Trends in Antibiotic Use in a Large Children’s Hospital in London (United Kingdom): 5 Years of Point Prevalence Surveys
Abstract
1. Introduction
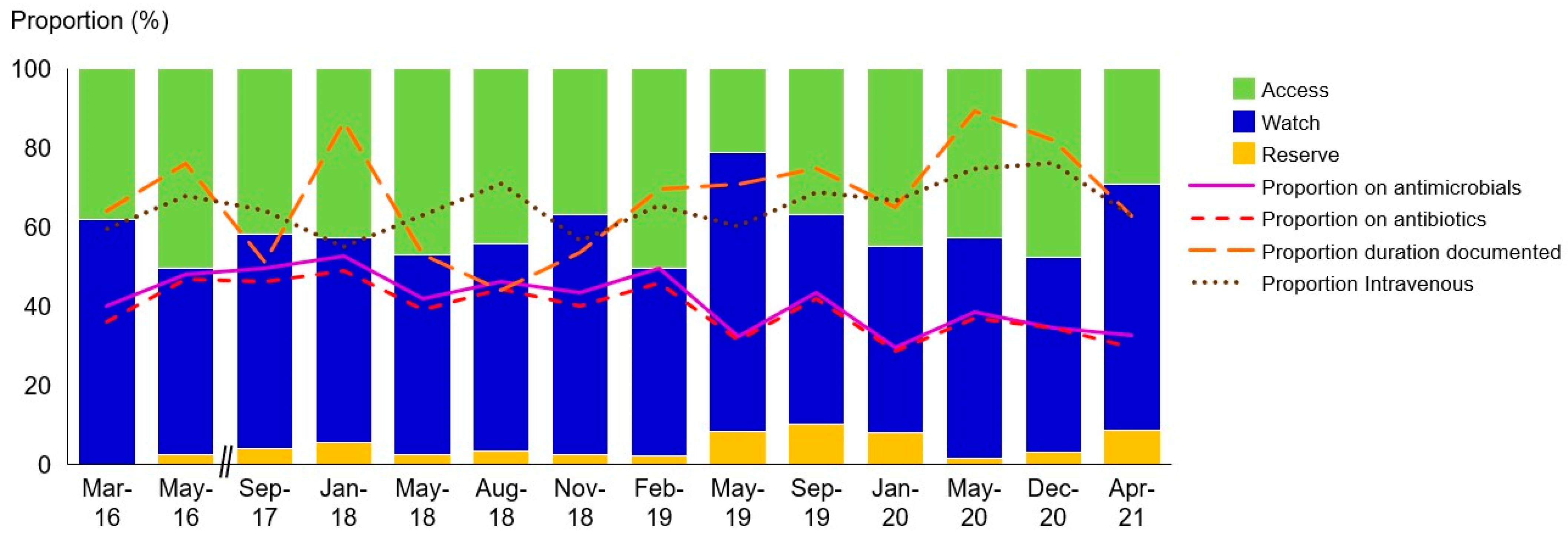
2. Results
3. Materials and Methods
3.1. Setting
3.2. Data Collection
3.3. Data Analysis and Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. UK-Adapted WHO AWaRe Categories [8]
| Access | Watch | Reserve |
| Amoxicillin/ampicillin | Other aminoglycosides | Aztreonam |
| All penicillins | Macrolides | Ceftobiprole |
| Co-trimoxazole | Most cephalosporines | Ceftaroline |
| Doxycycline | Chloramphenicol | Ceftazidime and avibactam |
| Flucloxacillin | Fluoroquinolones | Ceftolozane and tazobactam |
| Fosfomycin oral | Clindamycin | Colistin |
| Fusidate | Amoxicillin and clavulanate | Daptomycin |
| Gentamicin | Other tetracyclines | Carbapenems |
| Metronidazole | Fidaxomicin | Fosfomicin intravenously |
| Nitrofurantoin | Piperacillin and tazobactam | |
| Pivmecillinam | Linezolid/tedizolid | |
| Tetracycline | Temocillin | Televancin |
| Trimethoprim | Glycopeptides | Tigecycline |
Appendix B. Overview of the Recommended Empiric Antimicrobial Therapy at Evelina London Children’s Hospital at the Time of the Surveys
| Infection | Empiric Treatment |
| Community-acquired sepsis without localising signs | Age < 1 month: IV amoxicillin + IV cefotaxime Age > 1 month: IV ceftriaxone |
| Hospital-acquired sepsis without localising signs | Age < 1 month: IV amoxicillin + IV cefotaxime + IV gentamicin Age > 1 month: IV ceftriaxone + IV gentamicin |
| Febrile neutropenia | Piperacillin/tazobactam IV + gentamicin IV |
| Toxic shock syndrome | Age < 1 month: IV cefotaxime + IV amoxicillin + IV clindamycin Age > 1 month: IV ceftriaxone + IV clindamycin |
| Central line-associated blood stream infection | IV vancomycin + IV gentamicin |
| Retropharyngeal abscess | IV ceftriaxone + IV metronidazole |
| Acute sinusitis | PO amoxicillin |
| Acute otitis media | PO amoxicillin |
| Community-acquired pneumonia | Moderate illness: PO amoxicillin Severe illness: IV amoxicillin and clavulanate (+PO/IV clarithromycin if >5 years) |
| Aspiration pneumonia | PO amoxiclav |
| Hospital-acquired pneumonia | Moderate: PO amoxicillin and calvulanate Severe: IV amoxicillin and clavulanate + IV gentamicin |
| Throacic empyema | IV ceftriaxone |
| Urinary tract infection | If well: PO cefalexin Unwell: IV amoxicillin and clavulanate + IV gentamicin |
| Meningoencephalitis | <1 month: IV amoxicillin + IV cefotaxime + IV aciclovir >1 month: IV ceftriaxone + IV aciclovir +/− IV clarithromycin |
| Brain abscess | IV ceftriaxone + IV metronidazole |
| Mastoiditis | IV amoxicillin and clavulanate |
| Cellulitis | PO flocloxacillin If severe: IV flucloxacillin +/− IV clindamycin |
| Necrotising fasciitis | IV amoxicillin and clavulanate+ IV clindamycin |
| Purulent lymphadenitis | PO amoxicillin and clavulanate |
| Necrotising enterocolitis | IV amoxicillin + IV gentamicin + IV metronidazole |
| Orbital cellulitis | IV cefotaxime + IV/PO metronidazole |
| Periorbital cellulitis | PO/IV amoxicillin and clavulanate |
| Appendicitis and bacterial peritonitis | IV amoxicillin and clavulanate + IV gentamicin Severe: IV amoxicillin + IV gentamicin + IV metronidazole |
| Cholangitis | IV ceftriaxone + IV metronidazole |
Appendix C. Indications for Antimicrobial Prescribing during the Survey
| Category | Agent | Indication | Frequency | Adherent to Guidelines | Recommended by ID Team/Microbiologist | Guided by Microbiological Investigations | Guided by External Advice |
| Access | Amoxicillin | Intra-abdominal infections unspecified (3), appendicitis (1), and necrotizing enterocolitis (10) | 14 | 12 | 0 | 0 | 0 |
| Lower respiratory tract infection community-acquired pneumonia (8) and hospital-acquired pneumonia (1) | 9 | 9 | 0 | 0 | 0 | ||
| Surgical prophylaxis | 5 | 1 | 0 | 0 | 0 | ||
| Sepsis | 4 | 3 | 0 | 1 | 0 | ||
| Central nervous system condition | 1 | 1 | 0 | 0 | 0 | ||
| Urinary tract infection | 1 | 1 | 0 | 0 | 0 | ||
| Access | Co-trimoxazole | Medical prophylaxis | 31 | 23 | 0 | 3 | 1 |
| Lower respiratory tract infection | 4 | 0 | 0 | 3 | 0 | ||
| Surgical prophylaxis | 2 | 1 | 0 | 0 | 0 | ||
| Access | Flucloxacillin | Sepsis | 44 | 40 | 2 | 2 | 0 |
| Skin and soft tissue infection | 10 | 8 | 1 | 0 | 0 | ||
| Osteoarticular infection | 3 | 0 | 2 | 1 | 0 | ||
| Lower respiratory tract infection | 1 | 0 | 0 | 1 | 0 | ||
| Surgical prophylaxis | 1 | 1 | 0 | 1 | 0 | ||
| Access | Gentamicin | Sepsis | 97 | 87 | 0 | 5 | 0 |
| Intra-abdominal infection | 30 | 28 | 0 | 1 | 0 | ||
| Lower respiratory tract infection | 13 | 12 | 1 | 0 | 0 | ||
| Surgical prophylaxis | 12 | 4 | 0 | 0 | 1 | ||
| Urinary tract infection | 4 | 3 | 0 | 0 | 0 | ||
| Skin and soft tissue infection | 1 | 0 | 1 | 0 | 0 | ||
| Endocarditis | 1 | 1 | 0 | 1 | 0 | ||
| Febrile neutropenia | 1 | 1 | 0 | 0 | 0 | ||
| Access | Metronidazole | Intra-abdominal infection | 19 | 15 | 0 | 0 | 0 |
| Surgical prophylaxis | 6 | 1 | 0 | 1 | 0 | ||
| Sepsis | 3 | 1 | 0 | 2 | 0 | ||
| Ear, nose, and throat infection | 2 | 1 | 0 | 1 | 0 | ||
| Pleural empyema | 2 | 0 | 2 | 0 | 0 | ||
| Not documented | 2 | 0 | 1 | 0 | 0 | ||
| Osteomyelitis | 1 | 0 | 0 | 1 | 0 | ||
| Small intestinal bowel overgrowth | 1 | 0 | 0 | 0 | 0 | ||
| Extra-intestinal amoebiasis | 1 | 1 | 0 | 0 | 0 | ||
| Access | Nitrofurantoin | Medical prophylaxis | 4 | 0 | 0 | 3 | 0 |
| Urinary tract infection | 3 | 2 | 0 | 2 | 0 | ||
| Access | Penicillin G | Sepsis | 46 | 46 | 0 | 1 | 0 |
| Medical prophylaxis | 22 | 21 | 0 | 0 | 0 | ||
| Surgical prophylaxis | 1 | 1 | 0 | 0 | 0 | ||
| Not documented | 1 | 1 | 0 | 0 | 0 | ||
| Access | Trimethoprim | Medical prophylaxis | 27 | 27 | 0 | 0 | 0 |
| Urinary tract infection | 3 | 3 | 0 | 0 | 0 | ||
| Surgical prophylaxis | 2 | 0 | 0 | 0 | 0 | ||
| Watch | Amikacin | Sepsis | 4 | 0 | 3 | 1 | 0 |
| Lower respiratory tract infection: bronchiectasis | 1 | 0 | 0 | 0 | 0 | ||
| Urinary tract infection | 1 | 0 | 0 | 1 | 0 | ||
| Watch | Azithromycin | Medical prophylaxis | 41 | 2 | 1 | 1 | 3 |
| Lower respiratory tract infection | 2 | 1 | 1 | 0 | 0 | ||
| Watch | Clarithromycin | Central nervous system condition | 5 | 4 | 0 | 0 | 1 |
| Lower respiratory tract infection | 4 | 3 | 0 | 0 | 0 | ||
| Sickle-cell related infection | 2 | 0 | 0 | 0 | 0 | ||
| Sepsis | 1 | 0 | 0 | 0 | 0 | ||
| Watch | Erythromycin | Delayed gastric emptying | 15 | 15 | 0 | 0 | 0 |
| Watch | Amoxicillin and clavulanate | Lower respiratory tract infections | 87 | 69 | 2 | 2 | 1 |
| Surgical prophylaxis | 58 | 17 | 0 | 1 | 2 | ||
| Intra-abdominal infections | 23 | 22 | 0 | 1 | 0 | ||
| Skin and soft tissue infections | 13 | 4 | 1 | 0 | 0 | ||
| Sepsis | 11 | 1 | 0 | 0 | 0 | ||
| Urinary tract infection | 8 | 5 | 0 | 1 | 0 | ||
| Ear, nose, and throat infection | 7 | 4 | 0 | 0 | 1 | ||
| Medical prophylaxis | 5 | 5 | 0 | 0 | 0 | ||
| Not documented | 5 | 2 | 0 | 0 | 0 | ||
| Watch | Cephalexin | Medical prophylaxis | 15 | 6 | 0 | 1 | 0 |
| Urinary tract infection | 6 | 6 | 0 | 0 | 0 | ||
| Small intestinal bowel overgrowth | 2 | 0 | 0 | 0 | 0 | ||
| Watch | Cefotaxime | Sepsis | 63 | 45 | 0 | 7 | 0 |
| Central nervous system condition | 5 | 3 | 1 | 1 | 0 | ||
| Lower respiratory tract infection | 2 | 1 | 0 | 0 | 0 | ||
| Osteoarticular infections | 1 | 0 | 0 | 1 | 0 | ||
| Urinary tract infection | 1 | 1 | 0 | 0 | 0 | ||
| Surgical prophylaxis | 1 | 0 | 0 | 0 | 0 | ||
| Not documented | 1 | 0 | 1 | 0 | 0 | ||
| Watch | Ceftriaxone | Sepsis | 32 | 32 | 8 | 0 | 0 |
| Central nervous system condition | 10 | 10 | 0 | 0 | 0 | ||
| Osteoarticular infections | 6 | 6 | 2 | 2 | 0 | ||
| Sickle-cell related infections | 5 | 5 | 0 | 0 | 0 | ||
| Lower respiratory tract infections | 4 | 3 | 0 | 0 | 0 | ||
| Ear, nose, and throat infection | 3 | 3 | 1 | 1 | 0 | ||
| Toxic shock syndrome | 2 | 2 | 0 | 0 | 0 | ||
| Surgical site infection | 2 | 2 | 2 | 2 | 0 | ||
| Pleural empyema | 2 | 2 | 0 | 0 | 0 | ||
| Endocarditis | 2 | 2 | 1 | 1 | 0 | ||
| Urinary tract infection | 1 | 1 | 0 | 0 | 0 | ||
| Intra-abdominal infections | 1 | 1 | 0 | 0 | 0 | ||
| Surgical prophylaxis | 1 | 1 | 1 | 1 | 0 | ||
| Not documented | 1 | 1 | 0 | 0 | 0 | ||
| Watch | Cefuroxime | Surgical prophylaxis | 8 | 7 | 0 | 0 | 0 |
| Lower respiratory tract infection | 1 | 1 | 0 | 1 | 0 | ||
| Sepsis | 1 | 1 | 0 | 0 | 0 | ||
| Urinary tract infection | 1 | 1 | 0 | 0 | 0 | ||
| Watch | Ciprofloxacin | Pseudomonas aeruginosa eradication | 8 | 0 | 0 | 2 | 2 |
| Lower respiratory tract infection | 3 | 0 | 0 | 1 | 0 | ||
| Urinary tract infection | 2 | 1 | 0 | 1 | 0 | ||
| Febrile neutropenia | 1 | 0 | 0 | 1 | 0 | ||
| Watch | Moxifloxacin | Tuberculosis | 1 | 0 | 1 | 0 | 0 |
| Watch | Clindamycin | Sepsis | 6 | 5 | 4 | 0 | 0 |
| Toxic shock syndrome | 2 | 2 | 1 | 0 | 0 | ||
| Meningitis | 2 | 1 | 1 | 0 | 0 | ||
| Lower respiratory tract infection | 1 | 1 | 1 | 1 | 0 | ||
| Pleural empyema | 1 | 1 | 0 | 0 | 0 | ||
| Osteoarticular | 1 | 1 | 0 | 1 | 0 | ||
| Not documented | 1 | 1 | 0 | 1 | 0 | ||
| Watch | Piperacillin and tazobactam | Sepsis | 9 | 0 | 2 | 6 | 0 |
| Lower respiratory tract infection | 10 | 6 | 1 | 1 | 1 | ||
| Intra-abdominal infection | 1 | 0 | 0 | 0 | 0 | ||
| Osteomyelitis | 1 | 0 | 0 | 1 | 0 | ||
| Pseudomonas aeruginosa eradication therapy | 1 | 0 | 0 | 0 | 0 | ||
| Surgical prophylaxis | 1 | 0 | 0 | 1 | 0 | ||
| Febrile neutropenia | 1 | 0 | 0 | 0 | 0 | ||
| Watch | Teicoplanin | Surgical prophylaxis | 3 | 2 | 0 | 0 | 0 |
| Sepsis | 1 | 0 | 0 | 1 | 0 | ||
| Not documented | 1 | 0 | 0 | 0 | 0 | ||
| Watch | Tobramycin | Bronchiectasis | 2 | 2 | 0 | 1 | 0 |
| Watch | Vancomycin | Sepsis | 15 | 13 | 3 | 1 | 0 |
| Intra-abdominal infection | 3 | 2 | 0 | 2 | 0 | ||
| Enteritis | 3 | 0 | 0 | 0 | 1 | ||
| Skin and soft tissue infection | 2 | 0 | 1 | 1 | 0 | ||
| Central nervous system condition | 1 | 0 | 1 | 0 | 0 | ||
| Osteomyelitis | 1 | 0 | 0 | 1 | 0 | ||
| Pleural empyema | 1 | 0 | 0 | 1 | 0 | ||
| Reserve | Ceftazidime | Bronchiectasis | 2 | 1 | 0 | 1 | 0 |
| Surgical prophylaxis | 1 | 0 | 0 | 0 | 0 | ||
| Reserve | Colistin | Medical prophylaxis | 8 | 0 | 0 | 1 | 1 |
| Pseudomonas aeruginosa eradication therapy | 5 | 2 | 0 | 1 | 2 | ||
| Bronchiectasis | 1 | 0 | 0 | 0 | 0 | ||
| Reserve | Daptomycin | Endocarditis | 1 | 0 | 1 | 0 | 0 |
| Reserve | Meropenem | Sepsis | 14 | 1 | 6 | 4 | 0 |
| Lower respiratory tract infection | 4 | 0 | 1 | 2 | 0 | ||
| Intra-abdominal infection | 3 | 0 | 0 | 2 | 0 | ||
| Central nervous system condition | 3 | 0 | 2 | 0 | 1 | ||
| Skin and soft tissue infection | 3 | 2 | 1 | 0 | 0 | ||
| Pleural empyema | 1 | 0 | 0 | 1 | 0 | ||
| Surgical prophylaxis | 1 | 0 | 1 | 0 | 0 | ||
| Febrile neutropenia | 1 | 0 | 0 | 1 | 0 | ||
| Antivirals | Acyclovir | Central nervous system condition | 10 | 8 | 1 | 0 | 1 |
| Unspecified | 7 | 0 | 2 | 2 | 0 | ||
| Sepsis | 5 | 4 | 0 | 1 | 0 | ||
| Skin and soft tissue infection | 3 | 2 | 0 | 0 | 0 | ||
| Congenital varicella | 1 | 1 | 0 | 0 | 0 | ||
| Medical prophylaxis | 1 | 0 | 0 | 0 | 0 | ||
| Antivirals | Valacyclovir | Skin and soft tissue infection | 2 | 0 | 0 | 0 | 0 |
| Antivirals | Ganciclovir | Sepsis | 1 | 1 | 0 | 0 | 0 |
| Antivirals | Valganciclovir | Medical prophylaxis | 4 | 3 | 1 | 0 | 0 |
| Cytomegalovirus infection | 1 | 1 | 0 | 1 | 0 | ||
| Antivirals | Oseltamivir | Influenza treatment or prophylaxis | 26 | 26 | 0 | 0 | 0 |
| Antivirals | Zidovudine | Post exposure prophylaxis | 5 | 5 | 5 | 0 | 0 |
| Antifungals | Amphotericin B | Fungal infection, not specified otherwise | 2 | 0 | 1 | 1 | 0 |
| Antifungals | Fluconazole | Medical prophylaxis | 54 | 33 | 0 | 4 | 0 |
| Fungal infection, not otherwise specified | 15 | 11 | 0 | 1 | 0 | ||
| Sepsis | 2 | 0 | 0 | 1 | 0 | ||
| Intra-abdominal infection | 2 | 0 | 0 | 1 | 0 | ||
| Pleural empyema | 1 | 0 | 0 | 1 | 0 | ||
| Oral thrush | 1 | 0 | 0 | 0 | 0 | ||
| Not documented | 1 | 0 | 0 | 0 | 0 | ||
| Antifungals | Clotrimazole | Fungal infection, not further specified | 2 | 2 | 0 | 0 | 0 |
| Skin and soft tissue infection | 1 | 0 | 0 | 0 | 0 | ||
| Antifungals | Itraconazole | Medical prophylaxis | 1 | 0 | 0 | 1 | 0 |
| Antifungals | Nystatin | Oral thrush | 13 | 0 | 0 | 0 | 0 |
| Medical prophylaxis | 3 | 2 | 0 | 0 | 0 | ||
| Surgical prophylaxis | 2 | 1 | 0 | 0 | 0 | ||
| Not documented | 2 | 1 | 0 | 0 | 0 | ||
| Other antibiotics | Ethambutol | Tuberculosis | 1 | 1 | 1 | 0 | 0 |
| Other antibiotics | Isoniazid | Tuberculosis | 1 | 1 | 1 | 0 | 0 |
| Other antibiotics | Pyrazinamide | Tuberculosis | 2 | 2 | 2 | 0 | 0 |
| Other antibiotics | Pyrimethamine | Toxoplasmosis | 2 | 0 | 2 | 2 | 0 |
| Other antibiotics | Sulfadiazine | Toxoplasmosis | 2 | 0 | 2 | 2 | 0 |
| Other antibiotics | Rifampicin | Tuberculosis | 2 | 2 | 2 | 0 | 0 |
| Pruritus | 2 | 0 | 0 | 0 | 0 | ||
| Staphylococcus aureus sepsis | 1 | 0 | 0 | 1 | 0 | ||
| Other antibiotics | Rifaximin | Small intestinal bowel overgrowth | 1 | 1 | 0 | 0 | 0 |
References
- Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: Developing hospital-quality indicators of antibiotic prescribing for children. J. Antimicrob. Chemother. 2016, 71, 1106–1117. [Google Scholar] [CrossRef]
- Gerber, J.S.; Newland, J.G.; Coffin, S.E.; Hall, M.; Thurm, C.; Prasad, P.A.; Feudtner, C.; Zaoutis, T.E. Variability in Antibiotic Use at Children’s Hospitals. Pediatrics 2010, 126, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Romandini, A.; Pani, A.; Schenardi, P.A.; Pattarino, G.A.C.; De Giacomo, C.; Scaglione, F. Antibiotic resistance in pediatric infections: Global emerging threats, predicting the near future. Antibiotics 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Gray, J.; Bashir, S.; Ahmed, R.; Theodosiou, E. Critical points in the pathway of antibiotic prescribing in a children’s hospital: The Antibiotic Mapping of Prescribing (ABMAP) study. J. Hosp. Infect. 2019, 101, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Gerber, J.S.; Hersh, A.L. Inpatient antimicrobial stewardship in pediatrics: A systematic review. J. Pediatric Infect. Dis. Soc. 2015, 4, e127–e135. [Google Scholar] [CrossRef]
- Donà, D.; Barbieri, E.; Daverio, M.; Lundin, R.; Giaquinto, C.; Zaoutis, T.; Sharland, M. Erratum: Correction to: Implementation and impact of pediatric antimicrobial stewardship programs: A systematic scoping review (Antimicrobial resistance and infection control (2020) 9 (3)). Antimicrob. Resist. Infect. Control 2020, 9, 59. [Google Scholar] [CrossRef]
- WHO. Th The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book. 2022. Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 8 November 2023).
- Budd, E.; Cramp, E.; Sharland, M.; Hand, K.; Howard, P.; Wilson, P.; Wilcox, M.; Muller-Pebody, B.; Hopkins, S. Adaptation of the WHO Essential Medicines List for national antibiotic stewardship policy in England: Being AWaRe. J. Antimicrob. Chemother. 2019, 74, 3384–3389. [Google Scholar] [CrossRef]
- Grammatico-Guillon, L.; Abdurrahim, L.; Shea, K.; Astagneau, P.; Pelton, S. Scope of Antibiotic Stewardship Programs in Pediatrics. Clin. Pediatr. 2019, 58, 1291–1301. [Google Scholar] [CrossRef]
- Ebeledike, C.; Ahmad, T. Pediatric Pneumonia. [Updated 2023 Jan 16]. [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536940/ (accessed on 8 November 2023).
- Dalziel, S.R.; Haskell, L.; O’Brien, S.; Borland, M.L.; Plint, A.C.; Babl, F.E.; Oakley, E. Bronchiolitis. Lancet 2022, 400, 392–406. [Google Scholar] [CrossRef]
- Korppi, M. How to reduce the use of antibiotics in infant bronchiolitis? Acta Paediatr. Int. J. Paediatr. 2020, 109, 1086–1087. [Google Scholar] [CrossRef]
- Smith, L.; Leggett, C.; Borg, C. Administration of medicines to children: A practical guide. Aust. Prescr. 2022, 45, 188–192. [Google Scholar] [CrossRef]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020, 46, 10–67. [Google Scholar] [CrossRef]
- Cowart, M.C.; Heath, T.S.; Shipman, A. The Effect of Rapid Initiation Versus Delayed Initiation of Antibiotics in Pediatric Patients With Sepsis. J. Pediatr. Pharmacol. Ther. 2022, 27, 45–50. [Google Scholar] [CrossRef]
- Stocker, M.; van Herk, W.; El Helou, S.; Dutta, S.; Fontana, M.S.; Schuerman, F.A.B.A.; van den Tooren-de Groot, R.K.; Wieringa, J.W.; Janota, J.; van der Meer-Kappelle, L.H.; et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: A multicentre, randomised controlled trial (NeoPIns). Lancet 2017, 390, 871–881. [Google Scholar] [CrossRef]
- Zihlmann-Ji, J.; Braun, C.; Buettcher, M.; Hodel, M.; Lehnick, D.; Stocker, M. Reduction of Duration of Antibiotic Therapy for Suspected Early-Onset Sepsis in Late-Preterm and Term Newborns After Implementation of a Procalcitonin-Guided Algorithm: A Population-Based Study in Central Switzerland. Front. Pediatr. 2021, 9, 702133. [Google Scholar] [CrossRef]
- Nair, V.; Loganathan, P.; Soraisham, A.S. Azithromycin and Other Macrolides for Prevention of Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Neonatology 2014, 106, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Katundu, D.R.; Chussi, D.; van der Gaast-de Jongh, C.E.; Rovers, M.M.; de Jonge, M.I.; Hannink, G.; van Heerbeek, N. Effect of prophylactic amoxicillin on tonsillar bacterial pathogens after (adeno)tonsillectomy in children. Int. J. Infect. Dis. 2023, 133, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.E.; Rickert, S.M.; Jones, J. Duration-related efficacy of postoperative antibiotics following pediatric tonsillectomy: A prospective, randomized, placebo-controlled trial. Arch. Otolaryngol.—Head Neck Surg. 2009, 135, 984–987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nthumba, P.M.; Huang, Y.; Perdikis, G.; Kranzer, K. Surgical Antibiotic Prophylaxis in Children Undergoing Surgery: A Systematic Review and Meta-Analysis. Surg. Infect. 2022, 23, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Ferreras-Antolín, L.; Irwin, A.; Atra, A.; Dermirjian, A.; Drysdale, S.B.; Emonts, M.; McMaster, P.; Paulus, S.; Patel, S.; Kinsey, S.; et al. Neonatal Antifungal Consumption Is Dominated by Prophylactic Use; Outcomes From The Pediatric Antifungal Stewardship. Pediatr. Infect. Dis. J. 2019, 38, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, S.; Niittynen, I.; Airaksinen, M.; Holmström, A.R. Systemic causes of in-hospital intravenous medication errors: A systematic review. J. Patient Saf. 2021, 17, E1660–E1668. [Google Scholar] [CrossRef]
- Tribble, A.C.; Lee, B.R.; Flett, K.B.; Handy, L.K.; Gerber, J.S.; Hersh, A.L.; Kronman, M.P.; Terrill, C.M.; Sharland, M.; Newland, J.G.; et al. Appropriateness of Antibiotic Prescribing in United States Children’s Hospitals: A National Point Prevalence Survey. Clin. Infect. Dis. 2020, 71, E226–E234. [Google Scholar] [CrossRef]
- Wang, C.N.; Tong, J.; Yi, B.; Huttner, B.D.; Cheng, Y.; Li, S.; Wan, C.; Zhu, Q.; Zhou, Q.; Zhao, S.; et al. Antibiotic Use Among Hospitalized Children and Neonates in China: Results From Quarterly Point Prevalence Surveys in 2019. Front. Pharmacol. 2021, 12, 601561. [Google Scholar] [CrossRef]
- Yang, M.C.; Su, Y.T.; Chen, P.H.; Tsai, C.C.; Lin, T.I.; Wu, J.R. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front. Cell. Infect. Microbiol. 2023, 13, 1200617. [Google Scholar] [CrossRef]
- Aronson, P.L.; Kerns, E.; Jennings, B.; Magee, S.; Wang, M.E.; McDaniel, C.E. Trends in Prevalence of Bacterial Infections in Febrile Infants During the COVID-19 Pandemic. Pediatrics 2022, 150, e2022059235. [Google Scholar] [CrossRef]
- Meeker, D.; Linder, J.A.; Fox, C.R.; Friedberg, M.W.; Persell, S.D.; Goldstein, N.J.; Knight, T.K.; Hay, J.W.; Doctor, J.N. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA 2016, 315, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.S.; Prasad, P.A.; Fiks, A.G.; Localio, A.R.; Bell, L.M.; Keren, R.; Zaoutis, T.E. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014, 312, 2569–2570. [Google Scholar] [CrossRef] [PubMed]
- Primhak, S.; Pool, N.; Sam, M.S.Y.; Duffy, E.; Ritchie, S.R.; Webb, R.; Wilson, E.; Voss, L.; Best, E.J. Improved paediatric antimicrobial prescribing with a smartphone application: A before and after interventional study. Arch. Dis. Child. 2023, 108, 899–903. [Google Scholar] [CrossRef] [PubMed]
| Antimicrobial Agent | Category | Target for Improvement |
|---|---|---|
| Co-trimoxazole | Access | 33/37 prescriptions served prophylactic indications (both medical and surgical). Therefore, frequent revisions of indications for long-term prophylaxis could potentially decrease the number of prophylactic prescriptions. |
| Amoxicillin and clavulanate | Watch | 87/217 prescriptions had lower respiratory tract infection labelled as an indication, which is the first line agent for severe community-acquired pneumonia and hospital-acquired pneumonia. Specific criteria to classify cases as severe may increase the prescription of the first-line agent, amoxicillin. Intra-abdominal infections were labelled as an indication for 23/217 prescriptions. The combination of amoxicillin, gentamicin, and metronidazole (all Access agents) results in similar coverage. |
| Azithromycin | Watch | 41/43 prescriptions were labelled with a prophylactic indication. Restricting indications and establishing scheduled revision dates for long-term prophylactic use has the potential to decrease the number of prescriptions. |
| Cefotaxime | Watch | Among the 74 prescriptions, 63 were labelled with sepsis as an indication. However, microbiological findings guided this indication for only seven prescriptions. Reconsideration of the working diagnosis of sepsis is advisable if the blood culture remains negative, potentially allowing for earlier treatment discontinuation. Additionally, for infants older than 28 days who are not on parenteral nutrition or on any calcium-containing infusion fluids, cefotaxime can be replaced by ceftriaxone, offering the advantage of easier administration (once daily versus four times per day). |
| Ciprofloxacin | Watch | The use of fluoroquinolones should be restricted, given the very low barrier to resistance. Therefore, it is not a standard agent in our formulary, but it is considered a case-by-case scenario if it is the only available agent via the oral route. |
| Meropenem | Reserve | Carbapenems serve as last-resort agents; nevertheless, microbiological findings guided only 10 out of 30 meropenem prescriptions in this study. Introducing scheduled review dates following meropenem prescriptions could provide an opportunity to evaluate and consider alternative agents. |
| Fluconazole | Antifungals | 54/76 prescriptions provide prophylactic indications, in particular in the neonatal population. Scheduled revision dates for long-term prophylactic use can reduce the number of prescriptions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meesters, K.; Chappell, F.; Demirjian, A. Trends in Antibiotic Use in a Large Children’s Hospital in London (United Kingdom): 5 Years of Point Prevalence Surveys. Antibiotics 2024, 13, 172. https://doi.org/10.3390/antibiotics13020172
Meesters K, Chappell F, Demirjian A. Trends in Antibiotic Use in a Large Children’s Hospital in London (United Kingdom): 5 Years of Point Prevalence Surveys. Antibiotics. 2024; 13(2):172. https://doi.org/10.3390/antibiotics13020172
Chicago/Turabian StyleMeesters, Kevin, Faye Chappell, and Alicia Demirjian. 2024. "Trends in Antibiotic Use in a Large Children’s Hospital in London (United Kingdom): 5 Years of Point Prevalence Surveys" Antibiotics 13, no. 2: 172. https://doi.org/10.3390/antibiotics13020172
APA StyleMeesters, K., Chappell, F., & Demirjian, A. (2024). Trends in Antibiotic Use in a Large Children’s Hospital in London (United Kingdom): 5 Years of Point Prevalence Surveys. Antibiotics, 13(2), 172. https://doi.org/10.3390/antibiotics13020172






