Pharmacovigilance Strategies to Address Resistance to Antibiotics and Inappropriate Use—A Narrative Review
Abstract
1. Introduction
2. The Role of PV
3. PV Strategies Useful for Monitoring the Use of ABs and Combating AMR
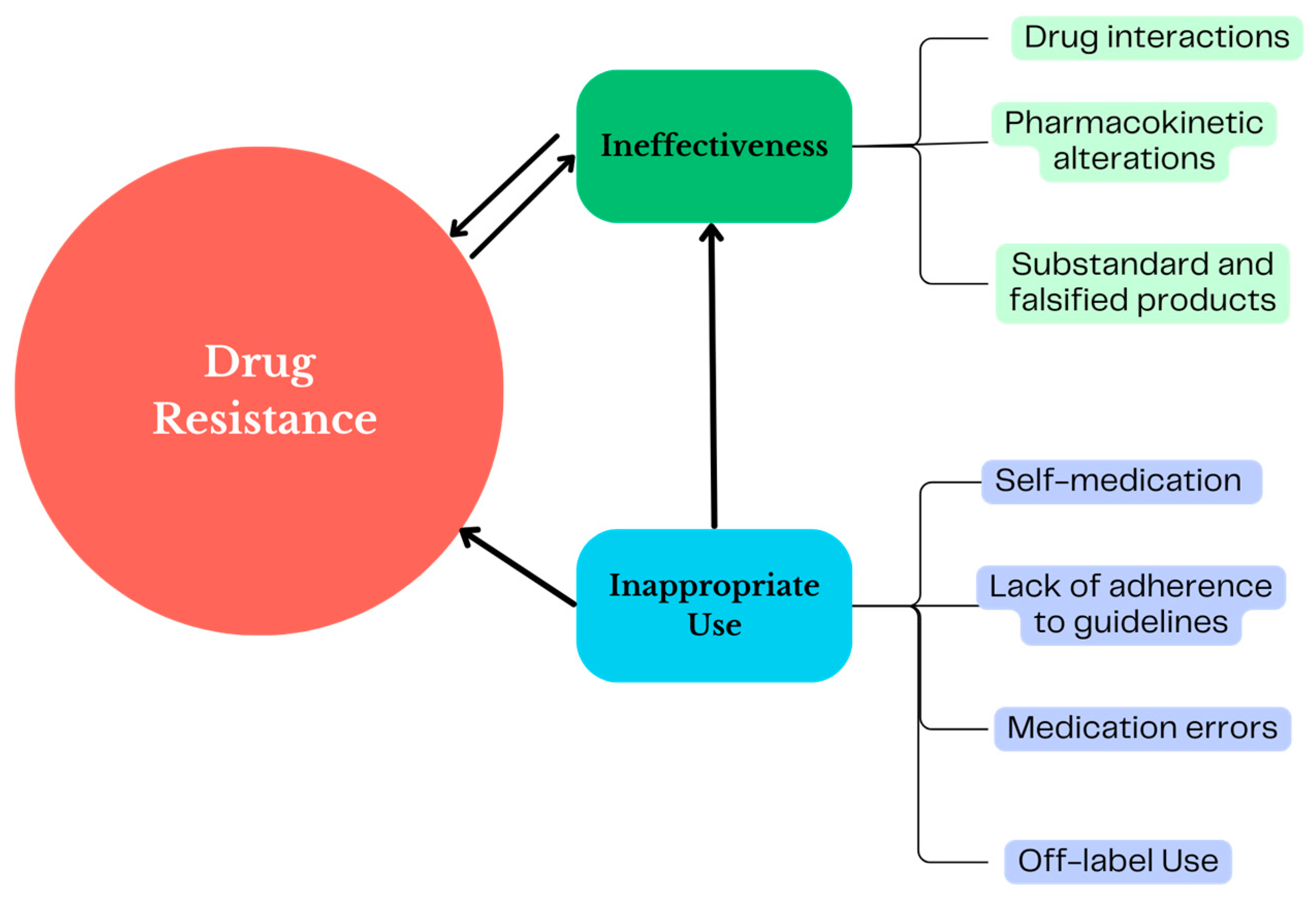
4. PV as a Tool to Identify the Ineffectiveness of Antibiotics
5. PV as a Tool to Identify Inappropriate Uses of Antibiotics
Off-Label Use of Antibiotics
6. Discussion
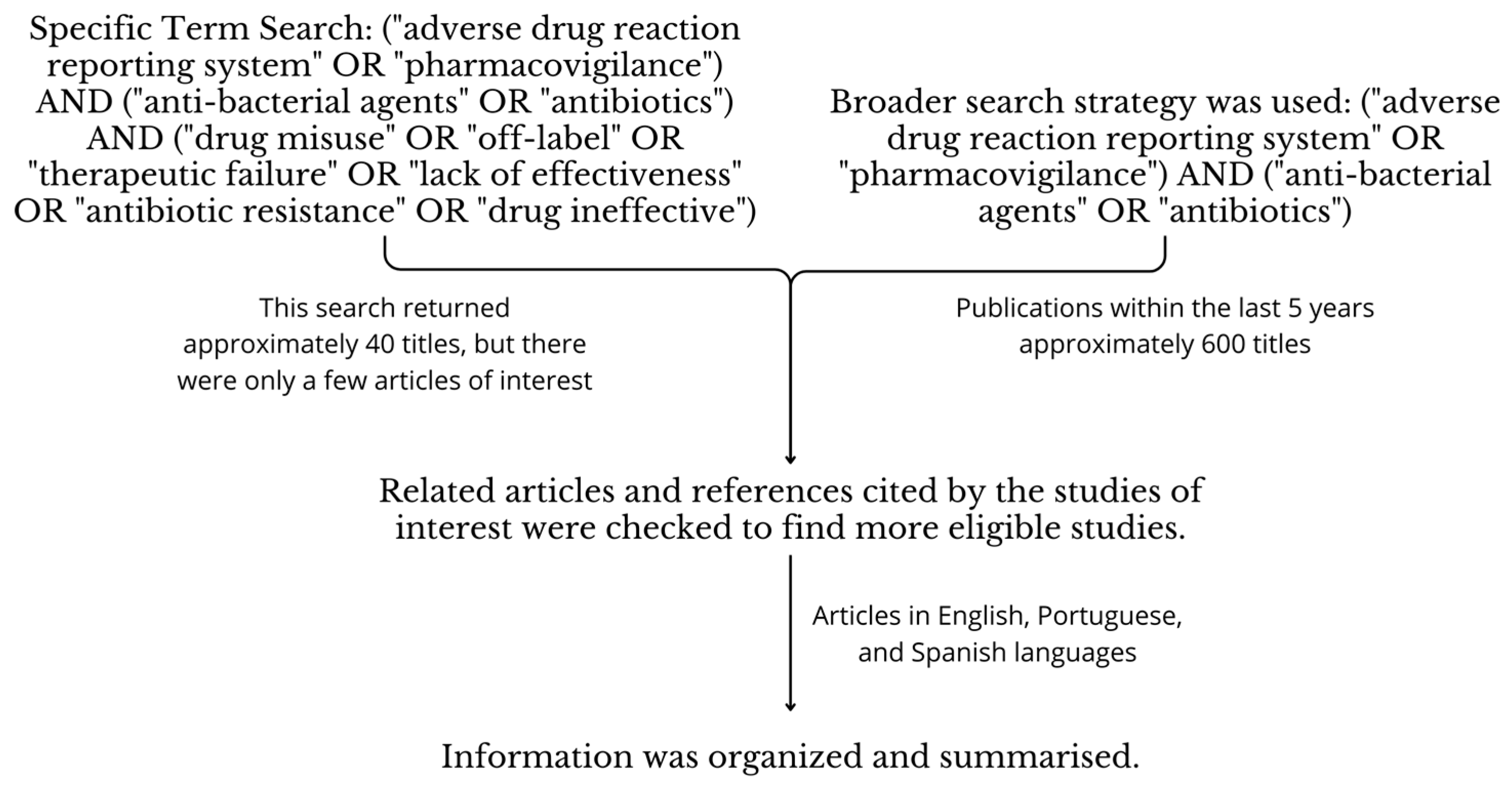
7. Materials and Methods
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kua, K. Antibiotics and Antibiotic Resistance- Flipsides of the Same Coin. Curr. Pharm. Des. 2022, 28, 2312–2329. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Di Domenico, M.; Montagnani, M.; Jirillo, E. Antibiotic Resistance and Microbiota Response. Curr. Pharm. Des. 2023, 29, 356–364. [Google Scholar] [CrossRef]
- Fishbein, S.R.S.; Mahmud, B.; Dantas, G. Antibiotic Perturbations to the Gut Microbiome. Nat. Rev. Microbiol. 2023, 21, 772–788. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of Antibiotic Residues in Animal Food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.M.; Kurui, P.; Sharma, G.; Ochieng, L.; Nganga, F.; Gudda, F.; Muthini, J.M.; Grace, D.; Dione, M.; Moodley, A.; et al. Antibiotic Quality and Use Practices amongst Dairy Farmers and Drug Retailers in Central Kenyan Highlands. Sci. Rep. 2023, 13, 23101. [Google Scholar] [CrossRef]
- Matin, M.A.; Khan, W.A.; Karim, M.M.; Ahmed, S.; John-Langba, J.; Sankoh, O.A.; Gyapong, M.; Kinsman, J.; Wertheim, H. What Influences Antibiotic Sales in Rural Bangladesh? A Drug Dispensers’ Perspective. J. Pharm. Policy Pract. 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Malik, F.; Figueras, A. Analysis of the Antimicrobial Market in Pakistan: Is It Really Necessary Such a Vast Offering of “Watch” Antimicrobials? Antibiotics 2019, 8, 189. [Google Scholar] [CrossRef]
- Ateshim, Y.; Bereket, B.; Major, F.; Emun, Y.; Woldai, B.; Pasha, I.; Habte, E.; Russom, M. Prevalence of Self-Medication with Antibiotics and Associated Factors in the Community of Asmara, Eritrea: A Descriptive Cross Sectional Survey. BMC Public Health 2019, 19, 726. [Google Scholar] [CrossRef]
- Kirchhelle, C.; Atkinson, P.; Broom, A.; Chuengsatiansup, K.; Ferreira, J.P.; Fortané, N.; Frost, I.; Gradmann, C.; Hinchliffe, S.; Hoffman, S.J.; et al. Setting the Standard: Multidisciplinary Hallmarks for Structural, Equitable and Tracked Antibiotic Policy. BMJ Glob. Health 2020, 5, e003091. [Google Scholar] [CrossRef]
- Guinovart, M.C.; Figueras, A.; Llop, J.C.; Llor, C. Obtaining Antibiotics without Prescription in Spain in 2014: Even Easier Now than 6 Years Ago. J. Antimicrob. Chemother. 2015, 70, 1270–1271. [Google Scholar] [CrossRef]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of Antimicrobial Resistance in Low- and Middle-Income Countries: A Scattered Picture. Antimicrob. Resist. Infect. Control 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS). Available online: https://www.who.int/initiatives/glass (accessed on 10 February 2024).
- World Health Organization. GLASS Dashboard. Available online: https://worldhealthorg.shinyapps.io/glass-dashboard/_w_270f2329/#!/home (accessed on 12 April 2024).
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022—World. Available online: https://reliefweb.int/report/world/global-antimicrobial-resistance-and-use-surveillance-system-glass-report-2022 (accessed on 8 March 2024).
- World Health Organization. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 12 April 2024).
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H. Hospital Antibiotic Prescribing Patterns in Adult Patients According to the WHO Access, Watch and Reserve Classification (AWaRe): Results from a Worldwide Point Prevalence Survey in 69 Countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.O.; Barbosa, X.B.C.; Rebelo, S.R.; Fernandez-Llimos, F.; Lima, E.C. Geographical Variation in Antimicrobial Use and Multiresistant Pathogens in Brazilian Intensive Care Units: A Nationwide Study. J. Infect. Dev. Ctries 2023, 17, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.; Ramadan, M.; Haif, H.; Abdullah, B.; Mubarak, J.; Ahmad, W.; Mari, S.; Hassan, S.; Eid, R.; Hasan, M.; et al. Empowering Low- and Middle-Income Countries to Combat AMR by Minimal Use of Antibiotics: A Way Forward. Antibiotics 2023, 12, 1504. [Google Scholar] [CrossRef]
- Marin, G.H.; Giangreco, L.; Dorati, C.; Mordujovich, P.; Boni, S.; Mantilla-Ponte, H.; Alfonso Arvez, M.J.; López Peña, M.; Aldunate González, M.F.; Ching Fung, S.M.; et al. Antimicrobial Consumption in Latin American Countries: First Steps of a Long Road Ahead. J. Prim. Care Community Health 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sohaili, A.; Asin, J.; Thomas, P.P.M. The Fragmented Picture of Antimicrobial Resistance in Kenya: A Situational Analysis of Antimicrobial Consumption and the Imperative for Antimicrobial Stewardship. Antibiotics 2024, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Policy Guidance on Integrated Antimicrobial Stewardship Activities 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Lim, C.; Ashley, E.A.; Hamers, R.L.; Turner, P.; Kesteman, T.; Akech, S.; Corso, A.; Mayxay, M.; Okeke, I.N.; Limmathurotsakul, D.; et al. Surveillance Strategies Using Routine Microbiology for Antimicrobial Resistance in Low- and Middle-Income Countries. Clin. Microbiol. Infect. 2021, 27, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
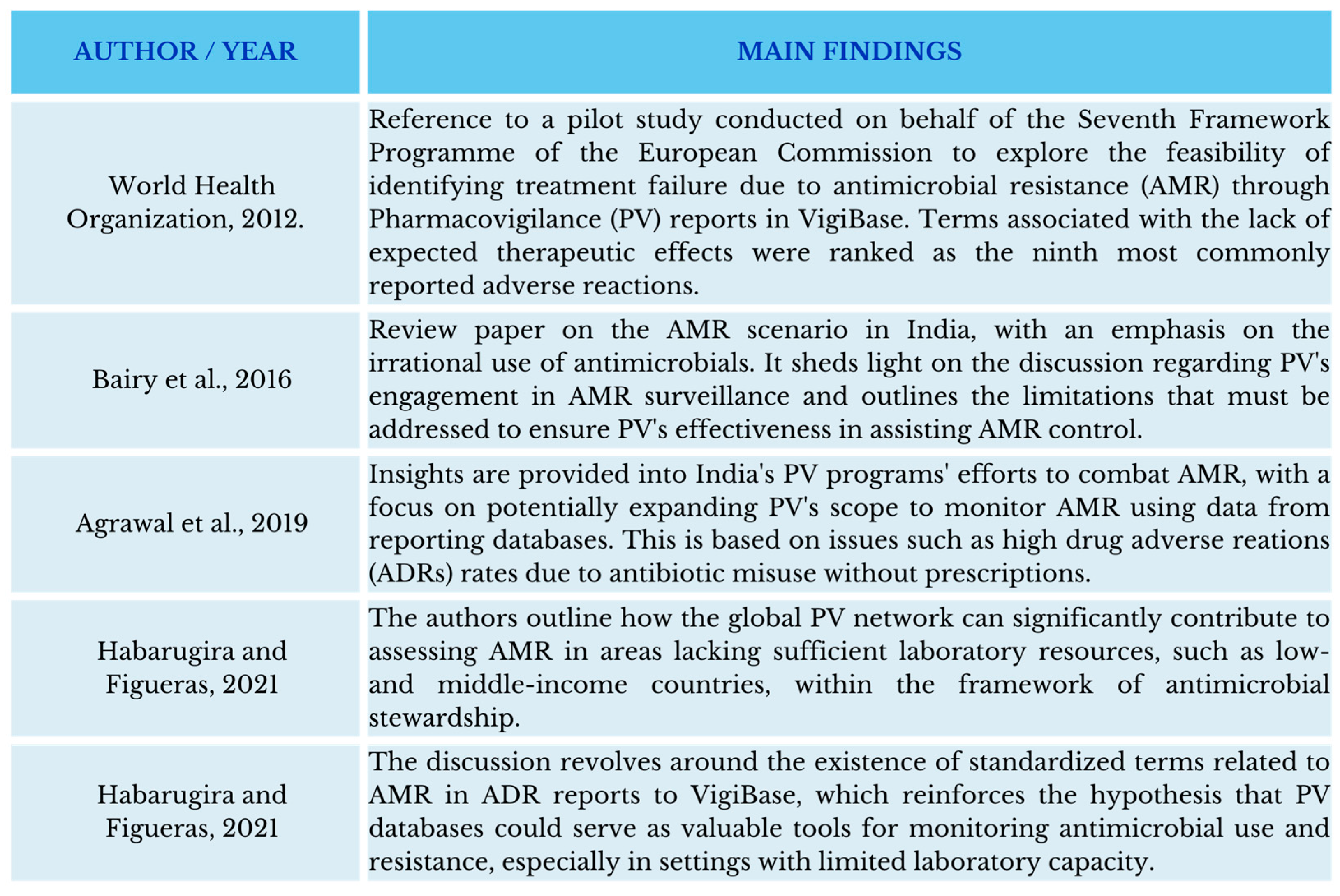
- Agrawal, V.; Shrivastava, T.P.; Adusumilli, P.K.; Vivekanandan, K.; Thota, P.; Bhushan, S. Pivotal Role of Pharmacovigilance Programme of India in Containment of Antimicrobial Resistance in India. Perspect. Clin. Res. 2019, 10, 140–144. [Google Scholar] [CrossRef]
- Bairy, L.K.; Nayak, V.; A, A.; Kunder, S.K. Advances in Pharmacovigilance Initiatives Surrounding Antimicrobial Resistance-Indian Perspective. Expert Opin. Drug Saf. 2016, 15, 1055–1062. [Google Scholar] [CrossRef]
- Gatti, M.; Raschi, E.; De Ponti, F. Relationship between Adverse Drug Reactions to Antibacterial Agents and the Klebsiella Pneumoniae Carbapenemase-Producing (KPC) Klebsiella Pneumoniae Outbreak: Insight from a Pharmacovigilance Study. BMC Pharmacol. Toxicol. 2019, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Habarugira, J.M.V.; Figueras, A. Antimicrobial Stewardship: Can We Add Pharmacovigilance Networks to the Toolbox? Eur. J. Clin. Pharmacol. 2021, 77, 787–790. [Google Scholar] [CrossRef]
- Habarugira, J.M.V.; Härmark, L.; Figueras, A. Pharmacovigilance Data as a Trigger to Identify Antimicrobial Resistance and Inappropriate Use of Antibiotics: A Study Using Reports from The Netherlands Pharmacovigilance Centre. Antibiotics 2021, 10, 1512. [Google Scholar] [CrossRef]
- Habarugira, J.M.V.; Figueras, A. Pharmacovigilance Network as an Additional Tool for the Surveillance of Antimicrobial Resistance. Pharmacoepidemiol. Drug Saf. 2021, 30, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pharmacovigilance. Available online: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance (accessed on 30 January 2024).
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Worldwide Withdrawal of Medicinal Products Because of Adverse Drug Reactions: A Systematic Review and Analysis. Crit. Rev. Toxicol. 2016, 46, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Farcaş, A.; Măhălean, A.; Bulik, N.B.; Leucuta, D.; Mogoșan, C. New Safety Signals Assessed by the Pharmacovigilance Risk Assessment Committee at EU Level in 2014-2017. Expert Rev. Clin. Pharmacol. 2018, 11, 1045–1051. [Google Scholar] [CrossRef]
- FDA Commissioner. Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions. Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse (accessed on 10 May 2024).
- Outterson, K.; Powers, J.H.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Kesselheim, A.S. Approval and Withdrawal of New Antibiotics and Other Antiinfectives in the U.S., 1980–2009. J. Law Med. Ethics 2013, 41, 688–696. [Google Scholar] [CrossRef]
- World Health Organization. The Importance of Pharmacovigilance. Available online: https://www.who.int/publications-detail-redirect/10665-42493 (accessed on 11 April 2024).
- Programme for International Drug Monitoring. Available online: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/networks/pidm (accessed on 8 March 2024).
- Wang, H.; Marquez, P.V.; Figueras, A.; Bieliaieva, K. Why Is the Safety of Medicines Important for Resilient Health Systems? A Synthesis Report; World Bank: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Uppsala Monitoring Centre Glossary. Available online: https://who-umc.org/pharmacovigilance-communications/glossary/ (accessed on 12 February 2024).
- Vintila, B.I.; Arseniu, A.M.; Butuca, A.; Sava, M.; Bîrluțiu, V.; Rus, L.L.; Axente, D.D.; Morgovan, C.; Gligor, F.G. Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database. Antibiotics 2023, 12, 918. [Google Scholar] [CrossRef]
- M Shaju, A.; Panicker, N.; Chandni, V.; Lakshmi Prasanna, V.M.; Nair, G.; Subeesh, V. Drugs-Associated with Red Man Syndrome: An Integrative Approach Using Disproportionality Analysis and Pharmip. J. Clin. Pharm. Ther. 2022, 47, 1650–1658. [Google Scholar] [CrossRef]
- Gatti, M.; Fusaroli, M.; Raschi, E.; Moretti, U.; Poluzzi, E.; De Ponti, F. Serious Adverse Events with Tedizolid and Linezolid: Pharmacovigilance Insights through the FDA Adverse Event Reporting System. Expert Opin. Drug Saf. 2021, 20, 1421–1431. [Google Scholar] [CrossRef]
- Gatti, M.; Raschi, E.; De Ponti, F. Serious Adverse Events with Novel Beta-Lactam/Beta-Lactamase Inhibitor Combinations: A Large-Scale Pharmacovigilance Analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, M.; Montané, E. Pharmacoepidemiology: An Overview. J. Clin. Med. 2023, 12, 7033. [Google Scholar] [CrossRef] [PubMed]
- García-Abeijon, P.; Costa, C.; Taracido, M.; Herdeiro, M.T.; Torre, C.; Figueiras, A. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf. 2023, 46, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Alomar, M.; Palaian, S.; Al-Tabakha, M.M. Pharmacovigilance in Perspective: Drug Withdrawals, Data Mining and Policy Implications. F1000Research 2019, 8, 2109. [Google Scholar] [CrossRef] [PubMed]
- Christ, P.; Dubrall, D.; Schmid, M.; Sachs, B. Comparative Analysis of Information Provided in German Adverse Drug Reaction Reports Sent by Physicians, Pharmacists and Consumers. Drug Saf. 2023, 46, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Zhang, R.; Yu, L.; Hu, T.; Wu, B. Seizures Associated with Antibiotics: A Real-World Disproportionality Analysis of FAERS Database. Expert Opin. Drug Saf. 2023, 22, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, J.; Xiao, M.; Shan, H.; Liu, M.; Lu, Y.; Zou, Y.; Wu, B. Severe Cutaneous Adverse Reactions Due to Antibiotics Therapy: A Pharmacovigilance Analysis of FDA Adverse Event Reporting System Events. Expert Opin. Drug Saf. 2023, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Song, Y.; Bai, Z.; Xi, X.; Liu, F.; Zhang, Y.; Qin, C.; Du, D.; Du, Q.; Liu, S. Real-World Data in Pharmacovigilance Database Provides a New Perspective for Understanding the Risk of Clostridium Difficile Infection Associated with Antibacterial Drug Exposure. Antibiotics 2023, 12, 1109. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Shi, D.; Dai, Y. Linezolid and the Risk of QT Interval Prolongation: A Pharmacovigilance Study of the Food and Drug Administration Adverse Event Reporting System. Br. J. Clin. Pharmacol. 2023, 89, 1386–1392. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.; Ma, J.; Zhang, A.; Li, Z.; Qi, G.; Li, Z.; Wei, F.; Zhong, L. Hepatobiliary Calculi Associated with Ceftriaxone Treatment: An Analysis of FAERS Data from 2004 to 2021. J. Infect. Chemother. 2023, 29, 136–142. [Google Scholar] [CrossRef]
- Seo, H.; Kim, E. Electrolyte Disorders Associated with Piperacillin/Tazobactam: A Pharmacovigilance Study Using the FAERS Database. Antibiotics 2023, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Huo, X.C.; Wang, S.X.; Wang, F.; Zhao, Q. Data Mining for Adverse Drug Reaction Signals of Daptomycin Based on Real-World Data: A Disproportionality Analysis of the US Food and Drug Administration Adverse Event Reporting System. Int. J. Clin. Pharm. 2022, 44, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Lopes, V.L.; Caffrey, A.R. Colistin-Associated Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Reactions: A Retrospective Case-Non-Case Pharmacovigilance Study. Expert Opin. Drug Saf. 2022, 21, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Cho, M.K.; Kim, S. Data Mining for Detecting Signals of Adverse Drug Reaction of Doxycycline Using the Korea Adverse Event Reporting System Database. J. Dermatolog. Treat. 2022, 33, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Recht, J.; Chansamouth, V.; White, N.J.; Ashley, E.A. Nitrofurantoin and Glucose-6-Phosphate Dehydrogenase Deficiency: A Safety Review. JAC Antimicrob. Resist. 2022, 4, dlac045. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Mitsuboshi, S.; Suzuki, K.; Nishihara, M.; Neo, M. Analysis of the Frequency of Ceftriaxone-Induced Encephalopathy Using the Japanese Adverse Drug Event Report Database. Int. J. Clin. Pharm. 2022, 44, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Kuula, L.S.M.; Backman, J.T.; Blom, M.L. Healthcare Costs and Mortality Associated with Serious Fluoroquinolone-Related Adverse Reactions. Pharmacol. Res. Perspect. 2022, 10, e00931. [Google Scholar] [CrossRef]
- Gatti, M.; Fusaroli, M.; Raschi, E.; Capelli, I.; Poluzzi, E.; De Ponti, F. Crystal Nephropathy and Amoxicillin: Insights from International Spontaneous Reporting Systems. J. Nephrol. 2022, 35, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.K.; Alami, A.; Gravel, C.A.; Tsui, D.; Bjerre, L.M.; Momoli, F.; Mattison, D.; Krewski, D. Systemic Quinolones and Risk of Retinal Detachment I: Analysis of Data from the US FDA Adverse Event Reporting System. Expert Opin. Drug Saf. 2022, 21, 269–276. [Google Scholar] [CrossRef]
- Mitsuboshi, S.; Katagiri, H. Risk of Kidney Injury in Patients on Concomitant Oral Vancomycin and Piperacillin-Tazobactam: Analysis of the Pharmacovigilance Database in Japan. Basic Clin. Pharmacol. Toxicol. 2022, 130, 208–212. [Google Scholar] [CrossRef]
- Rey, A.; Gras, V.; Moragny, J.; Choukroun, G.; Masmoudi, K.; Liabeuf, S. Use of the Capture-Recapture Method to Estimate the Frequency of Community- and Hospital-Acquired Drug-Induced Acute Kidney Injuries in French Databases. Front. Pharmacol. 2022, 13, 899164. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.-G.; Jeong, K.; Ryu, J.-Y.; Park, S.; Choi, Y.-S.; Kwack, W.-G.; Choi, Y.-J.; Chung, E.-K. Fatal Events Associated with Adverse Drug Reactions in the Korean National Pharmacovigilance Database. J. Pers. Med. 2021, 12, 5. [Google Scholar] [CrossRef]
- Asai, Y.; Yamamoto, T.; Abe, Y. Evaluation of the Expression Profile of Antibiotic-Induced Thrombocytopenia Using the Japanese Adverse Drug Event Report Database. Int. J. Toxicol. 2021, 40, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Largeau, B.; Agier, M.-S.; Beau-Salinas, F.; Pariente, A.; Maruani, A.; Vial, T.; Jonville-Béra, A.-P. Specific Features of Amoxicillin-Associated Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome: A Nationwide Study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2415–2420. [Google Scholar] [CrossRef]
- Ge, W.; Hu, H.; Li, C.; Wang, L.; Xia, J. Safety Profile of Carbapenems: Data Mining of the FDA Adverse Events Reporting System. Int. J. Clin. Pharmacol. Ther. 2021, 59, 594–602. [Google Scholar] [CrossRef]
- Yamada, T.; Mitsuboshi, S.; Suzuki, K.; Nishihara, M.; Uchiyama, K. Risk of Muscle Toxicity Events for Daptomycin with and without Statins: Analysis of the Japanese Adverse Event Report Database. Basic Clin. Pharmacol. Toxicol. 2021, 129, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Hasegawa, S.; Umetsu, R.; Shimada, K.; Mukai, R.; Tanaka, M.; Matsumoto, K.; Yoshida, Y.; Inoue, M.; Satake, R.; et al. Pharmacovigilance Study of Anti-Infective-Related Acute Kidney Injury Using the Japanese Adverse Drug Event Report Database. BMC Pharmacol. Toxicol. 2021, 22, 47. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Vu, D.H.; Nguyen, H.A.; Dao, V.T.; Montastruc, J.L.; Bagheri, H. Risk Comparison of Beta-Lactam-Induced Anaphylaxis: Therapeutic Stratification Analysis in a Vietnamese Pharmacovigilance Database. J. Clin. Pharm. Ther. 2021, 46, 950–956. [Google Scholar] [CrossRef]
- Rudolph, A.; Dahmke, H.; Kupferschmidt, H.; Burden, A.; Weiler, S. Coadministration of Tizanidine and Ciprofloxacin: A Retrospective Analysis of the WHO Pharmacovigilance Database. Eur. J. Clin. Pharmacol. 2021, 77, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Bera-Jonville, A.-P.; Montastruc, F.; Velly, L.; Micallef, J.; Guilhaumou, R. Serious Neurological Adverse Events of Ceftriaxone. Antibiotics 2021, 10, 540. [Google Scholar] [CrossRef]
- Kan, Y.; Nagai, J.; Uesawa, Y. Evaluation of Antibiotic-Induced Taste and Smell Disorders Using the FDA Adverse Event Reporting System Database. Sci. Rep. 2021, 11, 9625. [Google Scholar] [CrossRef] [PubMed]
- Contejean, A.; Tisseyre, M.; Canouï, E.; Treluyer, J.-M.; Kerneis, S.; Chouchana, L. Combination of Vancomycin plus Piperacillin and Risk of Acute Kidney Injury: A Worldwide Pharmacovigilance Database Analysis. J. Antimicrob. Chemother. 2021, 76, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Raschi, E.; De Ponti, F. Serotonin Syndrome by Drug Interactions with Linezolid: Clues from Pharmacovigilance-Pharmacokinetic/Pharmacodynamic Analysis. Eur. J. Clin. Pharmacol. 2021, 77, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, H.; Nagashima, T.; Minagawa, K.; Hayakawa, T.; Takahashi, Y.; Asai, S. Signal Detection of Potential Hepatotoxic Drugs: Case-Control Study Using Both a Spontaneous Reporting System and Electronic Medical Records. Biol. Pharm. Bull. 2021, 44, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, Y.; Zeng, Y.; Zhang, C.; Zhou, Z.; Shi, D. Linezolid and the Risk of Lactic Acidosis: Data Mining and Analysis of the FDA Adverse Event Reporting System. J. Clin. Pharm. Ther. 2020, 45, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Scavone, C.; Mascolo, A.; Ruggiero, R.; Sportiello, L.; Rafaniello, C.; Berrino, L.; Capuano, A. Quinolones-Induced Musculoskeletal, Neurological, and Psychiatric ADRs: A Pharmacovigilance Study Based on Data From the Italian Spontaneous Reporting System. Front. Pharmacol. 2020, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Villa Zapata, L.; Hansten, P.D.; Horn, J.R.; Boyce, R.D.; Gephart, S.; Subbian, V.; Romero, A.; Malone, D.C. Evidence of Clinically Meaningful Drug-Drug Interaction With Concomitant Use of Colchicine and Clarithromycin. Drug Saf. 2020, 43, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Zelmat, Y.; Rousseau, V.; Chebane, L.; Montastruc, J.-L.; Bagheri, H.; Sommet, A. Fluoroquinolone-Induced Photosensitivity: A Chemical Fragment-Based Approach by a Case/Non-Case Study in VigiBase(®). Drug Saf. 2020, 43, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.E.; Teng, C.; Patek, T.M.; Frei, C.R. Hypoglycemia Associated with Antibiotics Alone and in Combination with Sulfonylureas and Meglitinides: An Epidemiologic Surveillance Study of the FDA Adverse Event Reporting System (FAERS). Drug Saf. 2020, 43, 363–369. [Google Scholar] [CrossRef]
- Timbrook, T.T.; McKay, L.; Sutton, J.D.; Spivak, E.S. Disproportionality Analysis of Safety with Nafcillin and Oxacillin with the FDA Adverse Event Reporting System (FAERS). Antimicrob. Agents Chemother. 2020, 64, e01818-19. [Google Scholar] [CrossRef]
- Patek, T.M.; Teng, C.; Kennedy, K.E.; Alvarez, C.A.; Frei, C.R. Comparing Acute Kidney Injury Reports Among Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). Drug Saf. 2020, 43, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, G.; Andriani, L.A.; D’Annibali, O.; Motola, D.; Vaccheri, A. Cardiovascular Safety of Macrolide and Fluoroquinolone Antibiotics: An Analysis of the WHO Database of Adverse Drug Reactions. Pharmacoepidemiol. Drug Saf. 2019, 28, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.H.; Dayer, M.J.; Durkin, M.J.; Lockhart, P.B.; Baddour, L.M. Risk of Adverse Reactions to Oral Antibiotics Prescribed by Dentists. J. Dent. Res. 2019, 98, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Orion, K.; Mack, J.; Kullak-Ublick, G.A.; Weiler, S. Kounis Syndrome: A Retrospective Analysis of Individual Case Safety Reports from the International WHO Database in Pharmacovigilance. Int. J. Clin. Pharmacol. Ther. 2019, 57, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sommet, A.; Bénévent, J.; Rousseau, V.; Chebane, L.; Douros, A.; Montastruc, J.-L.; Montastruc, F. What Fluoroquinolones Have the Highest Risk of Aortic Aneurysm? A Case/Non-Case Study in VigiBase®. J. Gen. Intern. Med. 2019, 34, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Kheloufi, F.; Montastruc, F.; Bennis, Y.; Pizzoglio, V.; Micallef, J. Serious Central Nervous System Side Effects of Cephalosporins: A National Analysis of Serious Reports Registered in the French Pharmacovigilance Database. J. Neurol. Sci. 2019, 398, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.E. Increased Risk for Aseptic Meningitis after Amoxicillin or Amoxicillin-Clavulanic Acid in Males: A Signal Revealed by Subset Disproportionality Analysis within a Global Database of Suspected Adverse Drug Reactions. Pharmacoepidemiol. Drug Saf. 2019, 28, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Reveles, K.R.; Obodozie-Ofoegbu, O.O.; Frei, C.R. Clostridium Difficile Infection Risk with Important Antibiotic Classes: An Analysis of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2019, 16, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.R.; Slattery, J.; Pacurariu, A.; Pinheiro, L.; McGettigan, P.; Kurz, X. Relative and Absolute Risk of Tendon Rupture with Fluoroquinolone and Concomitant Fluoroquinolone/Corticosteroid Therapy: Population-Based Nested Case-Control Study. Clin. Drug Investig. 2019, 39, 205–213. [Google Scholar] [CrossRef]
- Teng, C.; Walter, E.A.; Gaspar, D.K.S.; Obodozie-Ofoegbu, O.O.; Frei, C.R. Torsades de Pointes and QT Prolongation Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2019, 16, 1018–1022. [Google Scholar] [CrossRef]
- Morales, D.; Pacurariu, A.; Slattery, J.; Pinheiro, L.; McGettigan, P.; Kurz, X. Association Between Peripheral Neuropathy and Exposure to Oral Fluoroquinolone or Amoxicillin-Clavulanate Therapy. JAMA Neurol. 2019, 76, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Abu Esba, L.C.; Al Mardawi, G.; AlJasser, M.I.; Aljohani, B.; Abu Alburak, A. Adverse Drug Reactions Spontaneously Reported at a Tertiary Care Hospital and Preventable Measures Implemented. J. Clin. Pharm. Ther. 2021, 46, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kanwal, P.; Goyal, P.; Singh, P.; Yakhmi, S.; Jain, S.; Kaushal, S. Spontaneous Adverse Drug Reaction Monitoring in a Tertiary Care Centre. Curr. Drug Saf. 2020, 15, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, L.; Hansen, E.H. Adverse Drug Reactions Reported for Systemic Antibacterials in Danish Children over a Decade. Br. J. Clin. Pharmacol. 2010, 70, 765–768. [Google Scholar] [CrossRef]
- Rosli, R.; Ming, L.C.; Abd Aziz, N.; Manan, M.M. A Retrospective Analysis of Spontaneous Adverse Drug Reactions Reports Relating to Paediatric Patients. PLoS ONE 2016, 11, e0155385. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Nguyen, T.N.T.; Tran, N.T.K.; Nguyen, L.T.; Vu, H.D.; Nguyen, A.H.; Trinh, N.T.H. Preventability of Adverse Drug Reactions Related to Antibiotics: An Assessment Based on Spontaneous Reporting System. Ther. Innov. Regul. Sci. 2023, 57, 1104–1112. [Google Scholar] [CrossRef]
- World Health Organization. The Evolving Threat of Antimicrobial Resistance: Options for Action. In The Evolving Threat of Antimicrobial Resistance: Options for Action; World Health Organization-Regional Office for Africa: Brazzaville, Congo, 2012; Available online: https://www.afro.who.int/publications/evolving-threat-antimicrobial-resistance-options-action (accessed on 11 April 2024).
- English|MedDRA. Available online: https://www.meddra.org/how-to-use/support-documentation/english/welcome (accessed on 5 April 2024).
- Ruíz-Garzón, J.A.; Calderón-Ospina, C.A. Consideraciones acerca del reporte y la evaluación del fallo terapéutico en farmacovigilancia. Rev. Fac. Med. 2019, 67, 475–480. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic Issues for Antibiotics in the Critically Ill Patient. Crit. Care Med. 2009, 37, 840–851; quiz 859. [Google Scholar] [CrossRef]
- Gallelli, L.; Palleria, C.; De Vuono, A.; Mumoli, L.; Vasapollo, P.; Piro, B.; Russo, E. Safety and Efficacy of Generic Drugs with Respect to Brand Formulation. J. Pharmacol. Pharmacother. 2013, 4, S110–S114. [Google Scholar] [CrossRef]
- Johnston, A.; Holt, D.W. Substandard Drugs: A Potential Crisis for Public Health. Br. J. Clin. Pharmacol. 2014, 78, 218–243. [Google Scholar] [CrossRef]
- World Health Organization. 1 in 10 Medical Products in Developing Countries Is Substandard or Falsified. Available online: https://www.who.int/news/item/28-11-2017-1-in-10-medical-products-in-developing-countries-is-substandard-or-falsified (accessed on 28 February 2024).
- Roberts, J.A.; Kruger, P.; Paterson, D.L.; Lipman, J. Antibiotic Resistance--What’s Dosing Got to Do with It? Crit. Care Med. 2008, 36, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- de S. Martins, T.S.; Figueras, A.; dos R. de Souza, L.; dos Santos, K.C.O.; de Oliveira, E.M.; Secoli, S.R. Nonadherence to Treatment Recommendations Is a Factor Contributing to the Clinical Failure of Daptomycin: Cohort Study in Brazil. Braz. J. Pharm. Sci. (Online) 2020, 56, e17184. [Google Scholar] [CrossRef]
- Mvalo, T.; Smith, A.G.; Eckerle, M.; Hosseinipour, M.C.; Kondowe, D.; Vaidya, D.; Liu, Y.; Corbett, K.; Nansongole, D.; Mtimaukanena, T.A.; et al. Antibiotic Treatment Failure in Children Aged 1 to 59 Months with World Health Organization-Defined Severe Pneumonia in Malawi: A CPAP IMPACT Trial Secondary Analysis. PLoS ONE 2022, 17, e0278938. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Placido, A.I.; Afreixo, V.; Ribeiro-Vaz, I.; Roque, F.; Herdeiro, M.T. Descriptive Analysis of Adverse Drug Reactions Reports of the Most Consumed Antibiotics in Portugal, Prescribed for Upper Airway Infections. Antibiotics 2022, 11, 477. [Google Scholar] [CrossRef]
- Soukavong, M.; Kim, J.; Park, K.; Yang, B.R.; Lee, J.; Jin, X.M.; Park, B.J. Signal Detection of Adverse Drug Reaction of Amoxicillin Using the Korea Adverse Event Reporting System Database. J. Korean Med. Sci. 2016, 31, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.H.L.A.; Silva, A.R.O.; Carvalho-Assef, A.P.D.; Lima, E.C.; da Silva, F.A.B. Potential Safety Signals for Antibacterial Agents from the Brazilian National Pharmacovigilance Database (Vigimed/VigiFlow). Front. Pharmacol. 2022, 13, 948339. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Al-Azzam, S.; Banat, H.A.; Jaber, J.M.; Araydah, M.; Alshogran, O.Y.; Aldeyab, M.A. Reporting Antimicrobial-Related Adverse Drug Events in Jordan: An Analysis from the VigiBase Database. Antibiotics 2023, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Baghel, R.; Thakur, S.; Adwal, S. Surveillance of Adverse Drug Reactions at an Adverse Drug Reaction Monitoring Centre in Central India: A 7-Year Surveillance Study. BMJ Open 2021, 11, e052737. [Google Scholar] [CrossRef] [PubMed]
- Kadoyama, K.; Sakaeda, T.; Tamon, A.; Okuno, Y. Adverse Event Profile of Tigecycline: Data Mining of the Public Version of the U.S. Food and Drug Administration Adverse Event Reporting System. Biol. Pharm. Bull. 2012, 35, 967–970. [Google Scholar] [CrossRef]
- Saleem, Z.; Ahsan, U.; Haseeb, A.; Altaf, U.; Batool, N.; Rani, H.; Jaffer, J.; Shahid, F.; Hussain, M.; Amir, A.; et al. Antibiotic Utilization Patterns for Different Wound Types among Surgical Patients: Findings and Implications. Antibiotics 2023, 12, 678. [Google Scholar] [CrossRef]
- Saleem, Z.; Godman, B.; Cook, A.; Khan, M.A.; Campbell, S.M.; Seaton, R.A.; Siachalinga, L.; Haseeb, A.; Amir, A.; Kurdi, A.; et al. Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future. Antibiotics 2022, 11, 1824. [Google Scholar] [CrossRef] [PubMed]
- Kamara, I.F.; Kanu, J.; Maruta, A.; Fofanah, B.D.; Kamara, K.N.; Sheriff, B.; Katawera, V.; D’Almeida, S.A.; Musoke, R.; Nuwagira, I.; et al. Antibiotic Use among Hospitalised Patients in Sierra Leone: A National Point Prevalence Survey Using the WHO Survey Methodology. BMJ Open 2023, 13, e078367. [Google Scholar] [CrossRef] [PubMed]
- Balon, M.; Tessier, S.; Damase-Michel, C.; Cottin, J.; Lambert, A.; Thompson, M.-A.; Benevent, J.; Lacroix, I. Adverse Drug Reactions in Pregnant Women: Do They Differ from Those in Non-Pregnant Women of Childbearing Age? Therapie 2023, 78, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kuzmina, A.V.; Asetskaya, I.L.; Zyryanov, S.K.; Polivanov, V.A. Detecting Medication Errors Associated with the Use of Beta-Lactams in the Russian Pharmacovigilance Database. BMC Pharmacol. Toxicol. 2021, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, D.; Ding, N.; Huang, K.; Xiong, Y.; Du, G.; Zeng, F. Safety Evaluation of Cephalosporins Based on Utilization and Adverse Drug Events: Analysis of Two Databases in China. Expert Opin. Drug Saf. 2012, 11, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Shalviri, G.; Yousefian, S.; Gholami, K. Adverse Events Induced by Ceftriaxone: A Ten Years Review of Reported Cases to Iranian Pharmacovigilance Centre. Pharmacoepidemiol. Drug Saf. 2010, 19, S136–S137. [Google Scholar] [CrossRef] [PubMed]
- Salvo, F.; Polimeni, G.; Moretti, U.; Conforti, A.; Leone, R.; Leoni, O.; Motola, D.; Dusi, G.; Caputi, A.P. Adverse Drug Reactions Related to Amoxicillin Alone and in Association with Clavulanic Acid: Data from Spontaneous Reporting in Italy. J. Antimicrob. Chemother. 2007, 60, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.; Barker, C.I.; Folgori, L.; Bielicki, J.A.; Bradley, J.S.; Lutsar, I.; Sharland, M. Global Shortage of Neonatal and Paediatric Antibiotic Trials: Rapid Review. BMJ Open 2017, 7, e016293. [Google Scholar] [CrossRef]
- Garazzino, S.; Lutsar, I.; Bertaina, C.; Tovo, P.-A.; Sharland, M. New Antibiotics for Paediatric Use: A Review of a Decade of Regulatory Trials Submitted to the European Medicines Agency from 2000—Why Aren’t We Doing Better? Int. J. Antimicrob. Agents 2013, 42, 99–118. [Google Scholar] [CrossRef]
- Thomas, S.H.L.; Yates, L.M. Prescribing without Evidence—Pregnancy. Br. J. Clin. Pharmacol. 2012, 74, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Jiang, T.; Xing, H.; Xu, J.; Li, C.; Ni, R.; Zhang, N.; Xiang, G.; Li, L.; et al. Opportunities and Challenges of Pharmacovigilance in Special Populations: A Narrative Review of the Literature. Ther. Adv. Drug Saf. 2023, 14, 1–21. [Google Scholar] [CrossRef]
- Wallerstedt, S.M.; Brunlöf, G.; Sundström, A. Rates of Spontaneous Reports of Adverse Drug Reactions for Drugs Reported in Children. Drug-Saf. 2011, 34, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, E.; Boussaha, I.; Dode, X.; Grenet, G.; Kassai, B.; Nguyen, K.A. Unlicensed/Off-Label Drug Prescriptions at Hospital Discharge in Children: An Observational Study Using Routinely Collected Health Data. Healthcare 2024, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Ufer, M.; Kimland, E.; Bergman, U. Adverse Drug Reactions and Off-Label Prescribing for Paediatric Outpatients: A One-Year Survey of Spontaneous Reports in Sweden. Pharmacoepidemiol. Drug Saf. 2004, 13, 147–152. [Google Scholar] [CrossRef]
- Dubrall, D.; Leitzen, S.; Toni, I.; Stingl, J.; Schulz, M.; Schmid, M.; Neubert, A.; Sachs, B. Descriptive Analysis of Adverse Drug Reaction Reports in Children and Adolescents from Germany: Frequently Reported Reactions and Suspected Drugs. BMC Pharmacol. Toxicol. 2021, 22, 56. [Google Scholar] [CrossRef]
- da C. Lima, E.; de Matos, G.C.; de L. Vieira, J.M.; da C.R. Gonçalves, I.C.; Cabral, L.M.; Turner, M.A. Suspected Adverse Drug Reactions Reported for Brazilian Children: Cross-Sectional Study. J. Pediatr. (Rio J.) 2019, 95, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Jasovský, D.; Aagaard, H.; Zorzet, A. Uppsala Reports Issue 74/Uppsala Monitoring Centre; Uppsala Monitoring Centre: Uppsala, Sweden, 2017; pp. 8–10. Available online: https://who-umc.org/media/2775/web_uppsalareports_issue74.pdf (accessed on 28 February 2024).
- Jean, S.-S.; Liu, I.-M.; Hsieh, P.-C.; Kuo, D.-H.; Liu, Y.-L.; Hsueh, P.-R. Off-Label Use versus Formal Recommendations of Conventional and Novel Antibiotics for the Treatment of Infections Caused by Multidrug-Resistant Bacteria. Int. J. Antimicrob. Agents 2023, 61, 106763. [Google Scholar] [CrossRef]
- Shenkutie, A.M.; Zhang, J.; Yao, M.; Asrat, D.; Chow, F.W.N.; Leung, P.H.M. Effects of Sub-Minimum Inhibitory Concentrations of Imipenem and Colistin on Expression of Biofilm-Specific Antibiotic Resistance and Virulence Genes in Acinetobacter Baumannii Sequence Type 1894. Int. J. Mol. Sci. 2022, 23, 12705. [Google Scholar] [CrossRef]
- Ismail, B.; Shafei, M.N.; Harun, A.; Ali, S.; Omar, M.; Deris, Z.Z. Predictors of Polymyxin B Treatment Failure in Gram-Negative Healthcare-Associated Infections among Critically Ill Patients. J. Microbiol. Immunol. Infect. 2018, 51, 763–769. [Google Scholar] [CrossRef]
- Vikan, M.; Haugen, A.S.; Bjørnnes, A.K.; Valeberg, B.T.; Deilkås, E.C.T.; Danielsen, S.O. The Association between Patient Safety Culture and Adverse Events—A Scoping Review. BMC Health Serv. Res. 2023, 23, 300. [Google Scholar] [CrossRef]
- Conteh, T.A.; Thomas, F.; Abiri, O.T.; Komeh, J.P.; Kanu, A.; Kanu, J.S.; Fofanah, B.D.; Thekkur, P.; Zachariah, R. Quality of Reporting of Adverse Drug Reactions to Antimicrobials Improved Following Operational Research: A before-and-after Study in Sierra Leone (2017–2023). Trop. Med. Infect. Dis. 2023, 8, 470. [Google Scholar] [CrossRef] [PubMed]
- Zabala, G.A.; Bellingham, K.; Vidhamaly, V.; Boupha, P.; Boutsamay, K.; Newton, P.N.; Caillet, C. Substandard and Falsified Antibiotics: Neglected Drivers of Antimicrobial Resistance? BMJ Glob. Health 2022, 7, e008587. [Google Scholar] [CrossRef] [PubMed]
- Kyriacos, S.; Mroueh, M.; Chahine, R.P.; Khouzam, O. Quality of Amoxicillin Formulations in Some Arab Countries. J. Clin. Pharm. Ther. 2008, 33, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A.T.M.; Smeets, N.J.L.; de Jong, E.F.M.; Kämink, J.L.; Kroeze, Y.; Draaisma, J.M.T.; van Puijenbroek, E.P.; te Loo, D.M.W.M. Quality of Active versus Spontaneous Reporting of Adverse Drug Reactions in Pediatric Patients: Relevance for Pharmacovigilance and Knowledge in Pediatric Medical Care. Pharmaceuticals 2022, 15, 1148. [Google Scholar] [CrossRef]
| MedDRA Terms | ||
|---|---|---|
| Drug-ineffective | ||
| Pathogen resistance | ||
| Drug resistance | ||
| Multidrug resistance | ||
| Product use issue * | ||
| Product use in inappropriate indication | ||
| Incorrect route of administration | ||
| Inappropriate prescribing * | ||
| Indicating antibiotics for patients with no sign of infection * | ||
| Inappropriate indication for antimicrobial prophylaxis * | ||
| Inappropriate indication * | ||
| Readministration of antibiotics causing prior allergy/allergies * | ||
| Inappropriate dosage * | ||
| Inappropriate dose and dosage regimen * | ||
| Contraindicated administration * | ||
| Late or irrational change of antibiotic in case of treatment failure * | ||
| Administration of an inappropriate treatment regimen for the disease/Inadequate treatment strategy * | ||
| Off-label use | ||
| AWaRe classification of the antibiotics included in the synthesized studies | ||
| Access | Watch | Reserve |
| Amikacin | Azithromycin | Aztreonam |
| Amoxicillin | Cefoperazone | Colistin |
| Amoxicillin/clavulanic acid | Ceftazidime | Daptomycin |
| Ampicillin | Ceftriaxone | Linezolid |
| Cefalotin | Cefuroxime | Polymyxin-B |
| Cefazolin | Ciprofloxacin | Tedizolid |
| Doxycycline | Clarithromycin | |
| Levofloxacin | ||
| Meropenem | ||
| Piperacillin/tazobactam | ||
| Tobramycin | ||
| Vancomycin | ||
| Ceftriaxone/tazobactam ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandes, V.; Figueras, A.; Lima, E.C. Pharmacovigilance Strategies to Address Resistance to Antibiotics and Inappropriate Use—A Narrative Review. Antibiotics 2024, 13, 457. https://doi.org/10.3390/antibiotics13050457
Sandes V, Figueras A, Lima EC. Pharmacovigilance Strategies to Address Resistance to Antibiotics and Inappropriate Use—A Narrative Review. Antibiotics. 2024; 13(5):457. https://doi.org/10.3390/antibiotics13050457
Chicago/Turabian StyleSandes, Valcieny, Albert Figueras, and Elisangela Costa Lima. 2024. "Pharmacovigilance Strategies to Address Resistance to Antibiotics and Inappropriate Use—A Narrative Review" Antibiotics 13, no. 5: 457. https://doi.org/10.3390/antibiotics13050457
APA StyleSandes, V., Figueras, A., & Lima, E. C. (2024). Pharmacovigilance Strategies to Address Resistance to Antibiotics and Inappropriate Use—A Narrative Review. Antibiotics, 13(5), 457. https://doi.org/10.3390/antibiotics13050457






