaceae | Galanthus transcaucasicus Fomin | Eastern and Southern Transcaucasia and Eastern Caucasus | Bulbs | 99% methanol extract | B. subtilis B. cereus S. aureus | -/82 -/71 -/35 | E. coli P. aeruginosa | -/85 -/46 | - | - | [28] |
| Flowers | B. subtilis B. cereus S. aureus | -/10 -/122 -/76 | E. coli P. aeruginosa | -/116 -/98 | - | - | [28] | ||||
| Shoots | B. subtilis B. cereus S. aureus | -/112 -/118 -/92 | E. coli P. aeruginosa | -/129 -/106 | - | - | [28] | ||||
| Bulbs | 96% ethanol extract | B. subtilis S. aureus | 9.28/- 1.17/- | - | - | C. albicans | 150 U/mL * | [29] | |||
| Apiaceae | Eryngium caucasicum Trautv. | Entire Caucasus | Roots | essential oil | S. aureus B. subtilis. | 0.5/19 0.5/18 | E. coli P. aeruginosa | 0.50/7 >1/9 | - | - | [30] |
| Aerial part | S. aureus B. subtilis | 0.5/19 0.4/18 | E. coli P. aeruginosa | 0.85/7 >1/9 | - | - | [31] | ||||
| Asteraceae | Artemisia fragransWilld. | South-Western Transcaucasia, Southern Transcaucasia, Eastern Transcaucasia | Aerial part | essential oil | S. aureus S. epidermidis B. subtilis | ni/12 ni/11 124.8 × 10−6/23 | E. coli K. pneumoniae P. vulgaris | ni/16.3 3.9 × 10−6/18.3 ni/21.3 | - | - | [32] |
| Leaves | c | S. aureus E. faecalis B. anthracis S. epidermidis S. saprophyticus | -/9 -/10 3.10/25 0.006/20 0.03/10 | E. coli P. aeruginosa S. flexneri S. paratyphi S. typhi | -/8 -/ni 0.0125/15 0.0125/15 0.025/10 | - | - | [33,34] | |||
| Roots | essential oil | S. aureus E. faecalis | -/12 -/14 | E. coli P. aeruginosa | -/9 -/ni | - | - | [34] | |||
| Stem | essential oil | B. anthracis E. faecalis S. aureus S. epidermidis S. saprophyticus | ni/ni 0.013/15 0.006/20 0.03/10 ni/ni | E. coli P. aeruginosa | 0.025/10 ni/ni | - | - | [33] | |||
| Flowers | essential oil | B. anthracis E. faecalis S. aureus S. epidermidis S. saprophyticus | ni/ni 0.006/20 0.003/25 0.006/20 25/10 | E. coli P. aeruginosa | 0.025/10 0.025/10 | - | - | [33] | |||
| Hypericaceae | Hypericum alpestre Steven. | Entire Caucasus | Aerial part | 99% methanol extract | S. aureus | 0.26/- | E. coli | 1.02/- | - | - | [35] |
| Aerial part | 99.8% acetone extract | S. aureus B. subtilis | 0.13/12 0.13/10 | E. coli P. aeruginosa S. typhimurium | 1.02/ni 0.06/21 0.51/ni | C. albicans C. guilliermondii | 1.02/ni 1.02/ni | [36] | |||
| Juglandaceae | Juglans regia L. | Entire Caucasus | Leaves | 99% methanol extract | - | - | Klebsiella sp. E. coli | -/15.3 -/21 | - | - | [37] |
| Leaves | 80% methanol extract | S. mutans S. salivarius S. sanguinis A. viscosus | 125/- 15.6/- 15.6/- 187.50/- | - | - | - | - | [38] | |||
| Bark | ethyl acetate extract | - | - | - | - | C. albicans C. dubliniensis C. glabrata P. guilliermondii | -/18.3 -/14.3 -/10.3 -/12.3 | [39] | |||
| 80% methanol extract | S. aureus Streptococcus spp. | -/18.4 -/12/0 | E. coli K. pneumoniae P. multocida M. haemolytica | -/ni -/ni -/17.1 -/16.6 | - | - | [40] | ||||
| Green husk | aqueous extract | B. cereus B. subtilis S. aureus | 0.1/>9 10/5 0.1/>9 | E. coli P. aeruginosa K. pneumoniae | 100/ni 100/5 100/ni | C. albicans C. neoformans | 100/ni- 100/ni- | [41] | |||
| Pterocarya fraxinifolia (Poir.) Spach | Entire Caucasus | Leaves | 99% methanol extract | Klebsiella sp. E. coli | -/16 -/21.7 | - | - | [37] | |||
| Stem | essential oil | S. epidermidis S. aureus B. subtilis | -/27 -/10 -/10 | S. paratyphi S. dysenteriae P. vulgaris E. coli K. pneumoniae | -/ni -/ni -/ni -/ni -/ni | C. albicans A. niger | -/ni -/11 | [42] | |||
| Lamiaceae | Clinopodium nepeta (L.) Kuntze | Western Transcaucasia | Aerial part | essential oil | B. cereus S. sanguinis | 2.50/- 2.50/- | E. coli P. aeruginosa S. typhimurium | 5/- 10/- 1.25/- | A. flavus A. terreus C. albicans M. canis M. gypseum T. mentagrophytes | 2/- 0.4/- 2/- 0.4/- 0.4/- 0.4/- | [43,44] |
| Mentha pulegium L. | Western and Eastern Ciscaucasia, Western Transcaucasia, South-Western Transcaucasia, Southern Transcaucasia, Eastern Transcaucasia | Leaves | 48% ethanol extract | S. pneumoniae | 0.11/17.2 | K. pneumoniae | 0.23/8.1 | - | - | [45] | |
| Flowering aerial part | essential oil | S. aureus S. epidermidis B. cereus L. monocytogenes E. faecalis | 0.5 */21 1 */19 1 */16 1 */8 4 **/10.7 | V. cholera E. coli S. typhimurium | 0.5 */13 4 */ni 2 */ni | A. niger C. albicans C. tropicalis | 0.25 */10 1 */16 16 **/18.6 | [46,47] | |||
| Thymus caucasicus Willd. ex Benth. | Western Transcaucasia, Central Transcaucasia, Eastern Transcaucasia | Aerial part | essential oil | S. faecalis S. typhi S. aureus | 0.005/- ni/- 0.1/- | E. coli | 0.005/- | C. albicans | ni/- | [48] | |
| Lauraceae | Laurus nobilis L. | Entire Caucasus | Leaves | essential oil | B. cereus E. faecalis E. faecium S. aureus L. monocytogenes | -/12 -/11.5 -/12 -/12.5 -/12.0 | S. abony P. vulgaris K. pneumoniae Shigella flexneri P. aeruginosa P. fluorescens P. mirabilis | -/9.5 -/8.5 ni ni ni ni ni | C. albicans C. glabrata C. tropicalis A. niger A. versicolor P. citrinum P. expansum | -/16.3 -/10 -/12 -/6 -/7.7 -/5.7 -/9.7 | [49,50] |
| 70% ethanol extract | - | - | E. coli S. typhi | -/11.3 -/14.5 | - | - | [51] | ||||
| 99.8% acetone extract | E. faecalis S. pneumoniae S. aureus | 0.13/24.0 0.13/37.2 0.25/16.7 | E. coli P. mirabilis | 0.25/23.3 0.13/24.0 | F. solani F. oxysporum A. alternata Bipolaris sp. | 0.25/- 4/- 32/- 0.5/- | [52] | ||||
| Liliaceae | Lilium monadelphum subsp. armenum (Miscz. ex Grossh.) Kudrjasch. | South-Western Transcaucasia, Southern Transcaucasia | Bulbs | 99.8% acetone extract | S. aureus B. subtilis | 0.51/10 0.51/9 | E. coli P. aeruginosa S. typhimurium | 0.51/9 0.13/11 0.51/9 | C. albicans C. guilliermondii | ni/ni ni/ni | [36] |
| Malvaceae | Alcea rosea L. | Eastern Caucasus, Eastern Transcaucasia | Flowers | 48% ethanol extract | S. pneumoniae | 375/6.9 | K. pneumoniae | 680/3.2 | - | - | [45] |
| Whole plant | 98% ethyl acetate extract | S. aureus | -/25 | P. vulgaris K. pneumoniae E. coli P. aeruginosa | -/13 -/18 -/28 -/20 | - | - | [53] | |||
| Polygonaceae | Rumex obtusifolius L. | Entire Caucasus | Leaf | 99.8% acetone extract | S. aureus B. subtilis | -/11 -/ni | E. coli P. aeruginosa S. typhimurium | -/ni -/12 -/10 | C. albicans C. guilliermondii | -/ni -/12 | [36] |
| Root | 99.8% acetone extract | S. aureus B. subtilis | -/10 -/9 | E. coli P. aeruginosa S. typhimurium | -/ni 64/12 512/ni | C. albicans C. guilliermondii | -/9 -/13 | [36] | |||
| Inflorescence | 99.8% acetone extract | S. aureus B. subtilis | -/11 -/11 | E. coli P. aeruginosa S. typhimurium | -/10 -/9 512/ni | C. albicans C. guilliermondii | -/ni -/9 | [36] | |||
| Seeds | 99.8% acetone extract | S. aureus B. subtilis | 0.256/12 0.128/10 | E. coli P. aeruginosa S. typhimurium | 512/10 128/10 512/12 | C. albicans C. guilliermondii | ni/ni ni/10 | [36] | |||
| Primulacea | Cyclamen coum Mill. | Eastern Transcaucasia | Bulb | 99% ethanol extract | E. faecalis B. subtilis S. epidermidis S. aureus | -/8 -/ni -/ni -/ni | E. coli P. aeruginosa S. typhimurium | -/ni -/ni -/ni | C. albicans | -/8 | [54] |
| Leaf | 99% ethanol extract | E. faecalis B. subtilis S. epidermidis S. aureus | -/ni -/ni -/ni -/ni | E. coli P. aeruginosa S. typhimurium | -/ni -/ni -/ni | C. albicans | -/ni | [54] | |||
| Flower | 99% ethanol extract | B. subtilis E. faecalis S. epidermidis S. aureus | -/ni -/ni -/ni -/ni | E. coli P. aeruginosa S. typhimurium | -/ni -/ni -/ni | C. albicans | -/11 | [54] | |||
| Aerial part | 99% methanol extract | S. aureus | 0.13/- | E. coli P. aeruginosa | 0.013/- 0.006/- | - | - | [55] | |||
| Primula macrocalyx Bunge | Western Transcaucasia, Central Transcaucasia, Eastern Transcaucasia, South-Western Transcaucasia, Southern Transcaucasia | Entire plant | 99% methanol extract | - | - | - | - | C. rugosa | -/20 | [56] | |
| Rosaceae | Agrimonia eupatoria L. | Entire Caucasus | Whole plant | 99% methanol extract | S. aureus | 0.26/- | E. coli | 0.51/- | - | - | [35] |
| 99.8% acetone extract | S. aureus B. subtilis | 0.26/10 0.13/11 | E. coli P. aeruginosa S. typhimurium | 0.51/10 0.13/11 0.51/10 | C. albicans C. guilliermondii | 0.51/9 0.26/12 | [36] | ||||
| Seeds | 99% methanol extract | B. cereus B. subtilis | 0.75/- 0.75/- | - | - | - | - | [57] | |||
| Aerial part | 99.8% acetone extract | B. cereus E. faecalis | 0.31/- 2.50/- | P. mirabilis K. pneumoniae S. enterica S. typhimurium | 2.5/- 10/- 10/- 10/- | P. italicum P. chrysogenum A. flavus A. niger | 2.50/- 2.50/- 10/- 20/- | [58] | |||
| 75% methanol extract | S. aureus | + | - | - | - | - | [59] | ||||
| Filipendula ulmaria (L.) Maxim. | Western Transcaucasia, Central Transcaucasia, Eastern Transcaucasia | Leaves | 60% ethanol extract | B. subtilis | -/ni | P. aeruginosa | -/10.7 | - | - | [60] | |
| Stem | 60% ethanol extract | B. subtilis | -/ni | P. aeruginosa | -/6.9 | - | - | [60] | |||
| Flowers | 60% ethanol extract | B. subtilis | -/ni | P. aeruginosa | -/10.7 | - | - | [60] | |||
| Fruits | 60% ethanol extract | B. subtilis | -/13.1 | P. aeruginosa | -/13.3 | - | - | [60] | |||
| Roots | 60% ethanol extract | B. subtilis | -/ni | P. aeruginosa | -/11.1 | - | - | [60] | |||
| Aerial part | 99% methanol extract | E. faecalis | 0.31/- | E. coli P. aeruginosa K. pneumoniae | 0.16/- 5/- 5/- | C. albicans T. harzianum T. longibrachiatum P. cyclopium P. canescens A. niger A. glaucus F. oxysporum A. alternata D. stemonitis P. fastigiata | >10/- 2.5/- 5/- 2.5/- 10/- >10/- 5/- 2.5/- >10/- >10/- 5/- | [61] | |||
| Roots | 99% methanol extract | E. faecalis | 0.31/- | E. coli P. aeruginosa K. pneumoniae | 0.63/- 5/- 5/- | C. albicans T. harzianum T. longibrachiatum P. cyclopium P. canescens A. niger A. glaucus F. oxysporum A. alternata D. stemonitis P. fastigiata | >10/- 10/- 10/- 5/- >10/- >10/- >10/- 2.5/- >10/- >10/- 10/- | ||||
| Rosa canina L. | Entire Caucasus | Fruits | 96% ethanol extract | B. cereus C. perfringens L. monocytogenes | 0.13/8.33 0.51/4.00 0.26/6.00 | E. coli K. oxytoca P. aeruginosa | 0.03/14.33 0.06/12.00 0.13/9.67 | - | - | [62] | |
| Fruit | hexane/acetone/ethanol (2:1:1), 0.05% butylated hydroxytoluene extract | S. aureus E. faecalis B. cereus | 4/- 4/- 4/- | E. coli S. enteritidis E. aerogenes P. aeruginosa | 4/- 4/- 4/- 2/- | C. albicans | 2/- | [63] | |||
| Sanguisorba officinalis L. | Entire Caucasus | Aerial part | 99% methanol extract | S. aureus | 0.56/- | E. coli | 1.02/- | - | - | [35] | |
| 99.8% acetone extract | S. aureus B. subtilis | 0.13/13 0.13/13 | E. coli P. aeruginosa S. typhimurium | 0.256/102 0.064/12 0.256/11 | C. albicans C. guilliermondii | 0.51/10 0.51/10 | [36] | ||||
| Roots | 99.8% acetone extract | S. aureus B. subtilis | -/10 -/10 | E. coli P. aeruginosa S. typhimurium | -/10 -/10 -/10 | C. albicans C. guilliermondii | -/10 -/10 | [36] | |||
| Roots | 70% ethanol extract | S. aureus | -/5.50 | E. coli P. aeruginosa | -/6.56 -/6.55 | - | - | [64] | |||
| Leaves | S. aureus | -/8.43 | E. coli P. aeruginosa | -/11.46 -/11.33 | - | - | |||||
| Flowers | S. aureus | -/2.5 | E. coli P. aeruginosa | -/2.66 -/4.60 | - | - | |||||
| Sapindaceae | Acer cappadocicum Gled. | Entire Caucasus | Branches with leaves | 99% methanol extract | B. subtilis | -/16.5 | E. coli K. pneumoniae S. enterica A. baumannii | -/16.3 -/16.5 -/15 -/11 | F. fujikuroi R. oryzae P. ultimum | -/48% *** /52% *** /49% *** | [65] |
| Zygophyllaceae | Peganum harmala L. | Western Transcaucasia, Central Transcaucasia, Eastern Transcaucasia, South-Western Transcaucasia, Southern Transcaucasia | Seeds | 48% ethanol extract | S. pneumoniae | 0.08/27.2 | K. pneumoniae | 0.15/10.1 | - | - | [45] |
| Whole plant | 99% methanol extract | S. aureus | 0.15/- | E. coli P. aeruginosa | 5/- 1.5/- | C. albicans | 0.063/- | [66] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fik-Jaskółka, M.; Mittova, V.; Motsonelidze, C.; Vakhania, M.; Vicidomini, C.; Roviello, G.N. Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics. Antibiotics 2024, 13, 487. https://doi.org/10.3390/antibiotics13060487
Fik-Jaskółka M, Mittova V, Motsonelidze C, Vakhania M, Vicidomini C, Roviello GN. Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics. Antibiotics. 2024; 13(6):487. https://doi.org/10.3390/antibiotics13060487
Chicago/Turabian StyleFik-Jaskółka, Marta, Valentina Mittova, Catherine Motsonelidze, Malkhaz Vakhania, Caterina Vicidomini, and Giovanni N. Roviello. 2024. "Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics" Antibiotics 13, no. 6: 487. https://doi.org/10.3390/antibiotics13060487
APA StyleFik-Jaskółka, M., Mittova, V., Motsonelidze, C., Vakhania, M., Vicidomini, C., & Roviello, G. N. (2024). Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics. Antibiotics, 13(6), 487. https://doi.org/10.3390/antibiotics13060487










