Novel Siderophore Cephalosporin and Combinations of Cephalosporins with β-Lactamase Inhibitors as an Advancement in Treatment of Ventilator-Associated Pneumonia
Abstract
1. Introduction
2. Characteristics of Cefoperazone-Sulbactam
- Cefoperazone
- Sulbactam
2.1. Clinical Efficiency of Cefoperazone-Sulbactam
2.1.1. In Vitro Studies
2.1.2. Clinical Trials
3. Characteristics of Ceftolozane-Tazobactam
- Ceftolozane
- Tazobactam
3.1. Clinical Efficiency of Ceftolozane-Tazobactam
3.1.1. In Vitro Studies
3.1.2. Clinical Trials
4. Characteristics of Ceftazidime-Avibactam
- Ceftazidime
- Avibactam
4.1. Clinical Efficiency of Ceftazidime-Avibactam
4.1.1. In Vitro Studies
4.1.2. Clinical Trials
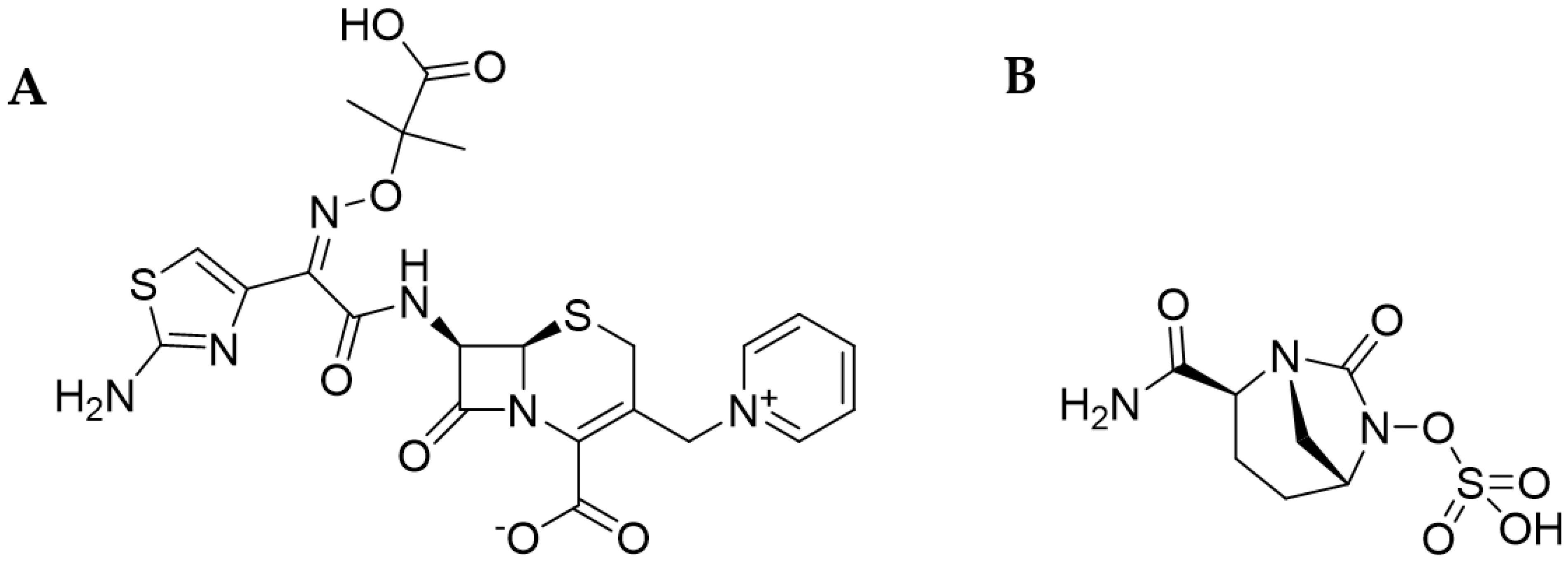
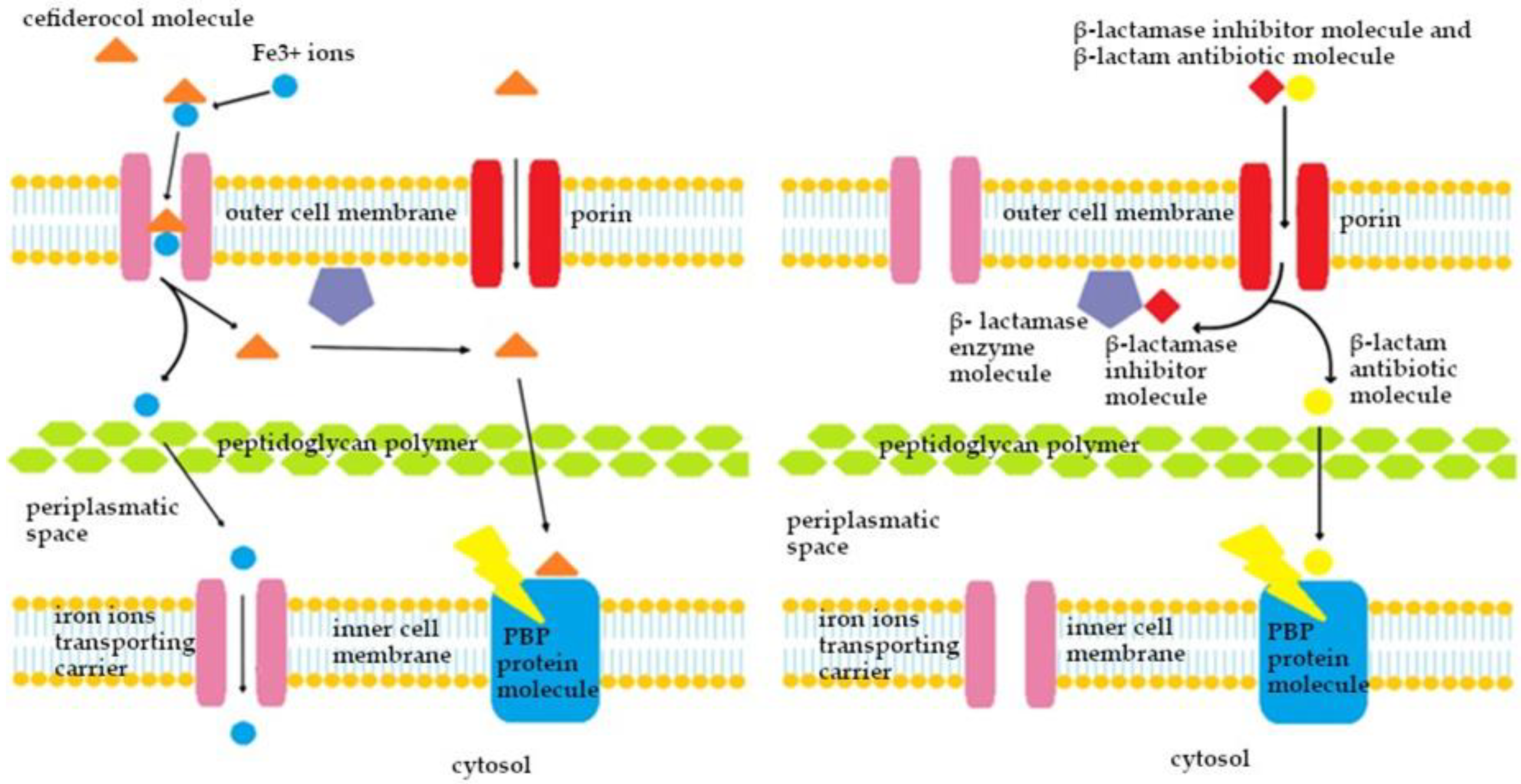
5. Characteristics of Cefiderocol
5.1. Clinical Efficiency of Cefiderocol
5.1.1. In Vitro Studies
5.1.2. Clinical Trials
5.1.3. Case Studies
5.1.4. Mechanism of Action of β-Lactam Drugs
6. Material and Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-Acquired, Ventilator-Associated, and Healthcare-Associated Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Kalanuria, A.A.; Zai, W.; Mirski, M. Ventilator-Associated Pneumonia in the ICU. Crit. Care 2014, 18, 208. [Google Scholar] [CrossRef]
- Shah, H.; Ali, A.; Patel, A.A.; Abbagoni, V.; Goswami, R.; Kumar, A.; Velasquez Botero, F.; Otite, E.; Tomar, H.; Desai, M.; et al. Trends and Factors Associated with Ventilator-Associated Pneumonia: A National Perspective. Cureus 2022, 14, e23634. [Google Scholar] [CrossRef]
- Gunalan, A.; Sastry, A.S.; Ramanathan, V.; Sistla, S. Early-vs Late-Onset Ventilator-Associated Pneumonia in Critically Ill Adults: Comparison of Risk Factors, Outcome, and Microbial Profile. Indian J. Crit. Care Med. 2023, 27, 411–415. [Google Scholar] [CrossRef]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-Associated Pneumonia in Adults: A Narrative Review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Sandiumenge, A.; Rello, J. Ventilator-Associated Pneumonia Caused by ESKAPE Organisms: Cause, Clinical Features, and Management. Curr. Opin. Pulm. Med. 2012, 18, 187–193. [Google Scholar] [CrossRef]
- Daubin, C.; Vincent, S.; Vabret, A.; Du Cheyron, D.; Parienti, J.J.; Ramakers, M.; Freymuth, F.; Charbonneau, P. Nosocomial Viral Ventilator-Associated Pneumonia in the Intensive Care Unit: A Prospective Cohort Study. Intensive Care Med. 2005, 31, 1116–1122. [Google Scholar] [CrossRef]
- Shorr, A.F.; Ilges, D.T.; Micek, S.T.; Kollef, M.H. The Importance of Viruses in Ventilator-Associated Pneumonia. Infect. Control Hosp. Epidemiol. 2023, 44, 1137–1142. [Google Scholar] [CrossRef]
- Fumagalli, J.; Panigada, M.; Klompas, M.; Berra, L. Ventilator-Associated Pneumonia among SARSCoV-2 Acute Respiratory Distress Syndrome Patients. Curr. Opin. Crit. Care 2022, 28, 74–82. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Zhang, J.; Xu, J.; Cheng, Q.; Li, Y.; Liang, S.; Li, H.; Gong, J.; Zhu, Y.; et al. Microbial Etiology and Prognostic Factors of Ventilator-Associated Pneumonia: A Multicenter Retrospective Study in Shanghai. Clin. Infect. Dis. 2018, 67, S146–S152. [Google Scholar] [CrossRef]
- Vincent, J.L.; Bihari, D.J.; Suter, P.M.; Bruining, H.A.; White, J.; Nicolas Chanoin, M.H.; Wolff, M.; Spencer, R.C.; Hemmer, M. The Prevalence of Nosocomial Infection in Intensive Care Units in Europe: Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA 1995, 274, 639–644. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Li, Y.; Roberts, J.; Walker, M.; Aslan, A.; Harris, P.; Sime, F. The Global Epidemiology of Ventilator-Associated Pneumonia Caused by Multi-Drug Resistant Pseudomonas aeruginosa: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2023, 139, 78–85. [Google Scholar] [CrossRef]
- Čiginskienė, A.; Dambrauskienė, A.; Rello, J.; Adukauskienė, D. Ventilator-Associated Pneumonia Due to Drug-Resistant Acinetobacter baumannii: Risk Factors and Mortality Relation with Resistance Profiles, and Independent Predictors of in-Hospital Mortality. Medicina 2019, 55, 49. [Google Scholar] [CrossRef]
- Awad, L.S.; Abdallah, D.I.; Mugharbil, A.M.; Jisr, T.H.; Droubi, N.S.; El-Rajab, N.A.; Moghnieh, R.A. An Antibiotic Stewardship Exercise in the ICU: Building a Treatment Algorithm for the Management of Ventilator-Associated Pneumonia Based on Local Epidemiology and the 2016 Infectious Diseases Society of America/American Thoracic Society Guidelines. Infect. Drug Resist. 2018, 11, 17–28. [Google Scholar] [CrossRef]
- Sader, H.S.; Carvalhaes, C.G.; Kimbrough, J.H.; Mendes, R.E.; Castanheira, M. Activity of Aztreonam-Avibactam against Enterobacterales Resistant to Recently Approved Beta-Lactamase Inhibitor Combinations Collected in Europe, Latin America, and the Asia-Pacific Region (2020–2022). Int. J. Antimicrob. Agents 2024, 63, 107113. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Topola, E. Meropenem/Vaborbactam: β-Lactam/β-Lactamase Inhibitor Combination, the Future in Eradicating Multidrug Resistance. Antibiotics 2023, 12, 1612. [Google Scholar] [CrossRef]
- Ku, Y.-H.; Yu, W.-L. Cefoperazone/Sulbactam: New Composites against Multiresistant Gram Negative Bacteria? Infect. Genet. Evol. 2021, 88, 104707. [Google Scholar] [CrossRef] [PubMed]
- Carvalhaes, C.G.; Castanheira, M.; Sader, H.S.; Flamm, R.K.; Shortridge, D. Antimicrobial Activity of Ceftolozane-Tazobactam Tested against Gram-Negative Contemporary (2015–2017) Isolates from Hospitalized Patients with Pneumonia in US Medical Centers. Diagn. Microbiol. Infect. Dis. 2019, 94, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, Against Gram-Negative Bacilli Isolated by Clinical Laboratories in North America and Europe in 2015-2016: SIDERO-WT-2015. Int. J. Antimicrob. Agents 2019, 53, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Cefiderocol: A Review in Serious Gram-Negative Bacterial Infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef]
- Ong’uti, S.; Czech, M.; Robilotti, E.; Holubar, M. Cefiderocol: A New Cephalosporin Stratagem Against Multidrug-Resistant Gram-Negative Bacteria. Clin. Infect. Dis. 2022, 74, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Carvalhaes, C.G.; Streit, J.M.; Castanheira, M.; Flamm, R.K. Antimicrobial Activity of Cefoperazone-Sulbactam Tested against Gram-Negative Organisms from Europe, Asia-Pacific, and Latin America. Int. J. Infect. Dis. 2020, 91, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Chen, C.-C.; Lu, Y.-C.; Lin, T.-P.; Chuang, Y.-C.; Tang, H.-J. Appropriate Composites of Cefoperazone–Sulbactam against Multidrug-Resistant Organisms. Infect. Drug Resist. 2018, 11, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Chen, C.-C.; Lu, Y.C.; Lai, C.-C.; Huang, H.-L.; Chuang, Y.-C.; Tang, H.-J. The Impact of Inoculum Size on the Activity of Cefoperazone-Sulbactam against Multidrug Resistant Organisms. J. Microbiol. Immunol. Infect. 2018, 51, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-Y.; Wang, F.-D.; Yen, Y.-F.; Lin, M.-L.; Liu, C.-Y. In Vitro Activities of Piperacillin or Cefoperazone Alone and in Combination with Beta-Lactamase Inhibitors against Gram-Negative Bacilli. New Microbiol. 2009, 32, 49–55. [Google Scholar] [PubMed]
- Jones, R.N.; Wilson, H.W.; Thornsberry, C.; Barry, A.L. In Vitro Antimicrobial Activity of Cefoperazone-Sulbactam Combinations against 554 Clinical Isolates Including a Review and β-Lactamase Studies. Diagn. Microbiol. Infect. Dis. 1985, 3, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, M.; Zhou, S. Observation of Clinical Efficacy of the Cefoperazone/Sulbactam Anti-infective Regimen in the Treatment of Multidrug-resistant Acinetobacter baumannii Lung Infection. J. Clin. Pharm. Ther. 2022, 47, 1020–1027. [Google Scholar] [CrossRef]
- Guclu, E.; Kaya, G.; Ogutlu, A.; Karabay, O. The Effect of Cefoperazone Sulbactam and Piperacillin Tazobactam on Mortality in Gram-Negative Nosocomial Infections. J. Chemother. 2020, 32, 118–123. [Google Scholar] [CrossRef]
- Lan, S.-H.; Chang, S.-P.; Lai, C.-C.; Lu, L.-C.; Tang, H.-J. Efficacy and Safety of Cefoperazone-Sulbactam in Empiric Therapy for Febrile Neutropenia. Medicine 2020, 99, e19321. [Google Scholar] [CrossRef] [PubMed]
- Brogden, R.N.; Carmine, A.; Heel, R.C.; Morley, P.A.; Speight, T.M.; Avery, G.S. Cefoperazone: A Review of Its In Vitro Antimicrobial Activity, Pharmacological Properties and Therapeutic Efficacy. Drugs 1981, 22, 423–460. [Google Scholar] [CrossRef]
- Williams, J.D. β-Lactamase Inhibition and In Vitro Activity of Sulbactam and Sulbactam/Cefoperazone. Clin. Infect. Dis. 1997, 24, 494–497. [Google Scholar] [CrossRef]
- Penwell, W.A.; Shapiro, A.B.; Giacobbe, R.A.; Gu, R.-F.; Gao, N.; Thresher, J.; McLaughlin, R.E.; Huband, M.; DeJonge, B.L.M.; Ehmann, D.E.; et al. Molecular Mechanisms of Sulbactam Antibacterial Activity and Resistance Determinants in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 59, 1680–1689. [Google Scholar] [CrossRef]
- Moussa, S.H.; Shapiro, A.B.; Mcleod, S.M.; Iyer, R.; Carter, N.M.; Tsai, Y.; Siu, L.K.; Alita, A. Contemporary Clinical Isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 10, 28. [Google Scholar]
- Namiduru, M.; Güngör, G.; Karaoğlan, I.; Dikensoy, Ö. Antibiotic Resistance of Bacterial Ventilator-Associated Pneumonia in Surgical Intensive Care Units. J. Int. Med. Res. 2004, 32, 78–83. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, D.; Xu, Y.; Gong, M.; Zhou, Y.; Fang, X. A Retrospective Analysis of Carbapenem-Resistant Acinetobacter baumannii-Mediated Nosocomial Pneumonia and the in Vitro Therapeutic Benefit of Cefoperazone/Sulbactam. Int. J. Infect. Dis. 2014, 23, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Capoor, M.R.; Nair, D.; Srivastava, L.; Gupta, B.; Aggarwal, P. Characterization and Changing Minimum Inhibitory Concentration (MIC) of Acinetobacter Species from a Tertiary Care Setup. J. Commun. Dis. 2005, 37, 99–107. [Google Scholar] [PubMed]
- Chiang, T.-T.; Tang, H.-J.; Chiu, C.-H.; Chen, T.-L.; Ho, M.-W.; Lee, C.-H.; Sheng, W.-H.; Yang, Y.-S. Antimicrobial Activities of Cefoperazone-Sulbactam in Comparison to Cefoperazone against Clinical Organisms from Medical Centers in Taiwan. J. Med. Sci. 2016, 36, 229. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Huang, Q.; Wang, Z.; Xu, Z.; Tu, C.; Wan, D.; He, M.; Yang, X.; Xu, H.; et al. Clinical Efficacy and In Vitro Drug Sensitivity Test Results of Azithromycin Combined with Other Antimicrobial Therapies in the Treatment of MDR P. Aeruginosa Ventilator-Associated Pneumonia. Front. Pharmacol. 2022, 13, 944965. [Google Scholar] [CrossRef]
- Xia, Y.-L.; Ge, M.; Wang, Z. Pathogenic Analysis of Ventilator-Associated Pneumonia in the Pediatric Intensive Care Unit in High-Altitude Areas. Zhongguo Dang Dai Er Ke Za Zhi 2014, 16, 787–790. [Google Scholar] [PubMed]
- Huang, C.-T.; Chen, C.-H.; Chen, W.-C.; Wang, Y.-T.; Lai, C.-C.; Fu, P.-K.; Kuo, L.-K.; Chen, C.-M.; Fang, W.-F.; Tu, C.-Y.; et al. Clinical Effectiveness of Cefoperazone-Sulbactam vs. Piperacillin-Tazobactam for the Treatment of Pneumonia in Elderly Patients. Int. J. Antimicrob. Agents 2022, 59, 106491. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-W.; Chen, Y.-H.; Lee, W.-S.; Lin, J.-C.; Huang, C.-T.; Lin, H.-H.; Liu, Y.-C.; Chuang, Y.-C.; Tang, H.-J.; Chen, Y.-S.; et al. Randomized Noninferiority Trial of Cefoperazone-Sulbactam versus Cefepime in the Treatment of Hospital-Acquired and Healthcare-Associated Pneumonia. Antimicrob. Agents Chemother. 2019, 63, e00023-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Tu, C.-Y.; Chen, W.-C.; Kuo, L.-K.; Wang, Y.-T.; Fu, P.-K.; Ku, S.-C.; Fang, W.-F.; Chen, C.-M.; Lai, C.-C. Clinical Efficacy of Cefoperazone-Sulbactam versus Piperacillin-Tazobactam in the Treatment of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Infect. Drug Resist. 2021, 14, 2251–2258. [Google Scholar] [CrossRef]
- Kara, İ.; Yildirim, F.; Bilaloglu, B.; Karamanlioglu, D.; Kayacan, E.; Dizbay, M.; Turkoglu, M.; Aygencel, G. Comparison of the Efficacy of Colistin Monotherapy and Colistin Combination Therapies in the Treatment of Nosocomial Pneumonia and Ventilator-Associated Pneumonia Caused by Acinetobacter baumannii. South Afr. J. Crit. Care 2015, 31, 51–58. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, J.; Wu, L.; Zhang, D.; Fu, L.; Xue, X. Comparison of the Treatment Efficacy between Tigecycline plus High-Dose Cefoperazone-Sulbactam and Tigecycline Monotherapy against Ventilator-Associated Pneumonia Caused by Extensively Drug-Resistant Acinetobacter baumannii. Int. J. Clin. Pharmacol. Ther. 2018, 56, 120–129. [Google Scholar] [CrossRef]
- Kanchanasuwan, S.; Kositpantawong, N.; Singkhamanan, K.; Hortiwakul, T.; Charoenmak, B.; Ozioma, F.N.; Doi, Y.; Chusri, S. Outcomes of Adjunctive Therapy with Intravenous Cefoperazone-Sulbactam for Ventilator-Associated Pneumonia Due to Carbapenem-Resistant Acinetobacter baumannii. Infect. Drug Resist. 2021, 14, 1255–1264. [Google Scholar] [CrossRef]
- Lv, Q.; Deng, Y.; Zhu, X.; Ruan, J.; Chen, L. Effectiveness of Cefoperazone-Sulbactam Alone and Combined with Tigecycline in the Treatment of Multi-Drug Resistant Acinetobacter baumannii Pulmonary Infection. J. Coll. Physicians Surg. Pak. 2020, 30, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Bassetti, M.; De Rosa, F.G.; Del Bono, V.; Grossi, P.A.; Menichetti, F.; Pea, F.; Rossolini, G.M.; Tumbarello, M.; Viale, P.; et al. Ceftolozane/Tazobactam: Place in Therapy. Expert Rev. Anti. Infect. Ther. 2018, 16, 307–320. [Google Scholar] [CrossRef]
- Lizza, B.D.; Betthauser, K.D.; Ritchie, D.J.; Micek, S.T.; Kollef, M.H. New Perspectives on Antimicrobial Agents: Ceftolozane-Tazobactam. Antimicrob. Agents Chemother. 2021, 65, e0231820. [Google Scholar] [CrossRef]
- Cluck, D.; Lewis, P.; Stayer, B.; Spivey, J.; Moorman, J. Ceftolozane-Tazobactam: A New-Generation Cephalosporin. Am. J. Health Pharm. 2015, 72, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-Generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, M.; Nelson, D.; O’Neal, M.; Gould, A.P.; Bouchard, J.; Nicolau, D.; Justo, J.A.; Hucks, J.; Bookstaver, P.B. Use of Continuous-Infusion Ceftolozane/Tazobactam for Resistant Gram-Negative Bacterial Infections: A Retrospective Analysis and Brief Review of the Literature. Int. J. Antimicrob. Agents 2020, 56, 106158. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Lungu, I.-A. The New Fifth-Generation Cephalosporins—A Balance between Safety and Efficacy. Rom. J. Pharm. Pract. 2020, 13, 121–126. [Google Scholar] [CrossRef]
- Craig, W.A.; Andes, D.R. In Vivo Activities of Ceftolozane, a New Cephalosporin, with and without Tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, Including Strains with Extended-Spectrum β-Lactamases, in the Thighs of Neutropenic Mice. Antimicrob. Agents Chemother. 2013, 57, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Haidar, G.; Philips, N.J.; Shields, R.K.; Snyder, D.; Cheng, S.; Potoski, B.A.; Doi, Y.; Hao, B.; Press, E.G.; Cooper, V.S.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin. Infect. Dis. 2017, 65, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Gato, E.; Pérez-Llarena, J.; Fernández-Cuenca, F.; Gude, M.J.; Oviaño, M.; Pachón, M.E.; Garnacho, J.; González, V.; Pascual, Á.; et al. High Incidence of MDR and XDR Pseudomonas aeruginosa Isolates Obtained from Patients with Ventilator-Associated Pneumonia in Greece, Italy and Spain as Part of the MagicBullet Clinical Trial. J. Antimicrob. Chemother. 2019, 74, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Flamm, R.K.; Dale, G.E.; Rhomberg, P.R.; Castanheira, M. Murepavadin Activity Tested against Contemporary (2016–17) Clinical Isolates of XDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2018, 73, 2400–2404. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Shortridge, D.; Arends, S.J.R.; Duncan, L.R.; Streit, J.M.; Flamm, R.K. Activity of Ceftolozane-Tazobactam and Comparators When Tested against Bacterial Surveillance Isolates Collected from Patients at Risk of Infections Caused by Resistant Gram-Negative Pathogens. Diagn. Microbiol. Infect. Dis. 2020, 98, 115101. [Google Scholar] [CrossRef]
- Idowu, T.; Zhanel, G.G.; Schweizer, F. A Dimer, but Not Monomer, of Tobramycin Potentiates Ceftolozane against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa and Delays Resistance Development. Antimicrob. Agents Chemother. 2020, 64, e02055-19. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Young, K.; Motyl, M.R.; Sahm, D.F. Activity of Ceftolozane/Tazobactam against Gram-Negative Isolates from Patients with Lower Respiratory Tract Infections—SMART United States 2018–2019. BMC Microbiol. 2021, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, D.; Pfaller, M.A.; Arends, S.J.R.; Raddatz, J.; DePestel, D.D.; Flamm, R.K. Comparison of the In Vitro Susceptibility of Ceftolozane-Tazobactam with the Cumulative Susceptibility Rates of Standard Antibiotic Combinations When Tested Against Pseudomonas aeruginosa from ICU Patients with Bloodstream Infections or Pneumonia. Open Forum Infect. Dis. 2019, 6, ofz240. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, D.; Carvalhaes, C.G.; Streit, J.M.; Flamm, R.K. Susceptibility Trends of Ceftolozane/Tazobactam and Comparators When Tested against U.S. Gram-Negative Bacterial Surveillance Isolates (2012–2018). Diagn. Microbiol. Infect. Dis. 2021, 100, 115302. [Google Scholar] [CrossRef] [PubMed]
- Lob, S.H.; DePestel, D.D.; DeRyke, C.A.; Kazmierczak, K.M.; Young, K.; Motyl, M.R.; Sahm, D.F. Ceftolozane/Tazobactam and Imipenem/Relebactam Cross-Susceptibility Among Clinical Isolates of Pseudomonas aeruginosa from Patients with Respiratory Tract Infections in ICU and Non-ICU Wards—SMART United States 2017–2019. Open Forum Infect. Dis. 2021, 8, ofab320. [Google Scholar] [CrossRef] [PubMed]
- Candel, F.J.; Santerre Henriksen, A.; Longshaw, C.; Yamano, Y.; Oliver, A. In Vitro Activity of the Novel Siderophore Cephalosporin, Cefiderocol, in Gram-Negative Pathogens in Europe by Site of Infection. Clin. Microbiol. Infect. 2022, 28, 447.e1–447.e6. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Nováček, M.; Kivistik, Ü.; Réa-Neto, Á.; Shime, N.; Martin-Loeches, I.; Timsit, J.-F.; Wunderink, R.G.; Bruno, C.J.; Huntington, J.A.; et al. Ceftolozane-Tazobactam versus Meropenem for Treatment of Nosocomial Pneumonia (ASPECT-NP): A Randomised, Controlled, Double-Blind, Phase 3, Non-Inferiority Trial. Lancet Infect. Dis. 2019, 19, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.-F.; Huntington, J.A.; Wunderink, R.G.; Shime, N.; Kollef, M.H.; Kivistik, Ü.; Nováček, M.; Réa-Neto, Á.; Martin-Loeches, I.; Yu, B.; et al. Ceftolozane/Tazobactam versus Meropenem in Patients with Ventilated Hospital-Acquired Bacterial Pneumonia: Subset Analysis of the ASPECT-NP Randomized, Controlled Phase 3 Trial. Crit. Care 2021, 25, 290. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.G.; Bruno, C.; Castanheira, M.; Yu, B.; Huntington, J.A.; Carmelitano, P.; Rhee, E.G.; De Anda, C.; Motyl, M. Evaluating the Emergence of Nonsusceptibility among Pseudomonas aeruginosa Respiratory Isolates from a Phase-3 Clinical Trial for Treatment of Nosocomial Pneumonia (ASPECT-NP). Int. J. Antimicrob. Agents 2021, 57, 106278. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Kaye, K.S.; Veve, M.P.; Patel, T.S.; Gerlach, A.T.; Davis, S.L.; Puzniak, L.A.; File, T.M.; Olson, S.; Dhar, S.; et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-Based Regimens for the Treatment of Drug-Resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2020, 71, 304–310. [Google Scholar] [CrossRef]
- Mogyoródi, B.; Csékó, A.B.; Hermann, C.; Gál, J.; Iványi, Z.D. Ceftolozane/Tazobactam versus Colistin in the Treatment of Ventilator-Associated Pneumonia Due to Extensively Drug-Resistant Pseudomonas aeruginosa. Sci. Rep. 2022, 12, 4455. [Google Scholar] [CrossRef]
- Bassetti, M.; Castaldo, N.; Cattelan, A.; Mussini, C.; Righi, E.; Tascini, C.; Menichetti, F.; Mastroianni, C.M.; Tumbarello, M.; Grossi, P.; et al. Ceftolozane/Tazobactam for the Treatment of Serious Pseudomonas aeruginosa Infections: A Multicentre Nationwide Clinical Experience. Int. J. Antimicrob. Agents 2019, 53, 408–415. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Dhami, R.; Baxter, M.; Kosar, J.; Cervera, C.; Irfan, N.; Zvonar, R.; Borgia, S.; Tessier, J.-F.; Dow, G.; et al. Real-Life Experience with Ceftolozane/Tazobactam in Canada: Results from the CLEAR (Canadian LEadership on Antimicrobial Real-Life Usage) Registry. J. Glob. Antimicrob. Resist. 2021, 25, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Satlin, M.J.; Elabor, A.; Saraiya, N.; McCreary, E.K.; Molnar, E.; El-Beyrouty, C.; Jones, B.M.; Dixit, D.; Heil, E.L.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: A Multicenter Study. Open Forum Infect. Dis. 2018, 5, ofy280. [Google Scholar] [CrossRef] [PubMed]
- Holger, D.J.; Rebold, N.S.; Alosaimy, S.; Morrisette, T.; Lagnf, A.; Belza, A.C.; Coyne, A.J.K.; El Ghali, A.; Veve, M.P.; Rybak, M.J. Impact of Ceftolozane-Tazobactam vs. Best Alternative Therapy on Clinical Outcomes in Patients with Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Lower Respiratory Tract Infections. Infect. Dis. Ther. 2022, 11, 1965–1980. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, R.; Cai, Y. Resistance to Ceftazidime-Avibactam and Underlying Mechanisms. J. Glob. Antimicrob. Resist. 2020, 22, 18–27. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Lawson, C.D.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Lagacé-Wiens, P.R.S.; Denisuik, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; et al. Ceftazidime-Avibactam: A Novel Cephalosporin/β-Lactamase Inhibitor Combination. Drugs 2013, 73, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, M.; Mensa, J. Ceftazidime-Avibactam. Rev. Esp. Quimioter. 2021, 34 (Suppl. 1), 38–40. [Google Scholar] [CrossRef]
- Falcone, M.; Paterson, D. Spotlight on Ceftazidime/Avibactam: A New Option for MDR Gram-Negative Infections. J. Antimicrob. Chemother. 2016, 71, 2713–2722. [Google Scholar] [CrossRef]
- Carmeli, Y.; Armstrong, J.; Laud, P.J.; Newell, P.; Stone, G.; Wardman, A.; Gasink, L.B. Ceftazidime-Avibactam or Best Available Therapy in Patients with Ceftazidime-Resistant Enterobacteriaceae and Pseudomonas aeruginosa Complicated Urinary Tract Infections or Complicated Intra-Abdominal Infections (REPRISE): A Randomised, Pathogen-Directed, Phase 3 Study. Lancet Infect. Dis. 2016, 16, 661–673. [Google Scholar] [CrossRef]
- Drwiega, E.N.; Rodvold, K.A. Penetration of Antibacterial Agents into Pulmonary Epithelial Lining Fluid: An Update. Clin. Pharmacokinet. 2022, 61, 17–46. [Google Scholar] [CrossRef]
- Ehmann, D.E.; Jahić, H.; Ross, P.L.; Gu, R.F.; Hu, J.; Kern, G.; Walkup, G.K.; Fisher, S.L. Avibactam Is a Covalent, Reversible, Non-β-Lactam β-Lactamase Inhibitor. Proc. Natl. Acad. Sci. USA 2012, 109, 11663–11668. [Google Scholar] [CrossRef]
- Sader, H.S.; Castanheira, M.; Flamm, R.K.; Mendes, R.E.; Farrell, D.J.; Jones, R.N. Ceftazidime/Avibactam Tested against Gram-Negative Bacteria from Intensive Care Unit (ICU) and Non-ICU Patients, Including Those with Ventilator-Associated Pneumonia. Int. J. Antimicrob. Agents 2015, 46, 53–59. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Li, J.; Zhang, Y.; Yang, Y.; Dong, D.; Zhu, D.; He, P.; Hu, F. In Vitro and in Vivo Bactericidal Activity of Ceftazidime-Avibactam against Carbapenemase-Producing Klebsiella pneumoniae. Antimicrob. Resist. Infect. Control 2018, 7, 142. [Google Scholar] [CrossRef]
- Stone, G.G.; Newell, P.; Gasink, L.B.; Broadhurst, H.; Wardman, A.; Yates, K.; Chen, Z.; Song, J.; Chow, J.W. Clinical Activity of Ceftazidime/Avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: Pooled Data from the Ceftazidime/Avibactam Phase III Clinical Trial Programme. J. Antimicrob. Chemother. 2018, 73, 2519–2523. [Google Scholar] [CrossRef]
- Stone, G.G.; Bradford, P.A.; Tawadrous, M.; Taylor, D.; Cadatal, M.J.; Chen, Z.; Chow, J.W. In Vitro Activity of Ceftazidime-Avibactam against Isolates from Respiratory and Blood Specimens from Patients with Nosocomial Pneumonia, Including Ventilator-Associated Pneumonia, in a Phase 3 Clinical Trial. Antimicrob. Agents Chemother. 2020, 64, e02356-19. [Google Scholar] [CrossRef]
- Sader, H.S.; Castanheira, M.; Flamm, R.K. Antimicrobial Activity of Ceftazidime-Avibactam against Gram-Negative Bacteria Isolated from Patients Hospitalized with Pneumonia in U.S. Medical Centers, 2011 to 2015. Antimicrob. Agents Chemother. 2017, 61, e02083-16. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.-F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-Avibactam versus Meropenem in Nosocomial Pneumonia, Including Ventilator-Associated Pneumonia (REPROVE): A Randomised, Double-Blind, Phase 3 Non-Inferiority Trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Rank, D.; Melnick, D.; Rekeda, L.; Chen, X.; Riccobene, T.; Critchley, I.A.; Lakkis, H.D.; Taylor, D.; Talley, A.K. Randomized Trial of Ceftazidime-Avibactam vs Meropenem for Treatment of Hospital-Acquired and Ventilator-Associated Bacterial Pneumonia (REPROVE): Analyses per US FDA–Specified End Points. Open Forum Infect. Dis. 2019, 6, ofz149. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, J.; Liu, P.; Wang, T.; Wang, H.; Liu, Y.; Cao, Q.; Zuo, X. Ceftazidime-Avibactam-Based Versus Tigecycline-Based Regimen for the Treatment of Carbapenem-Resistant Klebsiella pneumoniae-Induced Pneumonia in Critically Ill Patients. Infect. Dis. Ther. 2021, 10, 2721–2734. [Google Scholar] [CrossRef]
- Tsolaki, V.; Mantzarlis, K.; Mpakalis, A.; Malli, E.; Tsimpoukas, F.; Tsirogianni, A.; Papagiannitsis, C.; Zygoulis, P.; Papadonta, M.-E.; Petinaki, E.; et al. Ceftazidime-Avibactam To Treat Life-Threatening Infections by Carbapenem-Resistant Pathogens in Critically Ill Mechanically Ventilated Patients. Antimicrob. Agents Chemother. 2020, 64, e02320-19. [Google Scholar] [CrossRef]
- van Duin, D.; Lok, J.J.; Earley, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 2018, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhang, J.; Wang, B.; Cai, J.; Wang, L.; Hou, K.; Zhang, Y.; Zhang, L.; Yang, Z.; He, J.; et al. Ceftazidime-Avibactam in Combination with In Vitro Non-Susceptible Antimicrobials Versus Ceftazidime-Avibactam in Monotherapy in Critically Ill Patients with Carbapenem-Resistant Klebsiella pneumoniae Infection: A Retrospective Cohort Study. Infect. Dis. Ther. 2021, 10, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Mantzarlis, K.; Manoulakas, E.; Parisi, K.; Sdroulia, E.; Zapaniotis, N.; Tsolaki, V.; Zakynthinos, E.; Makris, D. Meropenem plus Ertapenem and Ceftazidime-Avibactam plus Aztreonam for the Treatment of Ventilator Associated Pneumonia Caused by Pan-Drug Resistant Klebsiella pneumonia. Antibiotics 2024, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Burastero, G.J.; Orlando, G.; Santoro, A.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Faltoni, M.; Bacca, E.; Biagioni, E.; et al. Ceftazidime/Avibactam in Ventilator-Associated Pneumonia Due to Difficult-to-Treat Non-Fermenter Gram-Negative Bacteria in COVID-19 Patients: A Case Series and Review of the Literature. Antibiotics 2022, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Corbella, L.; Boán, J.; San-Juan, R.; Fernández-Ruiz, M.; Carretero, O.; Lora, D.; Hernández-Jiménez, P.; Ruiz-Ruigómez, M.; Rodríguez-Goncer, I.; Silva, J.T.; et al. Effectiveness of Ceftazidime-Avibactam for the Treatment of Infections Due to Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2022, 59, 106517. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.C.J.; Trinh, T.D.; Zasowski, E.J.; Lagnf, A.M.; Bhatia, S.; Melvin, S.M.; Steed, M.E.; Simon, S.P.; Estrada, S.J.; Morrisette, T.; et al. Real-World Experience With Ceftazidime-Avibactam for Multidrug-Resistant Gram-Negative Bacterial Infections. Open Forum Infect. Dis. 2019, 6, ofz522. [Google Scholar] [CrossRef] [PubMed]
- Domingues, S.; Lima, T.; Saavedra, M.J.; Da Silva, G.J. An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms. Life 2023, 13, 1427. [Google Scholar] [CrossRef] [PubMed]
- El-Lababidi, R.M.; Rizk, J.G. Cefiderocol: A Siderophore Cephalosporin. Ann. Pharmacother. 2020, 54, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, e0217120. [Google Scholar] [CrossRef]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69, S538–S543. [Google Scholar] [CrossRef]
- Katsube, T.; Nicolau, D.P.; Rodvold, K.A.; Wunderink, R.G.; Echols, R.; Matsunaga, Y.; Menon, A.; Portsmouth, S.; Wajima, T. Intrapulmonary Pharmacokinetic Profile of Cefiderocol in Mechanically Ventilated Patients with Pneumonia. J. Antimicrob. Chemother. 2021, 76, 2902–2905. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; Idowu, T.; Domalaon, R.; Schweizer, F.; Zhanel, M.A.; et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs 2019, 79, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Koh, Y.-S. A Novel Antibiotic Agent, Cefiderocol, for Multidrug-Resistant Gram-Negative Bacteria. J. Bacteriol. Virol. 2020, 50, 218–226. [Google Scholar] [CrossRef]
- Yamano, Y. In Vitro Activity of Cefiderocol Against a Broad Range of Clinically Important Gram-Negative Bacteria. Clin. Infect. Dis. 2019, 69, S544–S551. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Hackel, M.; Echols, R.; Yamano, Y.; Sahm, D. In Vitro Activity of Cefiderocol against Globally Collected Carbapenem-Resistant Gram-Negative Bacteria Isolated from Urinary Track Source: SIDERO-CR-2014/2016. Open Forum Infect. Dis. 2017, 4, S366. [Google Scholar] [CrossRef][Green Version]
- Shortridge, D.; Streit, J.M.; Mendes, R.; Castanheira, M. In Vitro Activity of Cefiderocol against U.S. and European Gram-Negative Clinical Isolates Collected in 2020 as Part of the SENTRY Antimicrobial Surveillance Program. Microbiol. Spectr. 2022, 10, e0271221. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus High-Dose, Extended-Infusion Meropenem for the Treatment of Gram-Negative Nosocomial Pneumonia (APEKS-NP): A Randomised, Double-Blind, Phase 3, Non-Inferiority Trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and Safety of Cefiderocol or Best Available Therapy for the Treatment of Serious Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria (CREDIBLE-CR): A Randomised, Open-Label, Multicentre, Pathogen-Focused, Descriptive, Phase 3 Trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Nicastro, M.; Leonildi, A.; Vecchione, A.; Casella, C.; Forfori, F.; Malacarne, P.; Guarracino, F.; Barnini, S.; et al. Cefiderocol as Rescue Therapy for Acinetobacter baumannii and Other Carbapenem-Resistant Gram-Negative Infections in Intensive Care Unit Patients. Clin. Infect. Dis. 2021, 72, 2021–2024. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Leonildi, A.; Della Sala, L.; Vecchione, A.; Barnini, S.; Farcomeni, A.; Menichetti, F. Cefiderocol- Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, e0214221. [Google Scholar] [CrossRef]
- Rando, E.; Segala, F.V.; Vargas, J.; Seguiti, C.; De Pascale, G.; Murri, R.; Fantoni, M. Cefiderocol for Severe Carbapenem-Resistant A. baumannii Pneumonia: Towards the Comprehension of Its Place in Therapy. Antibiotics 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Bruni, A.; Gullì, S.; Borrazzo, C.; Quirino, A.; Lionello, R.; Serapide, F.; Garofalo, E.; Serraino, R.; Romeo, F.; et al. Efficacy of Cefiderocol- vs Colistin-Containing Regimen for Treatment of Bacteraemic Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii in Patients with COVID-19. Int. J. Antimicrob. Agents 2023, 62, 106825. [Google Scholar] [CrossRef] [PubMed]
- Rando, E.; Cutuli, S.L.; Sangiorgi, F.; Tanzarella, E.S.; Giovannenze, F.; De Angelis, G.; Murri, R.; Antonelli, M.; Fantoni, M.; De Pascale, G. Cefiderocol-Containing Regimens for the Treatment of Carbapenem-Resistant A. baumannii Ventilator-Associated Pneumonia: A Propensity-Weighted Cohort Study. JAC-Antimicrobial Resist. 2023, 5, dlad085. [Google Scholar] [CrossRef] [PubMed]
- Meschiari, M.; Volpi, S.; Faltoni, M.; Dolci, G.; Orlando, G.; Franceschini, E.; Menozzi, M.; Sarti, M.; Del Fabro, G.; Fumarola, B.; et al. Real-Life Experience with Compassionate Use of Cefiderocol for Difficult-to-Treat Resistant Pseudomonas aeruginosa (DTR-P) Infections. JAC-Antimicrobial Resist. 2021, 3, dlab188. [Google Scholar] [CrossRef]
- Dalfino, L.; Stufano, M.; Bavaro, D.F.; Diella, L.; Belati, A.; Stolfa, S.; Romanelli, F.; Ronga, L.; Di Mussi, R.; Murgolo, F.; et al. Effectiveness of First-Line Therapy with Old and Novel Antibiotics in Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii: A Real Life, Prospective, Observational, Single-Center Study. Antibiotics 2023, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, C.; Rodríguez, M.; Merino, N.; Carmona, P.; Machuca, I.; Córdoba-Fernández, M.; Guzmán-Puche, J.; Domínguez, A.; López-Viñau, T.; García, L.; et al. Real-Life Use of Cefiderocol for Salvage Therapy of Severe Infections Due to Carbapenem-Resistant Gram-Negative Bacteria. Int. J. Antimicrob. Agents 2023, 62, 106818. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Tripiciano, C.; Romani, L.; Di Nardo, M.; Bottari, G.; Goffredo, B.M.; Simeoli, R.; Guzzo, I.; Lancella, L.; Antachopoulos, C.; et al. The Use of Cefiderocol as Salvage Therapy in an Infant Receiving ECMO and Continuous Renal Replacement Therapy. Antibiotics 2023, 13, 37. [Google Scholar] [CrossRef]
- Roy, A.M.; Hodgson, M.; Brickman, C.; MacDougall, C. Cefiderocol as Adjunctive Treatment of Necrotizing Ventilator-Associated Pneumonia Due to Extensively Drug-Resistant Pseudomonas aeruginosa. Infect. Dis. Clin. Pract. 2021, 29, e111–e113. [Google Scholar] [CrossRef]



| Cephalosporin-Inhibitor Combination/Antibiotic | Spectrum of Action According to Ambler Classification | Examples of β-Lactamases | References |
|---|---|---|---|
| Cefoperazone-sulbactam | class A | narrow spectrum: TEM-1 and TEM-2 | [18] |
| class C | AmpC | ||
| Ceftolozane-tazobactam | class A | extended spectrum (ESβL): SHV-2 and CTX-M-15 | [19,20] |
| class C | AmpC | ||
| Ceftazidime-avibactam | class A | narrow spectrum: TEM-1 | [21,22] |
| extended spectrum: SHV and CTX-M | |||
| KPC-2 and KPC-3 | |||
| class C | AmpC | ||
| class D | OXA-48 | ||
| Cefiderocol | class A | extended spectrum, e.g., SHV type | [23] |
| KPC | |||
| class B | MBL: VIM, IMP, and NDM | ||
| class C | AmpC | ||
| class D | OXA-48 and OXA-23 |
| Pathogen | Antibiotic Scheme Used In Vitro | MIC Value | Reference |
|---|---|---|---|
| MSSA | CFP-SBT | MIC50 = 2 µg/mL | [39] |
| CFP | MIC50 = 4 µg/mL | ||
| K. pneumoniae | CFP-SBT | MIC90 = 32 µg/mL | |
| CFP | MIC90 = 128 µg/mL | ||
| P. aeruginosa | CFP-SBT | MIC90 = 32 µg/mL | |
| CFP | MIC90 = 128 µg/mL | ||
| A. baumannii | CFP-SBT | MIC90 = 64 µg/mL | |
| CFP | MIC90 > 128 µg/mL | ||
| S. maltophila | CFP-SBT | MIC50 = 64 µg/mL | |
| CFP | MIC50 = 128 µg/mL | ||
| MDR P. aeruginosa | CFP-SBT | MIC50 = 64 µg/mL | [42] |
| MIC90 = 128 µg/mL | |||
| CFP-SBT + AZT | MIC50 = 16 µg/mL | ||
| MIC90 = 64 µg/mL | |||
| Carbapenem-resistant A. baumannii | CFP-SBT + MER | MIC50 = 16 µg/mL | [37] |
| MIC90 = 64 µg/mL |
| Number of Patients | Treatment Scheme in VABP Population | Outcomes of Trial | Etiology of Infection | Reference | ||
|---|---|---|---|---|---|---|
| N = 166 | CFP-SBT (N = 79) | CC: | 73.1% | P. aeruginosa A. baumannii | [43] | |
| CPM (N = 87) | 56.8% | |||||
| N = 317 | CFP-SBT (N = 167) | CC | 85% | K. pneumoniae P. aeruginosa A. baumannii | [42] | |
| 81% | ||||||
| PIP-TAZ (N = 150) | IHMR | 24% | ||||
| 23% | ||||||
| N = 65 | CFP-SBT (N = 37) | CC in VABP group: | 78.4% | A. baumannii | [44] | |
| 71.4% | P. aeruginosa | |||||
| PIP-TAZ (N = 28) | Total mortality ratio: | 13.5% | K. pneumoniae | |||
| 3.6% | P. aeruginosa | |||||
| N = 308 | CFP-SBT (N = 154) | CC: | 50% | MDR (70%): K. pneumoniae E. coli P. aeruginosa A. baumannii | [30] | |
| 51.2% | ||||||
| 14-MR: | 29.2% | |||||
| PIP-TAZ (N = 154) | 35% | |||||
| 28-MR: | 46.1% | |||||
| 42.8% | ||||||
| N = 42 | CFP-SBT + TGC | CC: | 85.7% | XDR A. baumannii | [46] | |
| TGC | 47.6% | |||||
| N = 80 | Combination therapy with CFP-SBT (N = 52) | 14-MR | 17% | Carbapenem-resistant A. baumannii | [47] | |
| 39% | ||||||
| 30-MR | 35% | |||||
| 61% | ||||||
| Combination therapy without CFP-SBT (N = 38) | IHMR | 39% | ||||
| 68% | ||||||
| Pathogen | Antibiotic Scheme Used In Vitro | MIC Value | Reference |
|---|---|---|---|
| XDR P. aeruginosa | CEF-TAZ | MIC50 = 2 mg/mL MIC90 > 32 mg/mL | [58] |
| MIC50 = 1 mg/mL MIC90 = 8 mg/mL | |||
| MDR P. aeruginosa | CEF-TAZ | MIC50 = 1 mg/L | [19] |
| MIC90 = 8 mg/L | |||
| XDR P. aeruginosa | CEF-TAZ | MIC50 = 2 mg/L | |
| MIC90 = 16 mg/L | |||
| Non-CRE E. coli | CEF-TAZ | MIC50 = 0.5 mg/L | |
| MIC90 = 2 mg/L | |||
| Non-CRE K. pneumoniae | CEF-TAZ | MIC50 = 1 mg/L | |
| MIC90 = 8 mg/L | |||
| ESβL non-CRE Enterobacterales | CEF-TAZ | 0.5–8 mg/L | [59] |
| P. aeruginosa | CEF-TAZ | MIC50 = 0.5 mg/L | [65] |
| MIC90 = 8 mg/L | |||
| K. pneumoniae | CEF-TAZ | MIC50 = 0.5 mg/L | |
| MIC90 > 64 mg/L |
| Number of Patients | Treatment Scheme in VABP Population | Outcomes of Trial | Etiology of Infections | Reference | |
|---|---|---|---|---|---|
| N = 726 (71% VABP) | CEF-TAZ (N = 362) | 28-MR | 24% | K. pneumoniae, E. coli P. aeruginosa (including ESβL) | [66] |
| 25.3% | |||||
| Clinical response at TOC | 54% | ||||
| MER (N = 364) | 53% | ||||
| Microbiological eradication ratio | 73.1% | ||||
| 68% | |||||
| N = 200 (52% VABP) | CEF-TAZ (N = 100) | CC | 81% | MDR/XDR P. aeruginosa | [69] |
| 61% | |||||
| Aminoglycosides (TOB, GEN, AKC)/COL (N = 100) | IHMR | 20% | |||
| 25% | |||||
| N = 51 | CEF-TAZ (N = 18) | CC | 72.2% | XDR P. aeruginosa | [70] |
| 30.3% | |||||
| Microbiological eradication ratio | 44.4% | ||||
| 15.2% | |||||
| COL (N = 33) | 28-MR | 27.8% | |||
| 33.3% | |||||
| Frequency of AEs | 55.5% | ||||
| 72.7% | |||||
| N = 205 (63/205 VABP) | CEF-TAZ (N = 63) | 30-MR | 35% | MDR P. aeruginosa | [73] |
| CC | 50% | ||||
| Microbiological eradication in the EOT | 53.4% | ||||
| N = 206 (46.6% VABP) | CEF-TAZ (N = 118) | Clinical failure | 23.7% | MDR/XDR P. aeruginosa | [74] |
| 48.9% | |||||
| 30-MR | 15.3% | ||||
| BAT (N = 88) | 20.5% | ||||
| Frequency of TEAEs | 10.2% | ||||
| 33% | |||||
| Pathogens | Antibiotic Scheme Used In Vitro | MIC Value | Reference |
|---|---|---|---|
| KPC Enterobacteriaceae | CAZ-AVI | MIC50 = 0.5 mg/L | [82] |
| MIC90 = 2 mg/L | |||
| CRE Enterobacteriaceae | MIC50 = 0.5 mg/L | ||
| MIC90 = 2 mg/L | |||
| P. aeruginosa | MIC50 = 2 mg/L | ||
| MIC90 = 4 mg/L | |||
| P. aeruginosa (MER-NS, CAZ-NS, or MDR strains) | MIC50 = 4 mg/L | ||
| MIC90 = 16 mg/L | |||
| XDR P. aeruginosa | MIC50 = 8 mg/L | ||
| MIC90 = 32 mg/L | |||
| A. baumannii | MIC50 = 16 mg/L | ||
| MIC90 > 32 mg/L | |||
| MDR P. aeruginosa | CAZ-AVI | MIC50 = 2 mg/L | [57] |
| MIC90 = 16 mg/L | |||
| KPC-2 K. pneumoniae | CAZ-AVI | MIC = 0.4–0.8 mg/L | [83] |
| OXA-232 K. pneumoniae | MIC = 0.2 mg/L | ||
| NDM K. pneumoniae | MIC = 0.5–256 mg/L | ||
| KPC-2 + NDM K. pneumoniae | MIC = 8–128 mg/L | ||
| MDR P. aeruginosa | CAZ-AVI | MIC50 = 8 mg/L | [84] |
| MIC90 = 64 mg/L | |||
| MDR K. pneumoniae | MIC50 = 0.5 mg/L | ||
| MIC90 = 1 mg/L | |||
| P. aeruginosa | CAZ-AVI | MIC50 = 2 mg/L | [86] |
| MIC90 = 4 mg/L | |||
| MER-NS P. aeruginosa | MIC50 = 4 mg/L MIC90 = 16 mg/L | ||
| PIP-TAZ-NS P. aeruginosa | |||
| MDR P. aeruginosa | |||
| XDR P. aeruginosa | MIC50 = 8 mg/L | ||
| MIC90 = 32 mg/L | |||
| A. baumannii | MIC50 = 16 mg/L | ||
| MIC90 > 32 mg/L | |||
| ESβL K. pneumoniae | MIC50 = 0.25 mg/L | ||
| MIC90 = 1 mg/L | |||
| ESβL E. coli | MIC50 = 0.12 mg/L | ||
| MIC90 = 0.5 mg/L |
| Number of Patients | Treatment Scheme in VABP Population | Outcomes of Trial | Etiology of Infection | Reference | |
|---|---|---|---|---|---|
| N = 726 | CAZ-AVI (N = 356) (33% VABP) | Clinical success in mITT population | 70.3% | K. pneumoniae P. aeruginosa | [87] |
| 74.2% | |||||
| Clinical success in CEP population | 77.5% | ||||
| 75.9% | |||||
| MER (N = 370) (35% VABP) | 28-MR | 9% | |||
| 7% | |||||
| Frequency of AEs | 75% | ||||
| 74% | |||||
| N = 105 (71.4% VABP) | CAZ-AVI (N = 43) | Clinical success | 51.2% | CRE K. pneumoniae | [89] |
| 29% | |||||
| Microbiological success | 74.4% | ||||
| TGC (N = 62) | 33.9% | ||||
| 28-MR | 69.8% | ||||
| 66.1% | |||||
| N = 77 (33.8% VABP) | CAZ-AVI (N = 41, 19/41 VABP) | CC | 80.5% | CRE: P. aeruginosa K. pneumoniae E. coli | [90] |
| 52.8% | |||||
| Microbiological eradication ratio | 94.3% | ||||
| BAT (N = 36, 7/36 VABP) | 67.7% | ||||
| 28-day survival ratio | 85.4% | ||||
| 61.1% | |||||
| N = 62 | CAZ-AVI monotherapy (N = 21) | 30-MR | 47.6% | CRE: P. aeruginosa K. pneumoniae E. coli | [92] |
| 24.4% | |||||
| CAZ-AVI combined therapy (N = 41) | Microbiological eradication ratio | 42.9% | |||
| 61% | |||||
| N = 61 | CAZ-AVI in monotherapy 53% CAZ-AVI in combined therapy 47% (scheme obtained, e.g., COL/MER/ATM) | CC by the day 14 | 54.1% | P. aeruginosa: MDR—91.8% XDR—8.2% | [95] |
| 30-MR | 13.1% | ||||
| Recurrence by the day 90 | 12.5% | ||||
| 30-day survival ratio | 93.8% (for CAZ-AVI monotherapy) | ||||
| N = 203 (37.4% LRTI include VABP) | CAZ-AVI monotherapy (N = 203) | Clinical success | 70.9% | CRE: P. aeruginosa K. pneumoniae | [96] |
| Recurrence by the day 30 ratio | 5.9% | ||||
| 30-MR | 17.2% | ||||
| Pathogens | Antibiotic Scheme Used In Vitro | MIC Value | References |
|---|---|---|---|
| K. pneumoniae | CFD | MIC90 = 1 µg/mL | [20,104] |
| P. aeruginosa | MIC90 = 0.5 µg/mL | ||
| A. baumannii | MIC90 = 1–4 µg/mL | ||
| S. maltophila | MIC90 = 0.25–0.5 µg/mL | ||
| MER-NS P. aeruginosa | MIC90 = 0.5–1 µg/mL | ||
| MER-NS A. baumannii | MIC90 = 1–4 µg/mL | ||
| carbapenem-NS Enterobacteriaceae | CFD | MIC90 = 4 µg/mL | [105] |
| P. aeruginosa | CFD | MIC50 = 0.12 mg/L | [106] |
| MIC90 = 0.5 mg/L | |||
| XDR P. aeruginosa | MIC50 = 0.12 mg/L | ||
| MIC90 = 1 mg/L | |||
| MER-NS P. aeruginosa | MIC50 = 0.12 mg/L | ||
| MIC90 = 1 mg/L | |||
| A. baumannii | MIC50 = 0.25 mg/L | ||
| MIC90 = 1 mg/L | |||
| MER-NS A. baumannii | MIC50 = 0.5 mg/L | ||
| MIC90 = 2 mg/L | |||
| S. maltophila | MIC50 = 0.12 mg/L | ||
| MIC90 = 0.5 mg/L |
| Number of Patients | Treatment Scheme in VABP Population | Outcomes of Trial | Etiology of Infection | Reference | |
|---|---|---|---|---|---|
| N = 300 | CFD (N = 148) (41% VABP) | 14-MR | 15% | K. pneumoniae P. aeruginosa A. baumannii (CRE strains: 70% ESβL producers: 43–67%) | [107] |
| 13% | |||||
| 28-MR | 23% | ||||
| 22% | |||||
| MER (N = 152) (44% VABP) | CC | 66% | |||
| 56% | |||||
| Microbiological eradication | 42% | ||||
| 34% | |||||
| N = 152 | CFD (N = 101) (24% VABP) | CC at TOC | 50% | CR-GNB With a predominance of CRAB (65% of isolates in the CFD and 53% in the BAT groups) | [108] |
| 53% | |||||
| Microbiological eradication at TOC | 23% | ||||
| 21% | |||||
| BAT (N = 51) (27% VABP) | 14-MR | 24% | |||
| 31% | |||||
| 28-MR | 14% | ||||
| 18% | |||||
| N = 35 (VABP group) | 12/35 CFD-based therapy | 30-MR | 58.3% | CRAB | [110] |
| 23/35 COL-based therapy | 56.5% | ||||
| N = 73 | CFD (N = 19)-based therapy | 14-MR | 5.2% | CRAB complicated with SARS-CoV2 | [112] |
| 75.9% | |||||
| COL (N = 54)-based therapy | 28-MR | 31.5% | |||
| 98.1% | |||||
| N = 90 | CFD-based therapy with additional inh. COL (N = 40) | Clinical failure | 25% | CRAB | [115] |
| 48% | |||||
| Microbiological failure | 30% | ||||
| COL-based therapy with additional inh. COL (N = 50) | 60% | ||||
| 14-MR | 10% | ||||
| 38% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viscardi, S.; Topola, E.; Sobieraj, J.; Duda-Madej, A. Novel Siderophore Cephalosporin and Combinations of Cephalosporins with β-Lactamase Inhibitors as an Advancement in Treatment of Ventilator-Associated Pneumonia. Antibiotics 2024, 13, 445. https://doi.org/10.3390/antibiotics13050445
Viscardi S, Topola E, Sobieraj J, Duda-Madej A. Novel Siderophore Cephalosporin and Combinations of Cephalosporins with β-Lactamase Inhibitors as an Advancement in Treatment of Ventilator-Associated Pneumonia. Antibiotics. 2024; 13(5):445. https://doi.org/10.3390/antibiotics13050445
Chicago/Turabian StyleViscardi, Szymon, Ewa Topola, Jakub Sobieraj, and Anna Duda-Madej. 2024. "Novel Siderophore Cephalosporin and Combinations of Cephalosporins with β-Lactamase Inhibitors as an Advancement in Treatment of Ventilator-Associated Pneumonia" Antibiotics 13, no. 5: 445. https://doi.org/10.3390/antibiotics13050445
APA StyleViscardi, S., Topola, E., Sobieraj, J., & Duda-Madej, A. (2024). Novel Siderophore Cephalosporin and Combinations of Cephalosporins with β-Lactamase Inhibitors as an Advancement in Treatment of Ventilator-Associated Pneumonia. Antibiotics, 13(5), 445. https://doi.org/10.3390/antibiotics13050445






