Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer
Abstract
1. Introduction
2. The Gastric Microbiota in Healthy Individuals
3. H. pylori and Cancer
4. Non-H. pylori Gastric Microbiota and Cancer
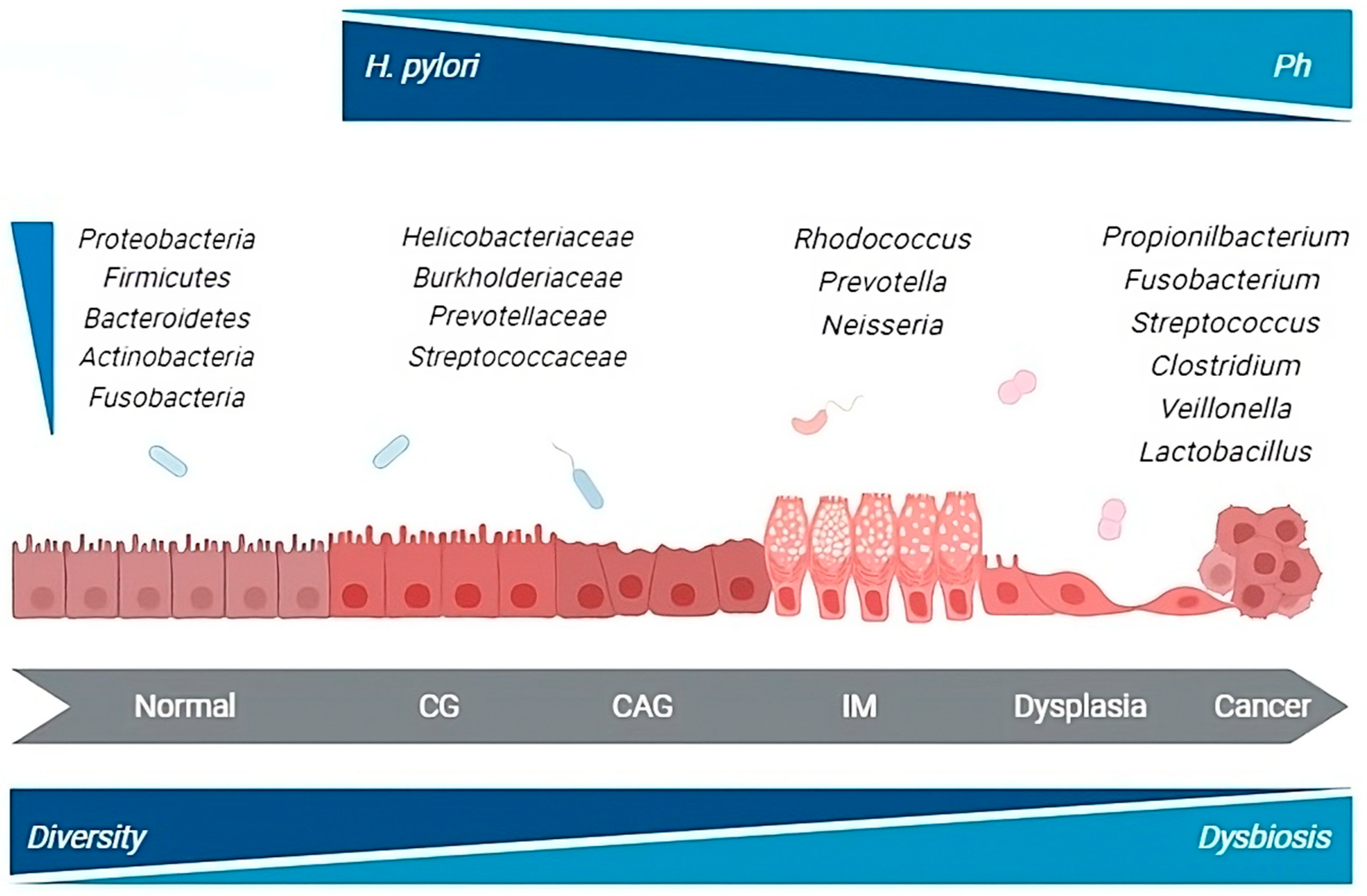
5. Microbiota Changes during the Multistep Process of Gastric Carcinogenesis
6. Effects of H. pylori Eradication on the Gastric Microbiome and Patient’s Outcome
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Graham, D.Y.; Malfertheiner, P. Helicobacter Pylori Research: Historical Insights and Future Directions. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. A Human Model of Gastric Carcinogenesis. Cancer Res. 1986, 48, 3554–3560. [Google Scholar]
- Yang, H.; Wei, B.; Hu, B. Chronic Inflammation and Long-Lasting Changes in the Gastric Mucosa after Helicobacter Pylori Infection Involved in Gastric Cancer. Inflamm. Res. 2021, 70, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N. The Magnitude of Association Between Helicobacter Pylori Infection and the Development of Gastric Cancer. Scand. J. Gastroenterol. 2002, 37, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.; Kwok, T.; Hartig, R.; König, W.; Backert, S. NF-κB Activation and Potentiation of Proinflammatory Responses by the Helicobacter Pylori CagA Protein. Proc. Natl. Acad. Sci. USA 2005, 102, 9300–9305. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Ooki, T.; Murata-Kamiya, N.; Komura, D.; Tahmina, K.; Wu, W.; Takahashi-Kanemitsu, A.; Knight, C.T.; Kunita, A.; Suzuki, N.; et al. Helicobacter Pylori CagA Elicits BRCAness to Induce Genome Instability That May Underlie Bacterial Gastric Carcinogenesis. Cell Host Microbe 2021, 29, 941–958.e10. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.; Tang, B.; Li, B.-S.; Xie, R.; Hu, C.-J.; Luo, G.; Qin, Y.; Dong, H.; Yang, S.-M. Helicobacter Pylori Virulence Factor CagA Promotes Tumorigenesis of Gastric Cancer via Multiple Signaling Pathways. Cell Commun. Signal. 2015, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I. Formation of Anion-Selective Channels in the Cell Plasma Membrane by the Toxin VacA of Helicobacter Pylori Is Required for Its Biological Activity. EMBO J. 1999, 18, 5517–5527. [Google Scholar] [CrossRef]
- El-Omar, E.M.; Carrington, M.; Chow, W.-H.; McColl, K.E.L.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.-C.; Rothman, N.; et al. Interleukin-1 Polymorphisms Associated with Increased Risk of Gastric Cancer. Nature 2000, 404, 398–402. [Google Scholar] [CrossRef]
- Hou, L.; El-Omar, E.M.; Chen, J.; Grillo, P.; Rabkin, C.S.; Baccarelli, A.; Yeager, M.; Chanock, S.J.; Zatonski, W.; Sobin, L.H.; et al. Polymorphisms in Th1-Type Cell-Mediated Response Genes and Risk of Gastric Cancer. Carcinogenesis 2007, 28, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Companioni, O.; Bonet, C.; Muñoz, X.; Weiderpass, E.; Panico, S.; Tumino, R.; Palli, D.; Agnoli, C.; Vineis, P.; Boutron-Ruault, M.C.; et al. Polymorphisms of Helicobacter Pylori Signaling Pathway Genes and Gastric Cancer Risk in the European Prospective Investigation into Cancer-Eurgast Cohort. Int. J. Cancer 2014, 134, 92–101. [Google Scholar] [CrossRef]
- Gonázlez, C.A.; López-Carrillo, L. Helicobacter Pylori, Nutrition and Smoking Interactions: Their Impact in Gastric Carcinogenesis. Scand. J. Gastroenterol. 2010, 45, 6–14. [Google Scholar] [CrossRef]
- Venneman, K.; Huybrechts, I.; Gunter, M.J.; Vandendaele, L.; Herrero, R.; Van Herck, K. The Epidemiology of Helicobacter Pylori Infection in Europe and the Impact of Lifestyle on Its Natural Evolution toward Stomach Cancer after Infection: A Systematic Review. Helicobacter 2018, 23, e12483. [Google Scholar] [CrossRef]
- Wang, L.L.; Yu, X.J.; Zhan, S.H.; Jia, S.J.; Tian, Z.B.; Dong, Q.J. Participation of Microbiota in the Development of Gastric Cancer. World J. Gastroenterol. 2014, 20, 4948–4952. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; MacHado, J.C.; Figueiredo, C. Gastric Microbial Community Profiling Reveals a Dysbiotic Cancer-Associated Microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Mowat, C.; Williams, C.; Gillen, D.; Hossack, M.; Gilmour, D.; Carswell, A.; Wirz, A.; Preston, T.; McColl, K.E.L. Omeprazole, Helicobacter Pylori Status, and Alterations in the Intragastric Milieu Facilitating Bacterial N-Nitrosation. Gastroenterology 2000, 119, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Keszei, A.P.; Goldbohm, R.A.; Schouten, L.J.; Jakszyn, P.; Van Den Brandt, P.A. Dietary N-Nitroso Compounds, Endogenous Nitrosation, and the Risk of Esophageal and Gastric Cancer Subtypes in the Netherlands Cohort Study. Am. J. Clin. Nutr. 2013, 97, 135–146. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhan, X.; Song, Y.; Xu, M.; Wang, S.; Huang, X.; Xu, Z.Z. Meta-Analysis Reveals Helicobacter Pylori Mutual Exclusivity and Reproducible Gastric Microbiome Alterations during Gastric Carcinoma Progression. Gut Microbes 2023, 15, 1024–1032. [Google Scholar] [CrossRef]
- Robin Warren, J.; Marshall, B. Unidentified Curved Bacilli on Gastric Epithelium in Active Chronic Gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar] [CrossRef]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular Analysis of the Bacterial Microbiota in the Human Stomach. Proc. Natl. Acad. Sci. USA 2006, 103, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Wong, G.L.H.; To, K.F.; Wong, V.W.S.; Lai, L.H.; Chow, D.K.L.; Lau, J.Y.W.; Sung, J.J.Y.; Ding, C. Bacterial Microbiota Profiling in Gastritis without Helicobacter Pylori Infection or Non-Steroidal Anti-Inflammatory Drug Use. PLoS ONE 2009, 4, e7985. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Cabrera-Rubio, R.; Mira, A.; Suárez, A.; Mayo, B. Microbiological Survey of the Human Gastric Ecosystem Using Culturing and Pyrosequencing Methods. Microb. Ecol. 2013, 65, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Nardone, G.; Compare, D.; Rocco, A. A Microbiota-Centric View of Diseases of the Upper Gastrointestinal Tract. Lancet Gastroenterol. Hepatol. 2017, 2, 298–312. [Google Scholar] [CrossRef]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the Upper Respiratory Tract Microbiotas as the Source of the Lung and Gastric Microbiotas in Healthy Individuals. MBio 2015, 6, e00037-15. [Google Scholar] [CrossRef]
- Sun, Q.-H.; Zhang, J.; Shi, Y.-Y.; Zhang, J.; Fu, W.-W.; Ding, S.-G. Microbiome Changes in the Gastric Mucosa and Gastric Juice in Different Histological Stages of Helicobacter Pylori-Negative Gastric Cancers. World J. Gastroenterol. 2022, 28, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.-H.; Kim, N.; Jo, H.J.; Kim, J.; Park, J.H.; Nam, R.H.; Seok, Y.-J.; Kim, Y.-R.; Lee, D.H. Analysis of Gastric Body Microbiota by Pyrosequencing: Possible Role of Bacteria Other Than Helicobacter Pylori in the Gastric Carcinogenesis. J. Cancer Prev. 2017, 22, 115–125. [Google Scholar] [CrossRef]
- Nardone, G.; Compare, D. The Human Gastric Microbiota: Is It Time to Rethink the Pathogenesis of Stomach Diseases? United Eur. Gastroenterol. J. 2015, 3, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, M.; Lee, J.H.; Choi, I.J.; Kim, Y.I.; Kim, J.S. Effect of the Interaction between Dietary Patterns and the Gastric Microbiome on the Risk of Gastric Cancer. Nutrients 2021, 13, 2692. [Google Scholar] [CrossRef]
- Yang, I.; Woltemate, S.; Piazuelo, M.B.; Bravo, L.E.; Yepez, M.C.; Romero-Gallo, J.; Delgado, A.G.; Wilson, K.T.; Peek, R.M.; Correa, P.; et al. Different Gastric Microbiota Compositions in Two Human Populations with High and Low Gastric Cancer Risk in Colombia. Sci. Rep. 2016, 6, 18594. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, A.; Suda, W.; Morita, H.; Takanashi, K.; Takagi, A.; Koga, Y.; Hattori, M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin. Transl. Gastroenterol. 2015, 6, e89. [Google Scholar] [CrossRef] [PubMed]
- Nakae, H.; Tsuda, A.; Matsuoka, T.; Mine, T.; Koga, Y. Gastric Microbiota in the Functional Dyspepsia Patients Treated with Probiotic Yogurt. BMJ Open Gastroenterol. 2016, 3, e000109. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Lin, J.T.; Ho, H.J.; Lai, Z.L.; Wang, C.B.; Tang, S.L.; Wu, C.Y. Gastric Microbiota and Predicted Gene Functions Are Altered after Subtotal Gastrectomy in Patients with Gastric Cancer. Sci. Rep. 2016, 6, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, T.; Llorca, L.; Perez-Perez, G. Impact of the Microbiota and Gastric Disease Development by Helicobacter Pylori. Curr. Top. Microbiol. Immunol. 2017, 400, 253–275. [Google Scholar] [PubMed]
- Yin, Y.N.; Wang, C.L.; Liu, X.W.; Cui, Y.; Xie, N.; Yu, Q.F.; Li, F.J.; Lu, F.G. Gastric and Duodenum Microflora Analysis after Long-Term Helicobacter Pylori Infection in Mongolian Gerbils. Helicobacter 2011, 16, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Schütte, K.; Koch, N.; Vilchez-Vargas, R.; Wos-Oxley, M.L.; Oxley, A.P.A.; Vital, M.; Malfertheiner, P.; Pieper, D.H. The Active Bacterial Assemblages of the Upper GI Tract in Individuals with and without Helicobacter Infection. Gut 2018, 67, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Contreras, A.; Goldfarb, K.C.; Godoy-Vitorino, F.; Karaoz, U.; Contreras, M.; Blaser, M.J.; Brodie, E.L.; Dominguez-Bello, M.G. Structure of the Human Gastric Bacterial Community in Relation to Helicobacter Pylori Status. ISME J. 2011, 5, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Llorca, L.; Pérez-Pérez, G.; Urruzuno, P.; Martinez, M.J.; Iizumi, T.; Gao, Z.; Sohn, J.; Chung, J.; Cox, L.; Simón-Soro, A.; et al. Characterisation of the Gastric Microbiota in a Pediatric Population According to Helicobacter Pylori Status. Pediatr. Infect. Dis. J. 2017, 36, 173–178. [Google Scholar] [CrossRef]
- Miao, R.; Wan, C.; Wang, Z. The Relationship of Gastric Microbiota and Helicobacter Pylori Infection in Pediatrics Population. Helicobacter 2020, 25, e12676. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A.; et al. Systematic Review: Gastric Microbiota in Health and Disease. Aliment. Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; Tohumcu, E.; Del Vecchio, L.E.; Porcari, S.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The Influence of Helicobacter Pylori on Human Gastric and Gut Microbiota. Antibiotics 2023, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Pereira, V.; Saxena, S.; Ghosh, T.S.; Anbumani, D.; Bag, S.; Das, B.; Nair, G.B.; Abraham, P.; Mande, S.S. Gastric Microbiome of Indian Patients with Helicobacter Pylori Infection, and Their Interaction Networks. Sci. Rep. 2017, 7, 15438. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.E.; Engstrand, L.; Nyrén, O.; Evans, D.J.; Lindgren, A.; Bergström, R.; Andersson, B.; Athlin, L.; Bendtsen, O.; Tracz, P. Helicobacter Pylori Infection: Independent Risk Indicator of Gastric Adenocarcinoma. Gastroenterology 1993, 105, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Ekström, A.M.; Held, M.; Hansson, L.E.; Engstrand, L.; Nyrén, O. Helicobacter Pylori in Gastric Cancer Established by CagA Immunoblot as a Marker of Past Infection. Gastroenterology 2001, 121, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.H. Pylori Infection and the Development of Gastric Cancer. Keio J. Med. 2002, 51, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Aparici, A.; Casaus, M.; Rodríguez, P.; Malagelada, J.R. Subjective Perception of Lactose Intolerance Does Not Always Indicate Lactose Malabsorption. Clin. Gastroenterol. Hepatol. 2010, 8, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; Satin, B.; Norais, N.; De Bernard, M.; Telford, J.L.; Rappuoli, R.; Montecucco, C. Selective Increase of the Permeability of Polarized Epithelial Cell Monolayers by Helicobacter Pylori Vacuolating Toxin. J. Clin. Investig. 1998, 102, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; de Bernard, M.; Milia, E.; Bugnoli, M.; Zerial, M.; Rappuoli, R.; Montecucco, C. Cellular Vacuoles Induced by Helicobacter Pylori Originate from Late Endosomal Compartments. Proc. Natl. Acad. Sci. USA 1994, 91, 9720–9724. [Google Scholar] [CrossRef]
- Burkitt, M.D.; Duckworth, C.A.; Williams, J.M.; Pritchard, D.M. Helicobacter Pylori-Induced Gastric Pathology: Insights from in Vivo and Ex Vivo Models. Dis. Model. Mech. 2017, 10, 89–104. [Google Scholar] [CrossRef]
- Raju, D.; Hussey, S.; Ang, M.; Terebiznik, M.R.; Sibony, M.; Galindo-Mata, E.; Gupta, V.; Blanke, S.R.; Delgado, A.; Romero-Gallo, J.; et al. Vacuolating Cytotoxin and Variants in Atg16L1 That Disrupt Autophagy Promote Helicobacter Pylori Infection in Humans. Gastroenterology 2012, 142, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Greenfield, L.K.; Bronte-Tinkew, D.; Capurro, M.I.; Rizzuti, D.; Jones, N.L. VacA Promotes CagA Accumulation in Gastric Epithelial Cells during Helicobacter Pylori Infection. Sci. Rep. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Rolig, A.S.; Cech, C.; Ahler, E.; Carter, J.E.; Ottemann, K.M. The Degree of Helicobacter Pylori-Triggered Inflammation Is Manipulated by Preinfection Host Microbiota. Infect. Immun. 2013, 81, 1382–1389. [Google Scholar] [CrossRef]
- Lofgren, J.L.; Whary, M.T.; Ge, Z.; Muthupalani, S.; Taylor, N.S.; Mobley, M.; Potter, A.; Varro, A.; Eibach, D.; Suerbaum, S.; et al. Lack of Commensal Flora in Helicobacter pylori–Infected INS-GAS Mice Reduces Gastritis and Delays Intraepithelial Neoplasia. Gastroenterology 2011, 140, 210–220.e4. [Google Scholar] [CrossRef] [PubMed]
- Lertpiriyapong, K.; Whary, M.T.; Muthupalani, S.; Lofgren, J.L.; Gamazon, E.R.; Feng, Y.; Ge, Z.; Wang, T.C.; Fox, J.G. Gastric Colonisation with a Restricted Commensal Microbiota Replicates the Promotion of Neoplastic Lesions by Diverse Intestinal Microbiota in the Helicobacter Pylori INS-GAS Mouse Model of Gastric Carcinogenesis. Gut 2014, 63, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Rickman, B.; Rogers, A.B.; Muthupalani, S.; Takaishi, S.; Peiying, Y.; Wang, T.C.; Fox, J.G. Combination of Sulindac and Antimicrobial Eradication of Helicobacter Pylori Prevents Progression of Gastric Cancer in Hypergastrinemic INS-GAS Mice. Cancer Res. 2009, 69, 8166–8174. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Matsunaga, A.; Fujimura, T.; Tsukamoto, T.; Taketo, M.M.; Oshima, M. Carcinogenesis in Mouse Stomach by Simultaneous Activation of the Wnt Signaling and Prostaglandin E2 Pathway. Gastroenterology 2006, 131, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Hioki, K.; Popivanova, B.K.; Oguma, K.; Van Rooijen, N.; Ishikawa, T.; Oshima, M. Prostaglandin E2 Signaling and Bacterial Infection Recruit Tumor-Promoting Macrophages to Mouse Gastric Tumors. Gastroenterology 2011, 140, 596–607.e7. [Google Scholar] [CrossRef]
- Kwon, S.-K.; Park, J.C.; Kim, K.H.; Yoon, J.; Cho, Y.; Lee, B.; Lee, J.-J.; Jeong, H.; Oh, Y.; Kim, S.-H.; et al. Human Gastric Microbiota Transplantation Recapitulates Premalignant Lesions in Germ-Free Mice. Gut 2022, 71, 1266–1276. [Google Scholar] [CrossRef]
- Shen, Z.; Dzink-Fox, J.; Feng, Y.; Muthupalani, S.; Mannion, A.J.; Sheh, A.; Whary, M.T.; Holcombe, H.R.; Piazuelo, B.M.; Bravo, L.E.; et al. Gastric Non-Helicobacter Pylori Urease-Positive Staphylococcus Epidermidis and Streptococcus Salivarius Isolated from Humans Have Contrasting Effects on H. Pylori-Associated Gastric Pathology and Host Immune Responses in a Murine Model of Gastric Cancer. mSphere 2022, 7, e0077221. [Google Scholar] [CrossRef]
- Hecht, S.S. DNA Adduct Formation from Tobacco-Specific N-Nitrosamines. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 1999, 424, 127–142. [Google Scholar] [CrossRef]
- Tsujiuchi, T.; Masaoka, T.; Sugata, E.; Onishi, M.; Fujii, H.; Shimizu, K.; Honoki, K. Hypermethylation of the Dal-1 Gene in Lung Adenocarcinomas Induced by N-Nitrosobis (2-Hydroxypropyl)amine in Rats. Mol. Carcinog. 2007, 46, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Smet, A.; Kupcinskas, J.; Link, A.; Hold, G.L.; Bornschein, J. The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse? Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Parra-Lara, L.G.; Falla-Martínez, J.C.; Isaza-Pierotti, D.F.; Mendoza-Urbano, D.M.; Tangua-Arias, A.R.; Bravo, J.C.; Bravo, L.E.; Zambrano, Á.R. Gastric Adenocarcinoma Burden, Trends and Survival in Cali, Colombia: A Retrospective Cohort Study. Front. Oncol. 2023, 13, 1069369. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Virulence-Associated Genotypes of Helicobacter Pylori: Do They Explain the African Enigma? Am. J. Gastroenterol. 2002, 97, 2839–2842. [Google Scholar] [CrossRef] [PubMed]
- Dicksved, J.; Lindberg, M.; Rosenquist, M.; Enroth, H.; Jansson, J.K.; Engstrand, L. Molecular Characterization of the Stomach Microbiota in Patients with Gastric Cancer and in Controls. J. Med. Microbiol. 2009, 58, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Rodríguez, N.; Goh, K.L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the Microbiome in Gastric Carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial Overgrowth and Diversification of Microbiota in Gastric Cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal Microbiome Dysbiosis in Gastric Carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Gunathilake, M.N.; Lee, J.; Choi, I.J.; Kim, Y.I.; Ahn, Y.; Park, C.; Kim, J. Association between the Relative Abundance of Gastric Microbiota and the Risk of Gastric Cancer: A Case-Control Study. Sci. Rep. 2019, 9, 13589. [Google Scholar] [CrossRef]
- Yu, G.; Torres, J.; Hu, N.; Medrano-Guzman, R.; Herrera-Goepfert, R.; Humphrys, M.S.; Wang, L.; Wang, C.; Ding, T.; Ravel, J.; et al. Molecular Characterisation of the Human Stomach Microbiota in Gastric Cancer Patients. Front. Cell. Infect. Microbiol. 2017, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Seo, I.; Jha, B.K.; Suh, S.-I.; Suh, M.-H.; Baek, W.-K. Microbial Profile of the Stomach: Comparison between Normal Mucosa and Cancer Tissue in the Same Patient. J. Bacteriol. Virol. 2014, 44, 162. [Google Scholar] [CrossRef][Green Version]
- Chen, X.-H.; Wang, A.; Chu, A.-N.; Gong, Y.-H.; Yuan, Y. Mucosa-Associated Microbiota in Gastric Cancer Tissues Compared With Non-Cancer Tissues. Front. Microbiol. 2019, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shao, L.; Liu, X.; Ji, F.; Mei, Y.; Cheng, Y.; Liu, F.; Yan, C.; Li, L.; Ling, Z. Alterations of Gastric Mucosal Microbiota across Different Stomach Microhabitats in a Cohort of 276 Patients with Gastric Cancer. EBioMedicine 2019, 40, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xin, Y.; Zhou, J.; Tian, Z.; Liu, C.; Yu, X.; Meng, X.; Jiang, W.; Zhao, S.; Dong, Q. Gastric Mucosa-Associated Microbial Signatures of Early Gastric Cancer. Front. Microbiol. 2020, 11, 1548. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The Gastric Precancerous Cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach Microbiota Composition Varies between Patients with Non-Atrophic Gastritis and Patients with Intestinal Type of Gastric Cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef] [PubMed]
- Eun, C.S.; Kim, B.K.; Han, D.S.; Kim, S.Y.; Kim, K.M.; Choi, B.Y.; Song, K.S.; Kim, Y.S.; Kim, J.F. Differences in Gastric Mucosal Microbiota Profiling in Patients with Chronic Gastritis, Intestinal Metaplasia, and Gastric Cancer Using Pyrosequencing Methods. Helicobacter 2014, 19, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Qin, Y.; Sham, P.C.; Lau, K.S.; Chu, K.-M.; Leung, W.K. Alterations in Gastric Microbiota After H. Pylori Eradication and in Different Histological Stages of Gastric Carcinogenesis. Sci. Rep. 2017, 7, 44935. [Google Scholar] [CrossRef]
- He, C.; Peng, C.; Shu, X.; Wang, H.; Zhu, Z.; Ouyang, Y.; Yang, X.; Xie, C.; Hu, Y.; Li, N.; et al. Convergent Dysbiosis of Gastric Mucosa and Fluid Microbiome during Stomach Carcinogenesis. Gastric Cancer 2022, 25, 837–849. [Google Scholar] [CrossRef]
- Lopes, C.; Chaves, J.; Ortigão, R.; Dinis-Ribeiro, M.; Pereira, C. Gastric Cancer Detection by Non-blood-based Liquid Biopsies: A Systematic Review Looking into the Last Decade of Research. United Eur. Gastroenterol. J. 2023, 11, 114–130. [Google Scholar] [CrossRef]
- Hu, Y.L.; Pang, W.; Huang, Y.; Zhang, Y.; Zhang, C.J. The Gastric Microbiome Is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Front. Cell. Infect. Microbiol. 2018, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.Y.; Tung, S.Y.; Pan, H.Y.; Yen, C.W.; Xu, H.W.; Lin, Y.J.; Deng, Y.F.; Hsu, W.T.; Wu, C.S.; Li, C. Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan. Sci. Rep. 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Seo, H.; Kang, C.S.; Shin, T.S.; Kim, J.W.; Park, J.M.; Kim, J.G.; Kim, Y.K. Dysbiotic Change in Gastric Microbiome and Its Functional Implication in Gastric Carcinogenesis. Sci. Rep. 2022, 12, 4285. [Google Scholar] [CrossRef] [PubMed]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.M.A.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M.; et al. Microbiome Differential Abundance Methods Produce Different Results across 38 Datasets. Nat. Commun. 2022, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, S.; Motooka, D.; Watanabe, S.; Kubo, R.; Jung, N.; Midorikawa, Y.; Shinozaki, N.O.; Sawai, Y.; Takeda, A.K.; Nakamura, S. Benchmark of 16S rRNA Gene Amplicon Sequencing Using Japanese Gut Microbiome Data from the V1–V2 and V3–V4 Primer Sets. BMC Genom. 2021, 22, 527. [Google Scholar] [CrossRef]
- Liu, C.; Ng, S.K.; Ding, Y.; Lin, Y.; Liu, W.; Wong, S.H.; Sung, J.J.Y.; Yu, J. Meta-Analysis of Mucosal Microbiota Reveals Universal Microbial Signatures and Dysbiosis in Gastric Carcinogenesis. Oncogene 2022, 41, 3599–3610. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, X.S.; Guo, G.Y.; Zhou, M.G.; Yu, B. Effect of Helicobacter Pylori Eradication on Human Gastric Microbiota: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 899248. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.M.; Kim, N.; Park, J.H.; Lee, D.H. Changes in Gastric Corpus Microbiota With Age and After Helicobacter Pylori Eradication: A Long-Term Follow-Up Study. Front. Microbiol. 2021, 11, 621879. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Coker, O.O.; Chu, E.; Szeto, C.H.; Luk, S.T.Y.; Lau, H.C.H.; Yu, J. Gastric Microbes Associated with Gastric Inflammation, Atrophy and Intestinal Metaplasia 1 Year after Helicobacter Pylori Eradication. Gut 2020, 69, 1572–1580. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A Bacterial Driver–passenger Model for Colorectal Cancer: Beyond the Usual Suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
| Author | Population | Country of Origin | Sample Type | Sequencing Methods | Results |
|---|---|---|---|---|---|
| Dicksved et al. [66] | GC (10) vs. FD (5) | Sweden | Biopsy samples from the antrum and corpus | T-RFLP, 16S rRNA sequencing | ↓ H. Pylori ↑ Streptococcus, Lactobacillus, Veillonella and Prevotella |
| Aviles-Jimenez et al. [77] | NAG (5), IM (5), GC (5) | Mexico | Biopsy samples from the antrum and corpus | Microarray G3 PhyloChip (16sRNA V3) | GC ↓ Porphyromonas, Neisseria and TM7 ↑ Lachnospiraceae and Lactobacillus coleohominis |
| Eun et al. [78] | GC (11), IM (10), CG (10) | Korea | Biopsy samples | 16S rRNA sequencing V5 | GC ↑ Bacilli class and Streptococcaceae family ↓ Epsilonproteobacteria class and Helicobacteriaceae family |
| Castaño-Rodríguez et al. [67] | GC (12), vs. FD (20) | Singapore and Malaysia | Biopsy samples from the antrum | 16S rRNA sequencing V4 | ↑ Lactococcus, Veillonella, Haemophilus, and Fusobacteriaceae |
| Ferreira et al. [17] | GC (54) vs. CG (81) | Portugal | Biopsy samples | 16S rRNA sequencing V5–V6 | ↓ H. Pylori ↑ Achromobacter, Citrobacter, Clostridium, Lactobacillus, and Rhodococcus |
| Coker et al. [69] | GC (20), IM (17), AG (23), SG (21) Validate: GC (19), AG (51), SG (56) | China | Biopsy samples from the antrum, body, and fundus | 16S rRNA sequencing V4 | GC ↑ Parvimonas micra, Dialister pneumosintes, Slackia exigua, Peptostreptococcus stomatis, Prevotella intermedia, Fusobacterium nucleatum, Prevotella oris, and Catonella morbi |
| Li et al. [79] | CG (9), IM (9), GC (7), H. pylori (−) control (8) | Hong Kong | Biopsy samples from the antrum and corpus | 16S rRNA sequencing V3–V4 | GC ↑ Flavobacterium, Klebsiella, Serratia marcescens, Stenotrophomnonas, Achromobacter, and Pseudomonas |
| Sun et al. [27] | H. pylori (−) CG (56), H. pylori (−) AG (9), H. pylori (−) IM (27), H. pylori (−) Dys (29), H. pylori (−) GC (13). | China | Biopsy samples and gastric juice | 16S rRNA sequencing V3–V4 | From AG to Dys ↑ Burkholderiaceae ↓ Streptococcaceae and Prevotellaceae GC ↑ Streptococcaceae and Lactobacillaceae |
| He et al. [80] | GC (61), IM (55), GC (64) | China | Biopsy samples and gastric juice | 16S rRNA sequencing V4 | GC classifiers in both GM and GF, including Lactobacillus, Veillonella, Gemella |
| Gunathilake et al. [70] | GC (268) vs. Controls (288) | Korea | Biopsy samples | 16S rRNA sequencing V3–V4 | ↑ Propionibacterium acnes, Prevotella copri ↓ Lactococcus lactis |
| Hu et al. [82] | SG (5), GC (6) | China | Gastric wash samples | Shotgun metagenomic sequencing | ↓ Sphingobium yanoikuyae, ↑ Aggregatibacter, Alloprevotella, and Neisseria |
| Hsieh et al. [83] | CG (9), IM (7), GC (11) | Taiwan | Biopsy samples | 16S rRNA sequencing V3–V4 | ↓ H. Pylori ↑ Fusobacterium, Lactobacillus and Clostridium |
| Yu et al. [71] | GC (160) | China and Mexico | Biopsy samples from non-malignant and tumor tissues | 16S rRNA sequencing V3–V4 | GC is dominated by Proteobacteria, Bacteroidetes (Chinese), or Firmicutes (Mexican) H. pylori abundance is lower in tumor tissue compared to matched non-malignant tissue |
| Liu et al. [74] | GC (276) | China | Biopsy samples from non-malignant and tumor tissues | 16S rRNA sequencing V3–V4 | ↓ H. pylori, Prevotella copri, and Bacteroides uniformis ↑ Prevotella melaninogenica, Streptococcus anginosus, and Propionibacterium acnes |
| Park et al. [84] | CG (16), GAD (16), EGC (36), AGC (20) | Korea | Gastric juice samples | 16S rRNA sequencing V3–V4 | CG ↑ Akkermansia and Lachnospiraceae NK4A136 Group GC ↑ Lactobacillus and Veillonella. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgamato, C.; Rocco, A.; Compare, D.; Priadko, K.; Romano, M.; Nardone, G. Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer. Antibiotics 2024, 13, 484. https://doi.org/10.3390/antibiotics13060484
Sgamato C, Rocco A, Compare D, Priadko K, Romano M, Nardone G. Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer. Antibiotics. 2024; 13(6):484. https://doi.org/10.3390/antibiotics13060484
Chicago/Turabian StyleSgamato, Costantino, Alba Rocco, Debora Compare, Kateryna Priadko, Marco Romano, and Gerardo Nardone. 2024. "Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer" Antibiotics 13, no. 6: 484. https://doi.org/10.3390/antibiotics13060484
APA StyleSgamato, C., Rocco, A., Compare, D., Priadko, K., Romano, M., & Nardone, G. (2024). Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer. Antibiotics, 13(6), 484. https://doi.org/10.3390/antibiotics13060484






