Is the Use of Monensin Another Trojan Horse for the Spread of Antimicrobial Resistance?
Abstract
1. Introduction
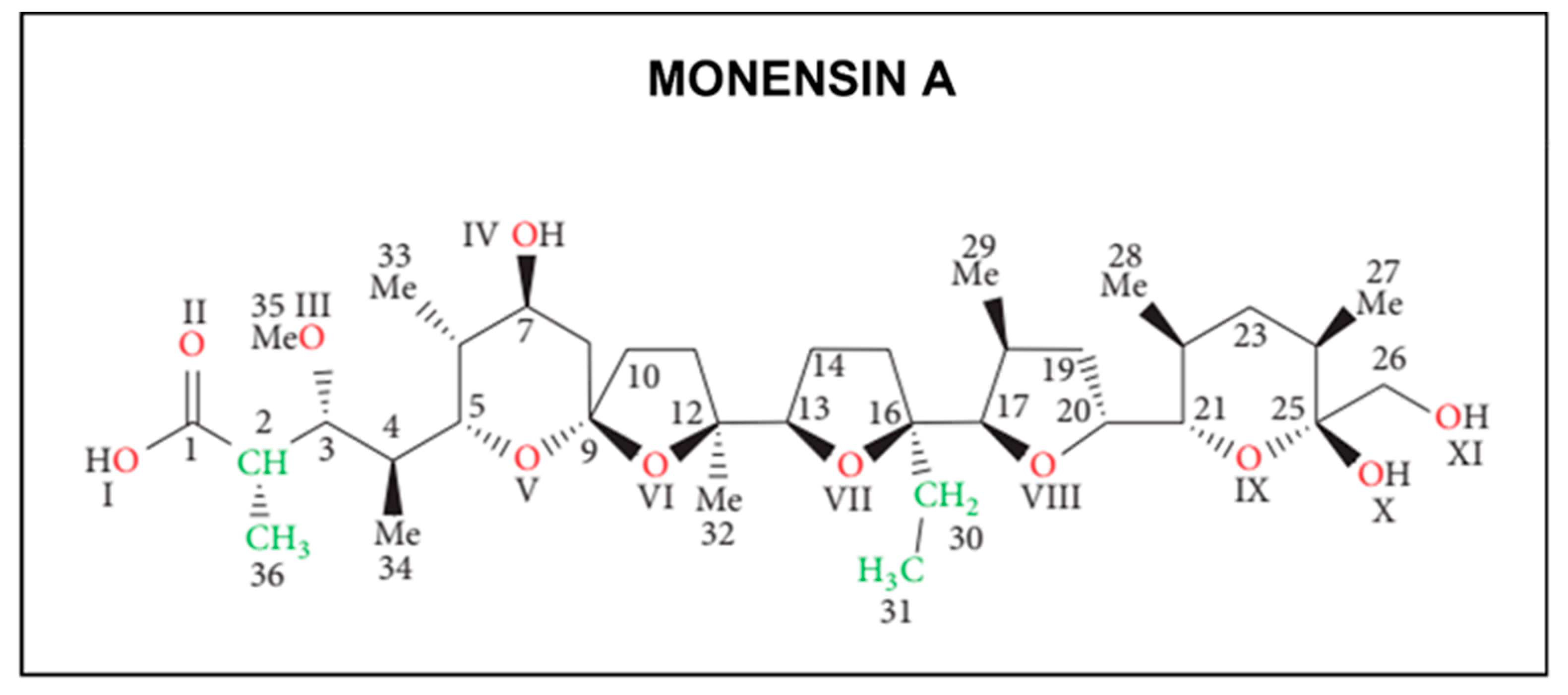
1.1. Chemical Structure and Pharmacological Properties
1.2. Antimicrobial Activity
2. Peer-Reviewed Results
2.1. Antimicrobial Resistance
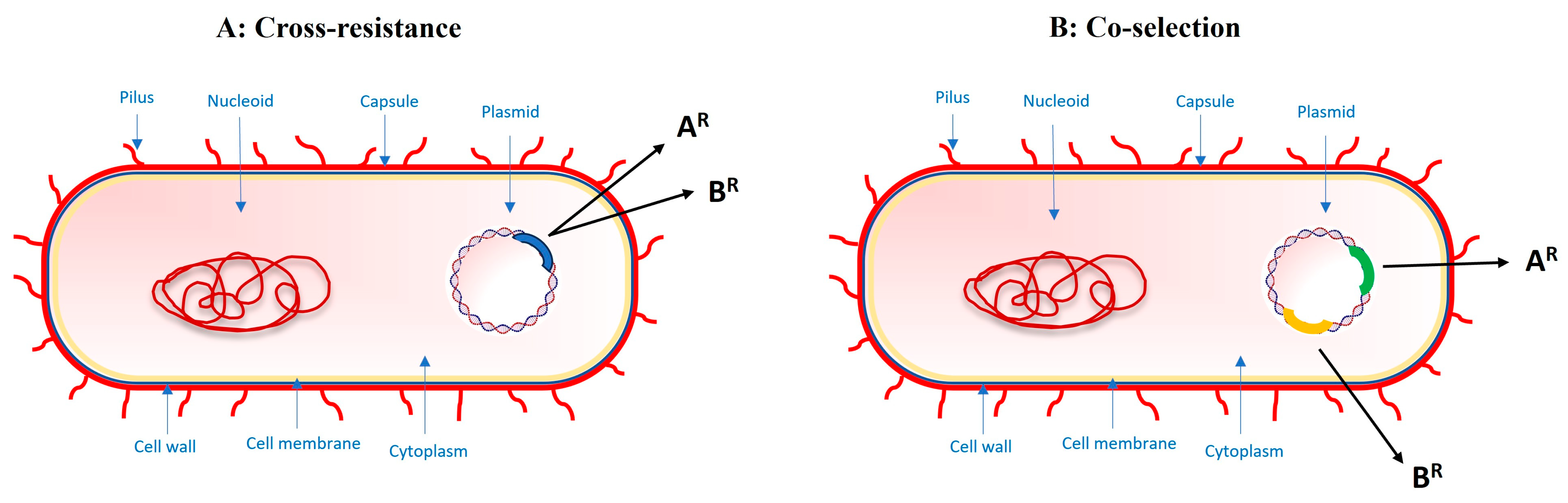
2.2. Cross-Resistance and Co-Selection
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wong, A. Unknown Risk on the Farm: Does Agricultural Use of Ionophores Contribute to the Burden of Antimicrobial Resistance. mSphere 2019, 4, e00433-19. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Commission Directive 97/72/EC of 15 December 1997 amending Council Directive 70/524/EEC concerning additives in animal feeding (Text with EEA relevance). Off. J. L 1997, 351, 0055–0059.
- FDA Department of Health and Human Services. Monensin approval. Fed. Regist. (Rules Regul.) 2004, 69, 68783–68784. [Google Scholar]
- European Council. Council Regulation (EC) No 108/2007 of 5 February 2007 Amending Regulation (EC) No 1356/2004 as Regards the Conditions for Authorisation of the Feed Additive Elancoban, Belonging to the Group of Coccidiostats and Other Medicinal Substances (Text. with EEA Relevance). Off. J. Eur. Union 2007, 4–5. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32007R0108&qid=1706387362985 (accessed on 1 January 2024).
- European Council. Council Regulation (EC) No 495/2011 of 20 May 2011 Amending Regulation (EC) No. 109/2007 as Regards the composition of the food additive monensin sodium. (Text. with EEA Relevance). Off. J. Eur. Union 2011, 9, 2009. [Google Scholar]
- European Medicines Agency Veterinary Medicines and Inspections. Committee for Medicinal Products for Veterinary Use Monensin (Cattle, Including Dairy Cows) Summary Report; EMEA/CVMP/185123/2007-Final; European Medicines Agency Veterinary Medicines and Inspections: London, UK, 2007. [Google Scholar]
- European Medicine Agency. European Public Assessment Reports (EPAR) European Medicine Agency. 2013. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/kexxtone (accessed on 20 December 2023).
- Agtarap, A.; Chamberlin, J.W.; Pinkerton, M.; Steinrauf, L. The structure of monensic acid, a new biologically active compound. J. Am. Chem. Soc. 1967, 89, 5737–5739. [Google Scholar] [CrossRef]
- Schmid, G.; Fukuyama, T.; Akasaka, K.; Kishi, Y. Total synthesis of monensin. 1. Stereocontrolled synthesis of the left half of monensin. J. Am. Chem. Soc. 1979, 101, 259–260. [Google Scholar] [CrossRef]
- Lutz, W.K.; Winkler, F.K.; Dunitz, J.D. Crystal structure of the antibiotic monensin similarities and differences betweeen free acid and metal complex. Helv. Chim. Acta 1971, 54, 1103–1108. [Google Scholar] [CrossRef]
- Collum, D.B.; McDonald, J.H.; Still, W.C. Synthesis of the polyether antibiotic monensin. 3. Coupling of precursors and transformation to monensin. J. Am. Chem. Soc. 1980, 102, 2120–2121. [Google Scholar] [CrossRef]
- Merion, M.; Sly, W.S. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J. Cell Biol. 1983, 96, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, H.H.; Morré, D.J.; Rowe, L.D. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta 1990, 1031, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Iacoangeli, A.; Melucci-Vigo, G.; Risuleo, G. The ionophore monensin inhibits mouse polyomavirus DNA replication and destabilizes viral early mRNAs. Biochimie 2000, 82, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Adovelande, J.; Schrével, J. Carboxylic ionophores in malaria chemotherapy: The effects of monensin and nigericin on Plasmodium falciparum in vitro and Plasmodium vinckei petteri in vivo. Life Sci. 1996, 59, PL309–PL315. [Google Scholar] [CrossRef] [PubMed]
- Shumard, R.F.; Callender, M.E. Monensin, a new biologically active compound. VI. Anticoccidial activity. Antimicrob. Agents Chemother. 1967, 7, 369–377. [Google Scholar] [PubMed]
- Donoho, A.L. Biochemical studies on the fate of monensin in animals and in the environment. J. Anim. Sci. 1984, 58, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Aowicki, D.; Huczyński, A. Structure and antimicrobial properties of monensin A and its derivatives: Summary of the achievements. Biomed. Res. Int. 2013, 2013, 742149. [Google Scholar] [CrossRef]
- Simjee, S.; Heffron, A.L.; Pridmore, A.; Shryock, T.R. Reversible monensin adaptation in Enterococcus faecium, Enterococcus faecalis and Clostridium perfringens of cattle origin: Potential impact on human food safety. J. Antimicrob. Chemother. 2012, 67, 2388–2395. [Google Scholar] [CrossRef]
- Duffield, T.F.; Bagg, R.N. Use of ionophores in lactating dairy cattle: A review. Can. Vet. J. 2000, 41, 388–394. [Google Scholar]
- Schären, M.; Drong, C.; Kiri, K.; Riede, S.; Gardener, M.; Meyer, U.; Hummel, J.; Urich, T.; Breves, G.; Dänicke, S. Differential effects of monensin and a blend of essential oils on rumen microbiota composition of transition dairy cows. J. Dairy Sci. 2017, 100, 2765–2783. [Google Scholar] [CrossRef]
- Russell, J.B.; Strobel, H.J. Effect of ionophores on ruminal fermentation. Appl. Environ. Microbiol. 1989, 55, 1–6. [Google Scholar] [CrossRef]
- Huczyński, A.; Stefańska, J.; Przybylski, P.; Brzezinski, B.; Bartl, F. Synthesis and antimicrobial properties of monensin A esters. Bioorg. Med. Chem. Lett. 2008, 18, 2585–2589. [Google Scholar] [CrossRef]
- Goodrich, R.D.; Garrett, J.E.; Gast, D.R.; Kirick, M.A.; Larson, D.A.; Meiske, J.C. Influence of monensin on the performance of cattle. J. Anim. Sci. 1984, 58, 1484–1498. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Yasui, T.; Ryan, C.M.; Mechor, G.D.; Overton, T.R. Performance of early-lactation dairy cows as affected by dietary starch and monensin supplementation. J. Dairy Sci. 2015, 98, 3335–3350. [Google Scholar] [CrossRef]
- Decloedt, A.; Verheyen, T.; De Clercq, D.; Sys, S.; Vercauteren, G.; Ducatelle, R.; Delahaut, P.; van Loon, G. Acute and long-term cardiomyopathy and delayed neurotoxicity after accidental lasalocid poisoning in horses. J. Vet. Intern. Med. 2012, 26, 1005–1011. [Google Scholar] [CrossRef]
- Ekinci, İ.B.; Chłodowska, A.; Olejnik, M. Ionophore Toxicity in Animals: A Review of Clinical and Molecular Aspects. Int. J. Mol. Sci. 2023, 24, 1696. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J.; DuTremblay, D.; DesCoteaux, L.; Baril, J.; Bagg, R.; Vessie, G.H. Case Report—Effects of an Unintended High Dose of Monensin on Milk Production and Milk Fat in a Dairy Herd. Bov. Pract. 2007, 41, 72–76. [Google Scholar] [CrossRef]
- Basaraba, R.J.; Oehme, F.W.; Vorhies, M.W.; Stokka, G.L. Toxicosis in cattle from concurrent feeding of monensin and dried distiller’s grains contaminated with macrolide antibiotics. J. Vet. Diagn. Investig. 1999, 11, 79–86. [Google Scholar] [CrossRef]
- Jones, A. Monensin toxicosis in 2 sheep flocks. Can. Vet. J. 2001, 42, 135–136. [Google Scholar]
- Ordidge, R.M.; Schubert, F.K.; Stoker, J.W. Death of horses after accidental feeding of monensin. Vet. Rec. 1979, 104, 375. [Google Scholar] [CrossRef]
- Van Vleet, J.F.; Ferrans, V.J. Ultrastructural alterations in the atrial myocardium of pigs with acute monensin toxicosis. Am. J. Pathol. 1984, 114, 367–379. [Google Scholar]
- Rozza, D.B.; Vervuert, I.; Kamphues, J.; da Cruz, C.E.; Driemeier, D. Monensin toxicosis in water buffaloes (Bubalus bubalis). J. Vet. Diagn. Investig. 2006, 18, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Mammi, L.M.E.; Guadagnini, M.; Mechor, G.; Cainzos, J.M.; Fusaro, I.; Palmonari, A.; Formigoni, A. The Use of Monensin for Ketosis Prevention in Dairy Cows during the Transition Period: A Systematic Review. Animals 2021, 11, 1988. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Evaluation of nonesterified fatty acids and beta-hydroxybutyrate in transition dairy cattle in the northeastern United States: Critical thresholds for prediction of clinical diseases. J. Dairy Sci. 2010, 93, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.Z.M.; De Oca Jiménez, R.M.; Cerrillo-Soto, M.A.; Kholif, A.E.; Salem, M.Z.M.; Elghandour, M.M.M.Y. Detection of sensitive and mutant ruminal bacteria isolates from sheep, cattle, and buffalo using 14 therapeutic antibiotics. Turk. J. Vet. Anim. Sci. 2014, 38, 514–519. [Google Scholar] [CrossRef]
- Simm, R.; Slettemeås, J.S.; Norström, M.; Dean, K.R.; Kaldhusdal, M.; Urdahl, A.M. Significant reduction of vancomycin resistant E. faecium in the Norwegian broiler population coincided with measures taken by the broiler industry to reduce antimicrobial resistant bacteria. PLoS ONE. 2019, 14, e0226101. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: New York, NY, USA, 1994. [Google Scholar]
- McCuddin, Z.P.; Carlson, S.A.; Rasmussen, M.A.; Franklin, S.K. Klebsiella to Salmonella gene transfer within rumen protozoa: Implications for antibiotic resistance and rumen defaunation. Vet. Microbiol. 2006, 114, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, N.; Alam, S.I.; Kumar, R.B.; Singh, L. Diversity and antibiotic susceptibility pattern of cultivable anaerobic bacteria from soil and sewage samples of India. Infect. Genet. Evol. 2011, 11, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Fulghum, R.S.; Baldwin, B.B.; Williams, P.P. Antibiotic susceptibility of anaerobic ruminal bacteria. Appl. Microbiol. 1968, 16, 301–307. [Google Scholar] [CrossRef]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug Efflux Pumps in Staphylococcus aureus: An Update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef]
- Russell, J.B.; Houlihan, A.J. Ionophore resistance of ruminal bacteria and its potential impact on human health. FEMS Microbiol. Rev. 2003, 27, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Russell, J.B. Selection of a highly monensin-resistant Prevotella bryantii subpopulation with altered outer membrane characteristics. Appl. Environ. Microbiol. 1999, 65, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, J.L.; Russell, J.B. The adaptation and resistance of Clostridium aminophilum F to the butyrivibriocin-like substance of Butyrivibrio fibrisolvens JL5 and monensin. FEMS Microbiol. Lett. 2002, 209, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Adams, K.A.; Russell, J.B. The ability of “low G þ C gram-positive” ruminal bacteria to resist monensin and counteract potassium depletion. Curr. Microbiol. 1999, 39, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; Wallace, R.J.; Watt, N.D. Properties of ionophore-resistant Bacteroides ruminicola enriched by cultivation in the presence of tetronasin. J. Appl. Microbiol. 1992, 72, 65–70. [Google Scholar] [CrossRef]
- Granados-Chinchilla, F.; Arias-Andrés, M.J.; Fernández Montes de Oca, M.L.; Rodríguez, C. Effect of the veterinary ionophore monensin on the structure and activity of a tropical soil bacterial community. J. Environ. Sci. Health B 2020, 55, 127–134. [Google Scholar] [CrossRef]
- Newbold, C.J.; Wallace, R.J.; Walker, N.D. The effect of tetronasin and monensin on fermentation, microbial numbers and the development of ionophore-resistant bacteria in the rumen. J. Appl. Bacteriol. 1993, 75, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Myrenås, M.; Ågren, J. Transferable genes putatively conferring elevated minimum inhibitory concentrations of narasin in Enterococcus faecium from Swedish broilers. Vet. Microbiol. 2016, 184, 80–83. [Google Scholar] [CrossRef]
- Jacob, M.E.; Fox, J.T.; Narayanan, S.K.; Drouillard, J.S.; Renter, D.G.; Nagaraja, T.G. Effects of feeding wet corn distillers grains with solubles with or without monensin and tylosin on the prevalence and antimicrobial susceptibilities of fecal foodborne pathogenic and commensal bacteria in feedlot cattle. J. Anim. Sci. 2008, 86, 1182–1190. [Google Scholar] [CrossRef]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B.; Ladely, S.R. Effects of tylosin use on erythromycin resistance in enterococci isolated from swine. Appl. Environ. Microbiol. 2004, 70, 4205–4210. [Google Scholar] [CrossRef]
- Jensen, L.B.; Frimodt-Møller, N.; Aarestrup, F.M. Presence of erm gene classes in gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol. Lett. 1999, 170, 151–158. [Google Scholar] [CrossRef]
- Roberts, M.C.; Sutcliffe, J.; Courvalin, P.; Jensen, L.B.; Rood, J.; Seppala, H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 1999, 43, 2823–2830. [Google Scholar] [CrossRef]
- Warsi, O.M.; Upterworth, L.M.; Breidenstein, A.; Lustig, U.; Mikkelsen, K.; Nagy, T.; Szatmari, D.; Ingmer, H.; Andersson, D.I. Staphylococcus aureus mutants resistant to the feed-additive monensin show increased virulence and altered purine metabolism. mBio 2024, e03155-23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Stephan, R.; Power, K.; Yan, Q.; Hächler, H.; Fanning, S. Nucleotide sequences of 16 transmissible plasmids identified in nine multidrug-resistant Escherichia coli isolates expressing an ESBL phenotype isolated from food-producing animals and healthy humans. J. Antimicrob. Chemother. 2014, 69, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.M.S.; Silva, N.M.V.; Ferreira, V.A.; Moreira Filho, A.L.B.; Givisiez, P.E.N.; Freitas Neto, O.C.; Berchieri Júnior, A.; Gebreyes, W.A.; de Oliveira, C.J.B. Residual concentrations of antimicrobial growth promoters in poultry litter favour plasmid conjugation among Escherichia coli. Lett. Appl. Microbiol. 2022, 74, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Zatyka, M.; Thomas, C.M. Control of genes for conjugative transfer of plasmids and other mobile elements. FEMS Microbiol. Rev. 1998, 21, 291–319. [Google Scholar] [CrossRef] [PubMed]
- Beaber, J.W.; Hochhut, B.; Waldor, M.K. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 2004, 427, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Sarker, Y.A.; Rashid, S.Z.; Sachi, S.; Ferdous, J.; Das Chowdhury, B.L.; Tarannum, S.S.; Sikder, M.H. Exposure pathways and ecological risk assessment of common veterinary antibiotics in the environment through poultry litter in Bangladesh. J. Environ. Sci. Health B 2020, 55, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Castro, M.J.L.; Fernández Cirelli, A. Degradation of monensin on soils: Influence of organic matter and water content. Chem. Ecol. 2010, 26, 27–33. [Google Scholar] [CrossRef]
- Song, W.; Ding, Y.; Chiou, C.T.; Li, H. Selected veterinary pharmaceuticals in agricultural water and soil from land application of animal manure. J. Environ. Qual. 2010, 39, 1211–1217. [Google Scholar] [CrossRef]
- Bak, S.A.; Hansen, M.; Krogh, C.A.; Brandt, A.; Halling-Sørensen, B.; Björklund, E. Development and validation of an SPE methodology combined with LC-MS/MS for the determination of four ionophores in aqueous environmental matrices. Intern. J. Environ. Anal. Chem. 2013, 93, 1500–1512. [Google Scholar] [CrossRef]
- ElSayed, E.M.; Prasher, S.O. Fate and transport of monensin in the presence of nonionic surfactant Brij35 in soil. Sci. Total Environ. 2014, 490, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Arikan, O.A.; Mulbry, W.; Rice, C.; Lansing, S. The fate and effect of monensin during anaerobic digestion of dairy manure under mesophilic conditions. PLoS ONE 2018, 13, e0192080. [Google Scholar] [CrossRef] [PubMed]
- Žižek, S.; Gombač, M.; Švara, T.; Pogačnik, M. Monensin—A Review of Factors Influencing its Presence in the Environment and Recommendations for Safe Storage and Use of Monensin-Contaminated Manure. Slov. Vet. Res. 2014, 51, 53–63. [Google Scholar]
- Sun, P.; Cabrera, M.L.; Huang, C.H.; Pavlostathis, S.G. Biodegradation of veterinary ionophore antibiotics in broiler litter and soil microcosms. Environ. Sci. Technol. 2014, 48, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, C.H.; Pavlostathis, S.G. Inhibition and biotransformation potential of veterinary ionophore antibiotics under different redox conditions. Environ. Sci. Technol. 2014, 48, 13146–13154. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, Y.; Wang, Y.; Qiu, J.; Li, Y. Soil Behaviour of the Veterinary Drugs Lincomycin, Monensin, and Roxarsone and Their Toxicity on Environmental Organisms. Molecules 2019, 24, 4465. [Google Scholar] [CrossRef]
- Sassman, S.A.; Lee, L.S. Sorption and degradation in soils of veterinary ionophore antibiotics: Monensin and lasalocid. Environ. Toxicol. Chem. 2007, 26, 1614–1621. [Google Scholar] [CrossRef]
- Aidara-Kane, A.; Angulo, F.J.; Conly, J.M.; Minato, Y.; Silbergeld, E.K.; McEwen, S.A.; Collignon, P.J.; WHO Guideline Development Group. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control 2018, 7, 7. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC (Text with EEA relevance). Off. J. Eur. Union 2019, 43–167. Available online: https://eur-lex.europa.eu/eli/reg/2019/6/oj (accessed on 20 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carresi, C.; Marabelli, R.; Roncada, P.; Britti, D. Is the Use of Monensin Another Trojan Horse for the Spread of Antimicrobial Resistance? Antibiotics 2024, 13, 129. https://doi.org/10.3390/antibiotics13020129
Carresi C, Marabelli R, Roncada P, Britti D. Is the Use of Monensin Another Trojan Horse for the Spread of Antimicrobial Resistance? Antibiotics. 2024; 13(2):129. https://doi.org/10.3390/antibiotics13020129
Chicago/Turabian StyleCarresi, Cristina, Romano Marabelli, Paola Roncada, and Domenico Britti. 2024. "Is the Use of Monensin Another Trojan Horse for the Spread of Antimicrobial Resistance?" Antibiotics 13, no. 2: 129. https://doi.org/10.3390/antibiotics13020129
APA StyleCarresi, C., Marabelli, R., Roncada, P., & Britti, D. (2024). Is the Use of Monensin Another Trojan Horse for the Spread of Antimicrobial Resistance? Antibiotics, 13(2), 129. https://doi.org/10.3390/antibiotics13020129






