Antimicrobial Susceptibility of Bacterial Pathogens from Patients with Ocular Surface Infections in Germany, 2020–2021: A Comparison with the Data from Three Previous National Studies
Abstract
1. Introduction
2. Results and Discussion
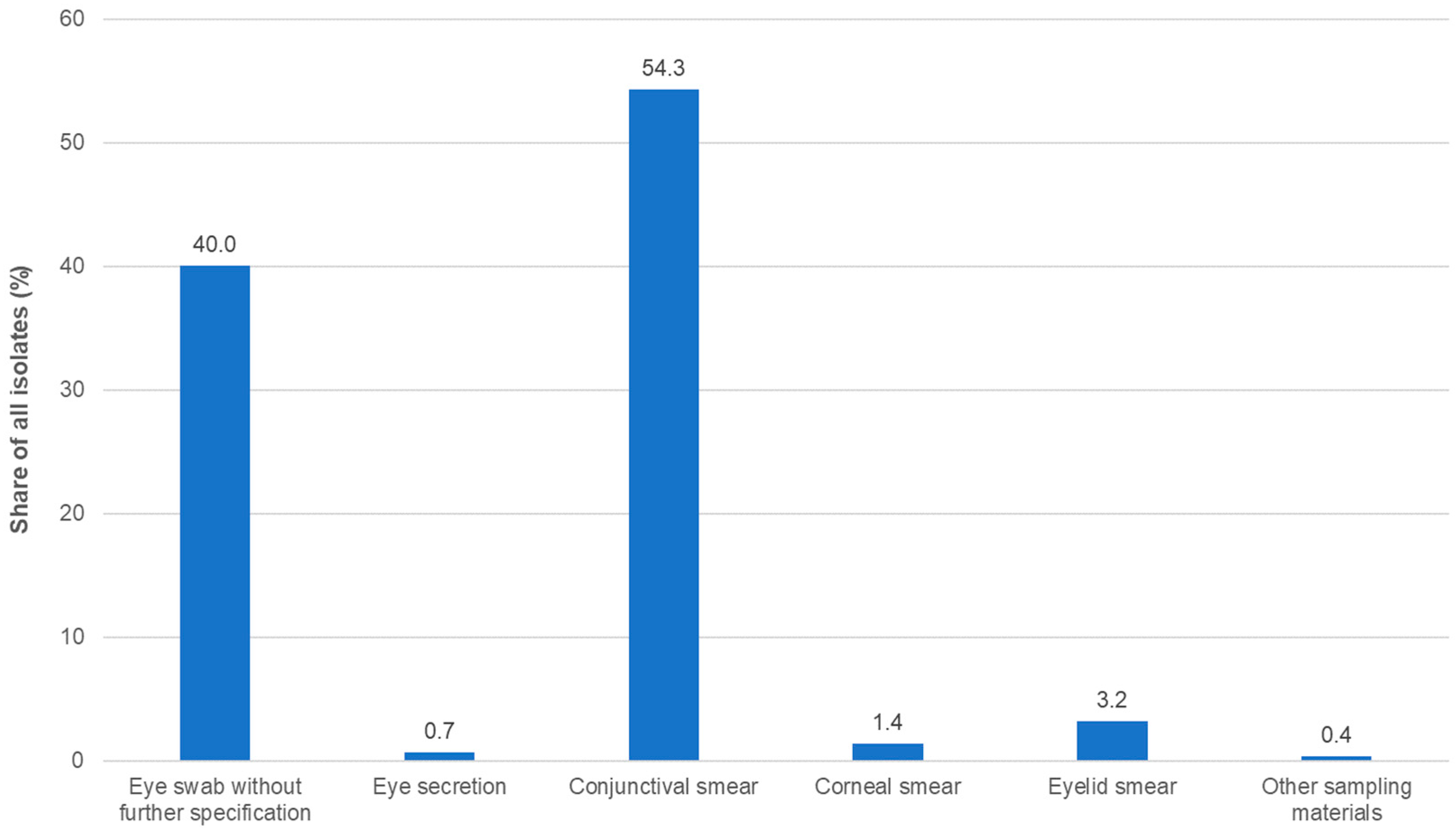
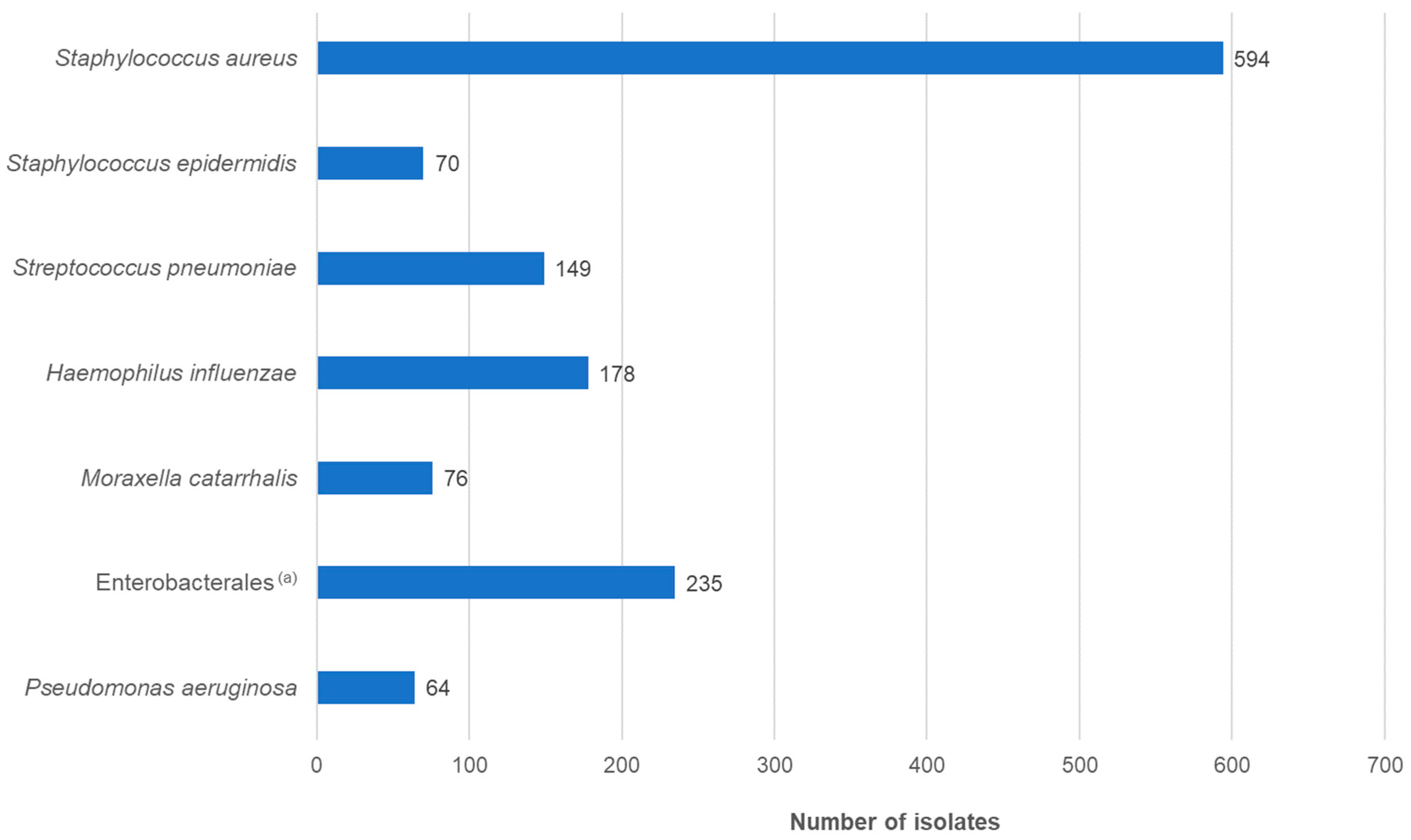
2.1. Bacterial Isolates and Patient Demographic Data
2.2. MIC Frequency Distributions and Ratios of Susceptibility to Resistant Isolates
2.2.1. Staphylococcus aureus
2.2.2. Staphylococcus epidermidis
2.2.3. Streptococcus pneumoniae
2.2.4. Haemophilus influenzae
2.2.5. Moraxella catarrhalis
2.2.6. Pseudomonas aeruginosa
2.2.7. Enterobacterales
3. Materials and Methods
3.1. Study Design
3.2. Clinical Isolates
3.3. Confirmation of Species Identification and Susceptibility Testing
3.4. Breakpoints
3.5. Quality Control
3.6. Statistical Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, S.; Cabrera-Aguas, M.; Khoo, P. Common eye infections. Aust. Prescr. 2018, 41, 67–72. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Liu, A.S.-H. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst. Rev. 2023, 2023, CD001211. [Google Scholar]
- EClinicalMedicine (editorial). Antimicrobial resistance: A top ten global public health threat. EClinicalMedicine 2021, 41, 101221. [Google Scholar] [CrossRef] [PubMed]
- Kresken, M.; Behrens-Baumann, W. Resistenzsituation bei bakteriellen Erregern von oberflächlichen Augeninfektionen gegenüber Antibiotika. Chemother. J. 2007, 16, 49–59. [Google Scholar]
- Kresken, M.; Körber-Irrgang, B.; Behrens-Baumann, W. Antibiotika-Resistenzsituation bakterieller Erreger von Patienten mit Infektionen der Augenoberfläche in Deutschland. Chemother. J. 2012, 21, 8–24. [Google Scholar]
- Körber-Irrgang, B.; Kresken, M. Multizentrische Studie zur Bestimmung der Antibiotika-Resistenzsituation bei Isolaten von Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Moraxella catarrhalis, Haemophilus influenzae und Pseudomonas aeruginosa als Ursache von Infektionen der Augenoberfläche in Deutschland (Ophthalmika-Studie III); Final Report June 3rd 2016; Antiinfectives Intelligence GmbH: Cologne, Germany, 2016. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). ECDC Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&HealthTopic=4 (accessed on 27 November 2023).
- Individuelle Datenbankabfrage—Arbeitsgemeinschaft “Empfindlichkeitsprüfung und Resistenz”, Paul-Ehrlich-Gesellschaft für Infektionstherapie e. V. Available online: https://www.p-e-g.org/resistenz/database/ (accessed on 27 November 2023).
- Robert Koch-Institute. ARS. Available online: https://ars.rki.de (accessed on 27 November 2023).
- Roth, M.; Goerke, P.; Holtmann, C.; Frings, A.; MacKenzie, C.R.; Geerling, G. Spectrum and resistance in bacterial infections of the ocular surface in a German tertiary referral center 2009–2019. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.E.; Niruttan, K.; Rawson, T.M.; Moore, L.S.P. Antibacterial resistance in ophthalmic infections: A multi-centre analysis across UK care settings. BMC Infect. Dis. 2019, 19, 768. [Google Scholar] [CrossRef] [PubMed]
- Daley, J.R.; Lee, M.R.; Kyu, M.; Wang, X.; Ly, M.; Samarawickrama, C. Epidemiology and Economic Cost Analysis of Microbial Keratitis from a Tertiary Referral Hospital in Australia. Pathogens 2023, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Sanfilippo, C.M.; Sahm, D.F.; DeCory, H.H. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol. 2020, 138, 439–450. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Expected Resistant Phenotypes. Version 1.2 January 2023. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Expert_Rules/2023/Expected_Resistant_Phenotypes_v1.2_20230113.pdf (accessed on 27 November 2023).
- ISO 20776-1:2019; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1. Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. International Organization for Standardization: Geneva, Switzerland, 2019.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Media Preparation for EUCAST Disk Diffusion Testing and for Determination of MIC Values by the Broth Microdilution Method. Version 7.0, 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/Media_preparation_v_7.0_EUCAST_AST_2022.pdf (accessed on 27 November 2023).
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial Wild Type Distributions of Microorganisms. Available online: https://mic.eucast.org/ (accessed on 30 November 2023).
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.1, 2023. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.1_Breakpoint_Tables.pdf (accessed on 27 November 2023).
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Routine and Extended Internal Quality Control for MIC Determination and Disk Diffusion as Recommended by EUCAST. Version 13.2, 2023. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/QC/v_13.2_EUCAST_QC_tables_routine_and_extended_QC.pdf (accessed on 27 November 2023).
- Sergeant; ESG. Epitools Epidemiological Calculators. Ausvet. 2018. Available online: https://epitools.ausvet.com.au/ (accessed on 27 November 2023).
| Number of Isolates | Antibiotic | MIC (mg/L) | % Susceptible Isolates (Wild-Types) | % Resistant Isolates (Non-Wild-Types) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50% | 90% | ||||||||||||||||||||
| OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | ||
| All | 436 | 395 | 360 | 594 | CXI 1 | n.t. | 4 | 4 | 4 | n.t. | 4 | 4 | 4 | n.t. | 91.6 | 96.4 | 96.6 | n.t. | 8.4 | 3.6 | 3.4 |
| OXA 1 | 0.5 | 0.5 | 0.5 | 0.25 | 2 | 2 | 1 | 0.5 | 90.1 | 91.6 | 96.4 | 96.8 | 9.9 | 8.4 | 3.6 | 3.2 | |||||
| CHL | 8 | 8 | 4 | 8 | 8 | 8 | 8 | 8 | 97.2 | 99.5 | 99.4 | 99.7 | 2.8 | 0.5 | 0.6 | 0.3 | |||||
| GEN | 0.5 | 0.5 | 0.5 | 0.5 | 2 | 1 | 0.5 | 1 | 91.1 | 96.2 | 98.1 | 98.1 | 8.9 | 3.8 | 1.9 | 1.9 | |||||
| KAN | 4 | 2 | 2 | 2 | 32 | 8 | 4 | 4 | 86.7 | 91.9 | 96.1 | 96.3 | 13.3 | 8.1 | 3.9 | 3.7 | |||||
| NEO | n.t. | n.t. | n.t. | 0.5 | n.t. | n.t. | n.t. | 1 | n.t. | n.t. | n.t. | 96.3 | n.t. | n.t. | n.t. | 3.7 | |||||
| LEV | 0.25 | 0.25 | 0.25 | 0.25 | 4 | 16 | 16 | 0.5 | 87.8 | 86.1 | 88.9 | 92.6 | 12.2 | 13.9 | 11.1 | 7.4 | |||||
| OFL | 0.5 | 0.5 | 0.25 | 0.5 | 8 | 16 | 16 | 1 | 86.9 | 86.1 | 88.9 | 92.4 | 13.1 | 13.9 | 11.1 | 7.6 | |||||
| OXY | n.t. | 0.25 | n.t. | 1 | n.t. | 0.5 | n.t. | 1 | n.t. | 95.7 | n.t. | 97.3 | n.t. | 4.3 | n.t. | 2.7 | |||||
| Methicillin-susceptible (MSSA) 1 | 393 | 362 | 347 | 574 | CHL | 8 | 8 | 4 | 8 | 8 | 8 | 8 | 8 | 98 | 100 | 99.4 | 99.7 | 2 | 0 | 0.6 | 0.3 |
| GEN | 0.5 | 0.5 | 0.5 | 0.5 | 2 | 1 | 0.5 | 0.5 | 94.1 | 97.5 | 98.6 | 98.3 | 5.9 | 2.5 | 1.4 | 1.7 | |||||
| KAN | 4 | 2 | 2 | 2 | 8 | 4 | 4 | 4 | 92.1 | 96.7 | 97.4 | 97.2 | 7.9 | 3.3 | 2.6 | 2.8 | |||||
| NEO | n.t. | n.t. | n.t. | 0.5 | n.t. | n.t. | n.t. | 1 | n.t. | n.t. | n.t. | 97.2 | n.t. | n.t. | n.t. | 2.8 | |||||
| LEV | 0.25 | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 93.6 | 93.1 | 91.6 | 93.6 | 6.4 | 6.9 | 8.4 | 6.4 | |||||
| OFL | 0.5 | 0.5 | 0.25 | 0.5 | 1 | 0.5 | 1 | 1 | 93.4 | 93.1 | 91.6 | 93.4 | 6.6 | 6.9 | 8.4 | 6.6 | |||||
| OXY | n.t. | 0.25 | n.t. | 1 | n.t. | 0.5 | n.t. | 1 | n.t. | 96.7 | n.t. | 97.7 | n.t. | 3.3 | n.t. | 2.3 | |||||
| Methicillin-resistant (MRSA) 1 | 43 | 33 | 13 | 20 | CHL | 8 | 8 | 8 | 8 | 16 | 8 | 8 | 8 | 90.7 | 93.9 | 100 | 100 | 9.3 | 6.1 | 0 | 0 |
| GEN | 1 | 0.5 | 0.5 | 0.5 | 64 | 32 | 16 | 1 | 62.8 | 81.8 | 84.6 | 95 | 37.2 | 18.2 | 15.4 | 5 | |||||
| KAN | 64 | 32 | 2 | 2 | 64 | 32 | 64 | 512 | 37.2 | 39.4 | 61.5 | 70 | 62.8 | 60.6 | 38.5 | 30 | |||||
| NEO | n.t. | n.t. | n.t. | 0.5 | n.t. | n.t. | n.t. | 32 | n.t. | n.t. | n.t. | 70 | n.t. | n.t. | n.t. | 30 | |||||
| LEV | 16 | 16 | 16 | 0.5 | 32 | 16 | 16 | 32 | 34.9 | 9.1 | 15.4 | 65 | 65.1 | 90.9 | 84.6 | 35 | |||||
| OFL | 32 | 16 | 32 | 0.5 | 32 | 16 | 32 | 32 | 27.9 | 9.1 | 15.4 | 65 | 72.1 | 90.9 | 84.6 | 35 | |||||
| OXY | n.t. | 0.5 | n.t. | 1 | n.t. | 32 | n.t. | 4 | n.t. | 84.8 | n.t. | 85 | n.t. | 15.2 | n.t. | 15 | |||||
| Species | Number of Isolates | Antibiotic | MIC (mg/L) | % Susceptible Isolates (Wild-Types) | % Resistant Isolates (Non-Wild-Types) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50% | 90% | ||||||||||||||||||||
| OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | ||
| S. epidermidis | 80 | 91 | 101 | 70 | CXI | n.t. | 2 | 2 | 2 | n.t. | 32 | 32 | 32 | n.t. | 70.6 | 60.4 | 71.4 | n.t. | 26.4 | 39.6 | 28.6 |
| OXA | 2 | 0.25 | 0.25 | 0.25 | 32 | 32 | 8 | 16 | 48.8 | 69.2 | 51.5 | 68.1 | 51.3 | 30.8 | 48.5 | 32.9 | |||||
| CHL | 4 | 4 | 4 | 4 | 8 | 4 | 4 | 8 | 92.5 | 98.9 | 99 | 98.6 | 7.5 | 1.1 | 1 | 1.4 | |||||
| GEN | 0.125 | 0.25 | 0.25 | 0.125 | 16 | 32 | 32 | 32 | 57.5 | 69.2 | 51.5 | 73.6 | 42.5 | 30.8 | 48.5 | 27.1 | |||||
| KAN | 4 | 2 | 2 | 1 | 64 | 32 | 64 | 256 | - | - | - | - | - | - | - | - | |||||
| NEO | n.t. | n.t. | n.t. | 0.125 | n.t. | n.t. | n.t. | 1 | n.t. | n.t. | n.t. | - | n.t. | n.t. | n.t. | - | |||||
| LEV | 0.25 | 0.25 | 0.25 | 0.25 | 8 | 8 | 8 | 4 | 70 | 69.2 | 68.3 | 68.1 | 28.7 | 30.8 | 31.7 | 32.9 | |||||
| OFL | 0.5 | 0.5 | 0.5 | 0.5 | 16 | 16 | 16 | 8 | - | - | - | - | - | - | - | - | |||||
| OXY | n.t. | 1 | n.t. | 1 | n.t. | 16 | n.t. | 32 | n.t. | 85.7 | n.t. | 59.7 | n.t. | 14.3 | n.t. | 41.4 | |||||
| S. pneumoniae | 187 | 212 | 240 | 149 | CHL | 2 | 2 | 2 | 2 | 4 | 2 | 2 | 4 | 98.9 | 99.5 | 100 | 100 | 1.1 | 0.5 | 0 | 0 |
| GEN | 4 | 4 | 4 | 8 | 8 | 8 | 8 | 8 | - | - | - | - | - | - | - | - | |||||
| KAN | 32 | 16 | 32 | 32 | 64 | 32 | 64 | 64 | - | - | - | - | - | - | - | - | |||||
| NEO | n.t. | n.t. | n.t. | 64 | n.t. | n.t. | n.t. | 64 | n.t. | n.t. | n.t. | - | n.t. | n.t. | n.t. | - | |||||
| LEV | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 1 | 98.4 | 100 | 99.6 | 100 | 1.6 | 0 | 0.4 | 0 | |||||
| OFL | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 98.4 | 100 | 99.6 | 100 | 1.6 | 0 | 0.4 | 0 | |||||
| OXY | n.t. | 0.25 | n.t. | 0.25 | n.t. | 0.5 | n.t. | 0.5 | n.t. | 92.5 | n.t. | 96.0 | n.t. | 7.5 | n.t. | 4.0 | |||||
| Species | Number of Isolates | Antibiotic | MIC (mg/L) | % Susceptible Isolates (Wild-Types) | % Resistant Isolates (Non-Wild-Types) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50% | 90% | ||||||||||||||||||||
| OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | OS1 | OS2 | OS3 | OS4 | ||
| H. influenzae | 164 | 234 | 325 | 178 | CHL | 0.5 | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 100 | 98.3 | 99.1 | 98.2 | 0 | 1.7 | 0.9 | 1.7 |
| GEN | 2 | 0.5 | 1 | 0.5 | 2 | 1 | 1 | 1 | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | |||||
| KAN | 2 | 1 | 2 | 2 | 4 | 2 | 4 | 4 | - | - | - | - | - | - | - | - | |||||
| NEO | n.t. | n.t. | n.t. | 2 | n.t. | n.t. | n.t. | 2 | n.t. | n.t. | n.t. | - | n.t. | n.t. | n.t. | - | |||||
| LEV | 0.016 | 0.031 | 0.063 | 0.016 | 0.031 | 0.031 | 0.063 | 0.031 | 98.8 | 97 | 96.9 | 96.1 | 1.2 | 3 | 3.1 | 3.9 | |||||
| OFL | 0.031 | 0.063 | 0.063 | 0.031 | 0.063 | 0.063 | 0.063 | 0.063 | 98.2 | 94.9 | 96 | 96.1 | 1.8 | 5.1 | 4 | 3.9 | |||||
| OXY | n.t. | 0.125 | n.t. | 0.5 | n.t. | 0.25 | n.t. | 0.5 | n.t. | 99.6 | n.t. | 98.3 | n.t. | 0.9 | n.t. | 1.7 | |||||
| M. catarrhalis | 33 | 59 | 50 | 76 | CHL | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 1 | 0.5 | 100 | 98.3 | 100 | 100 | 0 | 1.7 | 0 | 0 |
| GEN | 0.25 | 0.5 | 0.25 | 0.125 | 0.5 | 0.5 | 0.25 | 0.5 | - | - | - | - | - | - | - | - | |||||
| KAN | 1 | 1 | 1 | 0.5 | 2 | 2 | 1 | 1 | - | - | - | - | - | - | - | - | |||||
| NEO | n.t. | n.t. | n.t. | 0.25 | n.t. | n.t. | n.t. | 0.25 | n.t. | n.t. | n.t. | - | n.t. | n.t. | n.t. | - | |||||
| LEV | 0.063 | 0.063 | 0.063 | 0.063 | 0.063 | 0.063 | 0.063 | 0.063 | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | |||||
| OFL | 0.063 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | |||||
| OXY | n.t. | 0.25 | n.t. | 0.5 | n.t. | 0.5 | n.t. | 0.5 | n.t. | 100 | n.t. | 100 | n.t. | 0 | n.t. | 0 | |||||
| P. aeruginosa | 45 | 36 | 34 | 64 | CHL | 64 | 64 | 64 | 64 | 128 | 64 | 64 | 256 | - | - | - | - | - | - | - | - |
| GEN | 2 | 2 | 1 | 2 | 4 | 4 | 2 | 2 | 97.8 | 100 | 100 | 96.9 | 2.2 | 0 | 0 | 3.1 | |||||
| KAN | 64 | 32 | 64 | 64 | 64 | 32 | 64 | 128 | n.a. | n.a. | n.a. | 98.4 | n.a. | n.a. | n.a. | 1.6 | |||||
| NEO | n.t. | n.t. | n.t. | 8 | n.t. | n.t. | n.t. | 16 | n.t. | n.t. | n.t. | 100 | n.t. | n.t. | n.t. | 0 | |||||
| LEV | 0.5 | 0.5 | 0.5 | 0.5 | 4 | 4 | 1 | 1 | 86.7 | 88.9 | 100 | 96.9 | 13.3 | 11.1 | 0 | 3.1 | |||||
| OFL | 1 | 1 | 1 | 1 | 8 | 8 | 2 | 2 | 86.7 | 88.9 | 100 | 98.4 | 13.3 | 11.1 | 0 | 1.6 | |||||
| OXY | n.t. | 16 | n.t. | 16 | n.t. | 32 | n.t. | 32 | n.t. | n.a. | n.t. | 100 | n.t. | n.a. | n.t. | 0 | |||||
| Enterobacterales | 164 | 129 | n.t. | 235 | CHL | 8 | 4 | n.t. | 8 | 16 | 32 | n.t. | 16 | 91.5 | 88.4 | n.t. | 93.6 | 8.5 | 11.6 | n.t. | 6.4 |
| GEN | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 98.2 | 96.9 | 96.6 | 1.8 | 3.1 | 3.4 | |||||||||
| KAN | 2 | 2 | 2 | 8 | 4 | 4 | - | - | - | - | - | - | |||||||||
| NEO | n.t. | n.t. | 0.5 | n.t. | n.t. | 2 | n.t. | n.t. | 98.7 | n.t. | n.t. | 1.3 | |||||||||
| LEV | 0.063 | 0.063 | 0.063 | 0.125 | 0.125 | 0.25 | 98.2 | 94.6 | 91.5 | 1.8 | 5.4 | 8.5 | |||||||||
| OFL | 0.125 | 0.125 | 0.125 | 0.25 | 0.25 | 0.5 | 93.9 | 91.5 | 86.8 | 6.1 | 8.5 | 13.2 | |||||||||
| OXY | n.t. | 2 | 4 | n.t. | 32 | 128 | n.t. | - | - | n.t. | - | - | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohlfarth, E.; Kresken, M.; Deuchert, F. Antimicrobial Susceptibility of Bacterial Pathogens from Patients with Ocular Surface Infections in Germany, 2020–2021: A Comparison with the Data from Three Previous National Studies. Antibiotics 2024, 13, 471. https://doi.org/10.3390/antibiotics13060471
Wohlfarth E, Kresken M, Deuchert F. Antimicrobial Susceptibility of Bacterial Pathogens from Patients with Ocular Surface Infections in Germany, 2020–2021: A Comparison with the Data from Three Previous National Studies. Antibiotics. 2024; 13(6):471. https://doi.org/10.3390/antibiotics13060471
Chicago/Turabian StyleWohlfarth, Esther, Michael Kresken, and Fabian Deuchert. 2024. "Antimicrobial Susceptibility of Bacterial Pathogens from Patients with Ocular Surface Infections in Germany, 2020–2021: A Comparison with the Data from Three Previous National Studies" Antibiotics 13, no. 6: 471. https://doi.org/10.3390/antibiotics13060471
APA StyleWohlfarth, E., Kresken, M., & Deuchert, F. (2024). Antimicrobial Susceptibility of Bacterial Pathogens from Patients with Ocular Surface Infections in Germany, 2020–2021: A Comparison with the Data from Three Previous National Studies. Antibiotics, 13(6), 471. https://doi.org/10.3390/antibiotics13060471






