Prevalence of Vancomycin-Variable Enterococci from the Bloodstream in the Korea Global Antibiotic Resistance Surveillance System, 2017–2022
Abstract
1. Introduction
2. Results and Discussion
2.1. Prevalence of VVE in E. faecium and E. faecalis
2.2. Antimicrobial Resistance of VVE
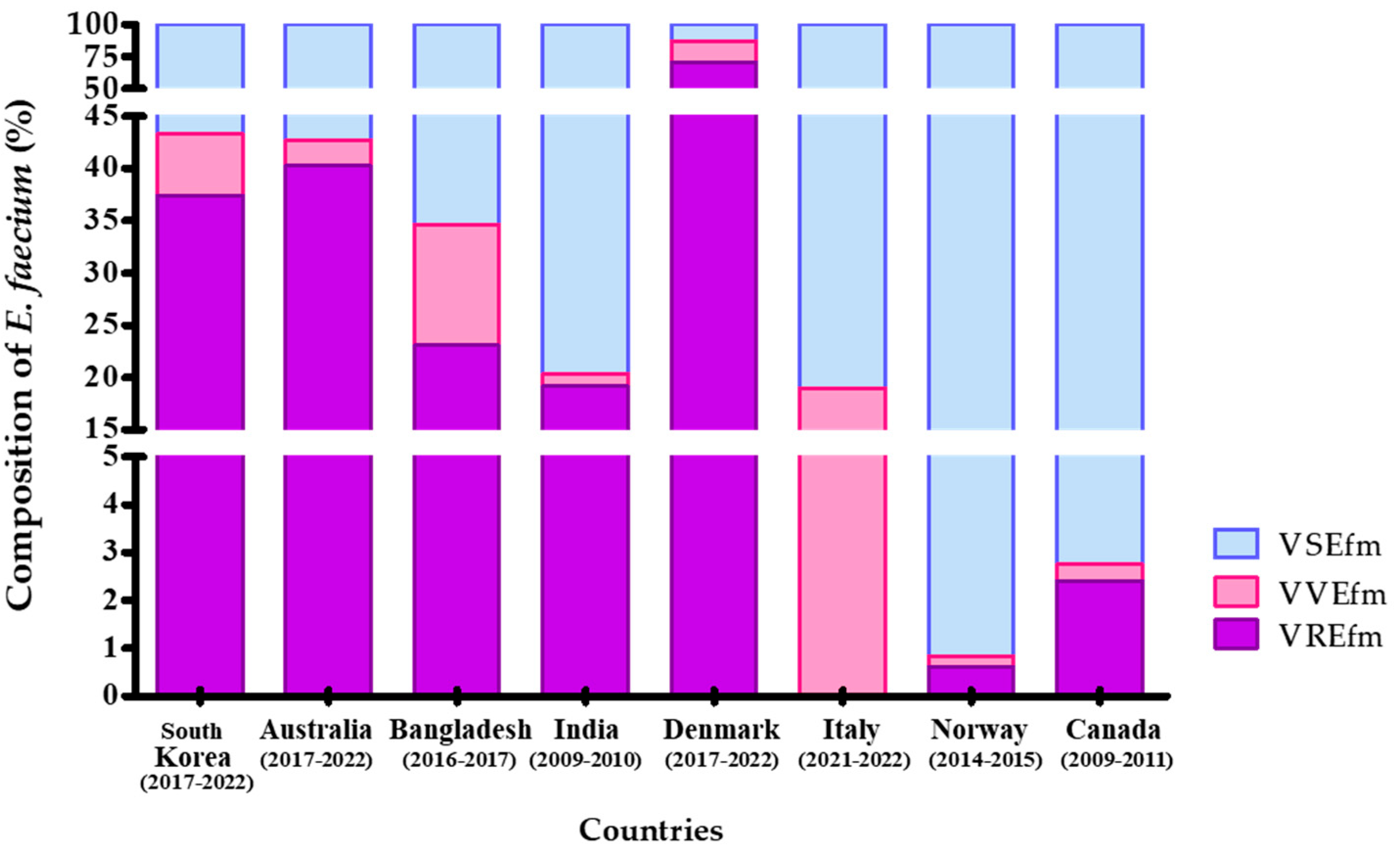
2.3. Comparative Analysis of VVEfm Survey Results Between Korea and Other Countries
2.4. Multilocus Sequence Type of VVE in E. faecium and E. faecalis
3. Materials and Methods
3.1. Bacterial Collection and Antimicrobial Susceptibility Test
3.2. vanA/B Gene PCR and Multilocus Sequence Typing (MLST)
3.3. VVE Reversion Rate
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-resistant Enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Ahn, S.H.; Lee, W.G.; Lee, E.H. Molecular epidemiology of vancomycin-resistant Enterococci isolated from non-tertiarycare and tertiarycare hospitals in Korea. Epidemiol. Infect. 2014, 142, 2372–2377. [Google Scholar] [CrossRef]
- World Health Organization. National Antimicrobial Resistance Surveillance in Korea 2018 Annual Report; Kor-GLASS & KARMS; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. National Antimicrobial Resistance Surveillance in Korea 2022 Annual Report; Kor-GLASS & KARMS; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Szakacs, T.A.; Kalan, L.; McConnell, M.J.; Eshaghi, A.; Shahinas, D.; McGeer, A.; Wright, G.D.; Low, D.E.; Patel, S.N. Outbreak of vancomycin-susceptible Enterococcus faecium containing the wild-type vanA gene. J. Clin. Microbiol. 2014, 52, 1682–1686. [Google Scholar] [CrossRef]
- Sivertsen, A.; Pedersen, T.; Larssen, K.W.; Bergh, K.; Rønning, T.G.; Radtke, A.; Hegstad, K. A silenced vanA gene cluster on a transferable plasmid caused an outbreak of vancomycin-variable Enterococci. Antimicrob. Agents Chemother. 2016, 60, 4119–4127. [Google Scholar] [CrossRef] [PubMed]
- Coccitto, S.N.; Cinthi, M.; Simoni, S.; Pocognoli, A.; Zeni, G.; Mazzariol, A.; Morroni, G.; Mingoia, M.; Giovanetti, E.; Brenciani, A.; et al. Genetic analysis of vancomycin-variable Enterococcus faecium clinical isolates in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 673–682. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Baig, S.; Kamel, Y.; Roer, L.; Pinholt, M.; Gumpert, H.; Røder, B.H.B.; Justesen, U.S.; Samulioniené, J.; Kjærsgaard, M.; et al. The emergence of vanA Enterococcus faecium in Denmark, 2005–2015. J. Antimicrob. Chemother. 2017, 72, 2184–2190. [Google Scholar] [CrossRef]
- Viswanath, L.S.; Sugumar, M.; Peela, S.C.M.; Walia, K.; Sistla, S. Detection of vancomycin variable Enterococci (VVE) among clinical isolates of Enterococcus faecium collected across India-first report from the subcontinent. Indian J. Med. Microbiol. 2022, 40, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.A.; Sagor, M.S.; Hossain, M.S.; Karim, M.R.; Mahmud, M.A.; Sarker, M.S.; Shownaw, F.A.; Mia, Z.; Card, R.M.; Agunos, A.; et al. High prevalence of vancomycin non-susceptible and multi-drug resistant Enterococci in farmed animals and fresh retail meats in Bangladesh. Vet. Res. Commun. 2022, 46, 811–822. [Google Scholar] [CrossRef]
- Wagner, T.M.; Janice, J.; Schulz, M.; Ballard, S.A.; da Silva, A.G.; Coombs, G.W.; Daley, D.A.; Pang, S.; Mowlaboccus, S.; Stinear, T.; et al. Reversible vancomycin susceptibility within emerging ST1421 Enterococcus faecium strains is associated with rearranged vanA-gene clusters and increased vanA plasmid copy number. Int. J. Antimicrob. Agents 2023, 62, 106849. [Google Scholar] [CrossRef]
- Kim, D.; Yoon, E.J.; Hong, J.S.; Choi, M.H.; Kim, H.S.; Kim, Y.R.; Kim, Y.A.; Uh, Y.; Shin, K.S.; Shin, J.H.; et al. Major bloodstream infection-causing bacterial pathogens and their antimicrobial resistance in South Korea, 2017–2019: Phase I report from Kor-GLASS. Front. Microbiol. 2021, 12, 799084. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, Y.S.; Lee, S.Y.; Yoo, J.S.; Yoo, J.I.; Kim, H.S.; Kim, O.; Yu, J.Y. Structure and transfer of the vanA cluster in vanA-positive, vancomycin-susceptible Enterococcus faecium, and its revertant mutant. Diagn. Microbiol. Infect. Dis. 2014, 80, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Chung, D.R.; Cho, S.Y.; Huh, K.; Kang, C.I.; Peck, K.R. Emergence of vancomycin-resistant Enterococcus faecium ST1421 lacking the pstS gene in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.J.; Song, W.; Kim, H.S.; Kim, H.S.; Kim, J.S. Impact of COVID-19 on antimicrobial consumption and spread of multidrug-resistance in bacterial infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef]
- The Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP). DANMAP 2022—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Copenhagen: DANMAP. 2022. Available online: https://www.danmap.org/-/media/institutter/foedevareinstituttet/danmap-site/report-2022/danmap_2022_low_version-3.pdf (accessed on 1 November 2023).
- Yoo, I.Y.; Kwon, J.A.; Lee, M.; Jung, S.H.; Kim, J.O.; Ha, S.I.; Park, Y.J. Prevalence and Molecular Characterization of Vancomycin Variable Enterococcus faecium Isolated From Clinical Specimens. Ann. Lab. Med. 2024, 44, 450. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Kurushima, J.; Nomura, T.; Tanimoto, K.; Tamai, K.; Yanagisawa, H.; Shirabe, K.; Ike, Y.; Tomita, H. Dissemination and genetic analysis of the stealthy vanB gene clusters of Enterococcus faecium clinical isolates in Japan. BMC Microbiol. 2018, 18, 213. [Google Scholar] [CrossRef]
- Kim, D.; Kang, D.Y.; Choi, M.H.; Hong, J.S.; Kim, H.S.; Kim, Y.R.; Kim, Y.A.; Uh, Y.; Shin, K.S.; Shin, J.H.; et al. Fitness costs of Tn1546-type transposons harboring the vanA operon by plasmid type and structural diversity in Enterococcus faecium. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 62. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; M100; CLSI: Wayne, PA, USA, 2023. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Clinical Breakpoints—Bacteria (v 6.0). AST Clinical Breakpoint. Available online: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/ (accessed on 1 January 2023).
- Mac, K.; Wichmann-Schauer, H.; Peters, J.; Ellerbroek, L. Species identification and detection of vancomycin resistance genes in enterococci of animal origin by multiplex PCR. Int. J. Food Microbiol. 2003, 88, 305–309. [Google Scholar] [CrossRef]
- Lee, W.G.; Lee, S.M.; Kim, Y.S. Molecular characterisation of Enterococcus faecium isolated from hospitalised patients in Korea. Lett. Appl. Microbiol. 2006, 43, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garbajosa, P.; Bonten, M.J.M.; Robinson, D.A.; Top, J.; Nallapareddy, S.R.; Torres, C.; Coque, T.M.; Cantón, R.; Baquero, F.; Murray, B.E.; et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J. Clin. Microbiol. 2006, 44, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C. Open-access bacterial population genomics: BIGSdb software, the PubMLST. org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]


| Enterococcus faecium (n = 2115) | Years | Antibiotic Resistant Rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | CIP | HL-GEN | HL-STR | TET | TEI | LIN | TIG | DAP | QDA | ||
| VSEfm (n = 1199) | 2017 (n = 186) | 83.9 | 84.4 | 18.3 | 1.6 | 17.7 | 0 | 0 | 0.5 | - | 3.2 |
| 2018 (n = 167) | 85.6 | 84.4 | 16.2 | 0 | 16.8 | 0 | 0 | 0 | - | 6.0 | |
| 2019 (n = 182) | 83.5 | 84.1 | 12.1 | 1.1 | 15.4 | 0 | 0 | 0.5 | - | 9.3 | |
| 2020 (n = 208) | 78.8 | 80.8 | 17.3 | 2.9 | 10.1 | 0 | 0 | 0 | 0 | 3.8 | |
| 2021 (n = 175) | 82.9 | 87.4 | 24.0 | 4.6 | 15.4 | 0 | 0 | 1.1 | 3.4 | 25.7 | |
| 2022 (n = 281) | 82.9 | 88.3 | 17.8 | 6.4 | 12.5 | 0 | 0 | 0 | 0.4 | 21.7 | |
| VVEfm (n = 124) | 2017 (n = 4) | 100 | 100 | 0 | 0 | 25.0 | 0 | 0 | 0 | - | 0 |
| 2018 (n = 1) | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | |
| 2019 (n = 0) | - | - | - | - | - | - | - | - | - | - | |
| 2020 (n = 10) | 90.0 | 90.0 | 70.0 | 10.0 | 0 | 0 | 0 | 0 | 0 | 10.0 | |
| 2021 (n = 78) | 76.9 | 84.6 | 15.4 | 5.1 | 9.0 | 0 | 0 | 0 | 5.1 | 25.6 | |
| 2022 (n = 31) | 90.3 | 93.5 | 29.0 | 6.5 | 12.9 | 0 | 0 | 0 | 0 | 12.9 | |
| VREfm (n = 792) | 2017 (n = 98) | 100 | 100 | 18.4 | 0 | 9.2 | 55.1 | 0 | 0 | - | 3.1 |
| 2018 (n = 109) | 99.1 | 99.1 | 29.4 | 0 | 14.7 | 68.8 | 0 | 0 | - | 6.4 | |
| 2019 (n = 126) | 100 | 100 | 40.5 | 0 | 7.1 | 84.9 | 0 | 0 | - | 7.1 | |
| 2020 (n = 137) | 97.1 | 97.8 | 40.9 | 1.5 | 7.3 | 88.1 | 0 | 0 | 0 | 5.1 | |
| 2021 (n = 153) | 100 | 100 | 25.5 | 3.9 | 9.2 | 37.9 | 0 | 0 | 2.0 | 52.9 | |
| 2022 (n = 169) | 99.4 | 100 | 42.0 | 1.8 | 16.0 | 68.6 | 0 | 0.6 | 0.6 | 24.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Nam, J.-H.; Kim, J.W.; Kim, S.H.; Yoo, J.S. Prevalence of Vancomycin-Variable Enterococci from the Bloodstream in the Korea Global Antibiotic Resistance Surveillance System, 2017–2022. Antibiotics 2024, 13, 1210. https://doi.org/10.3390/antibiotics13121210
Lee SY, Nam J-H, Kim JW, Kim SH, Yoo JS. Prevalence of Vancomycin-Variable Enterococci from the Bloodstream in the Korea Global Antibiotic Resistance Surveillance System, 2017–2022. Antibiotics. 2024; 13(12):1210. https://doi.org/10.3390/antibiotics13121210
Chicago/Turabian StyleLee, Sung Young, Ji-Hyun Nam, Jung Wook Kim, Soo Hyun Kim, and Jung Sik Yoo. 2024. "Prevalence of Vancomycin-Variable Enterococci from the Bloodstream in the Korea Global Antibiotic Resistance Surveillance System, 2017–2022" Antibiotics 13, no. 12: 1210. https://doi.org/10.3390/antibiotics13121210
APA StyleLee, S. Y., Nam, J.-H., Kim, J. W., Kim, S. H., & Yoo, J. S. (2024). Prevalence of Vancomycin-Variable Enterococci from the Bloodstream in the Korea Global Antibiotic Resistance Surveillance System, 2017–2022. Antibiotics, 13(12), 1210. https://doi.org/10.3390/antibiotics13121210






