The Influence of COVID-19 on Antimicrobial Resistance Trends at a Secondary Care Hospital in Slovenia: An Interrupted Time Series Analysis
Abstract
1. Introduction
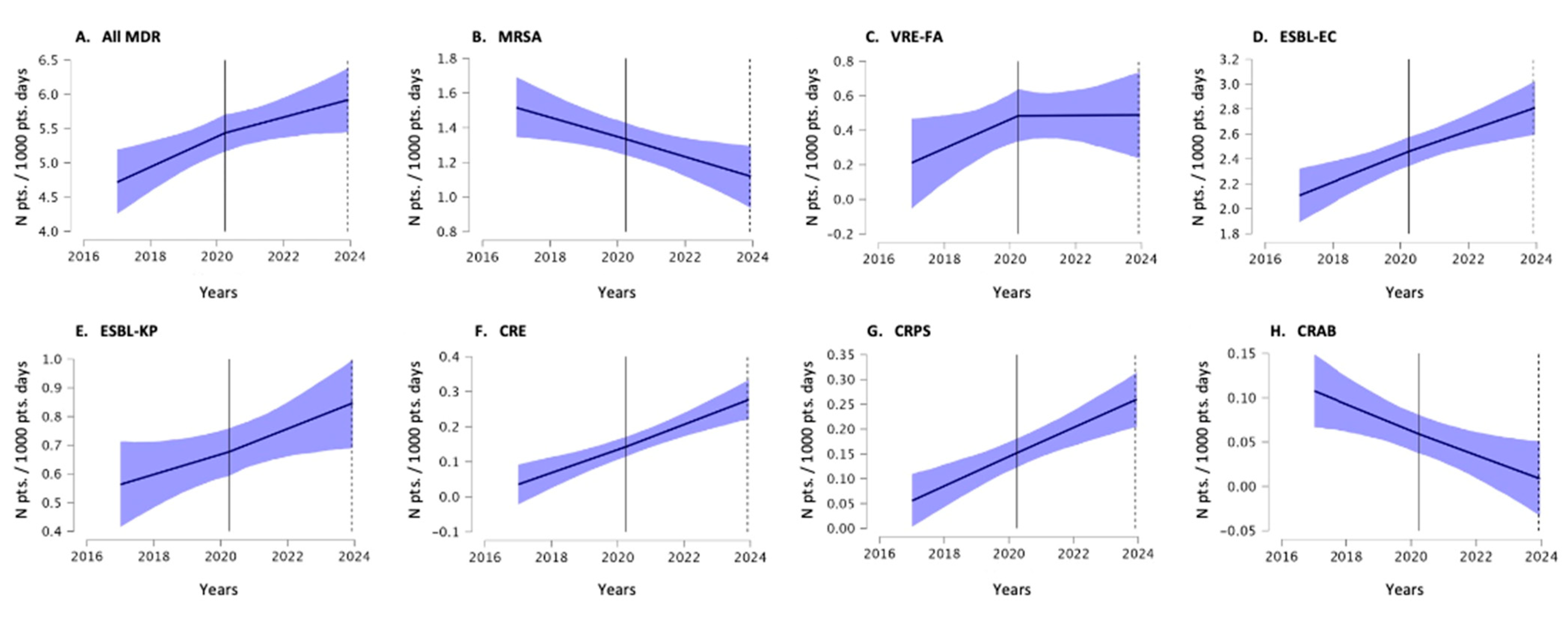
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Clinical Setting
4.2. Data Collection
4.3. Microbiological Method
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- World Bank. Drug-Restitant Infections—A Threat to Our Economic Future; World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Rehman, S. A parallel and silent emerging pandemic: Antimicrobial resistance (AMR) amid COVID-19 pandemic. J. Infect. Public Health 2023, 16, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Janezic, S.; Mahnic, A.; Kuhar, U.; Kovač, J.; Jenko Bizjan, B.; Koritnik, T.; Tesovnik, T.; Šket, R.; Krapež, U.; Slavec, B.; et al. SARS-CoV-2 Molecular epidemiology in Slovenia, January to September 2021. Eurosurveillance 2023, 28, 2200451. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Sledilnik. Available online: https://covid-19.sledilnik.org/sl/stats (accessed on 25 September 2024).
- Langford, B.J.; Soucy, J.-P.R.; Leung, V.; So, M.; Kwan, A.T.H.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.R.; Daneman, N. Antibiotic resistance associated with the COVID-19 pandemic: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baño, J. Impact of COVID-19 pandemic on multidrug resistant Gram positive and Gram negative pathogens: A systematic review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Gandhi, T.N.; Petty, L.A.; Patel, P.K.; Prescott, H.C.; Malani, A.N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S.A. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): A multi-hospital cohort study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Bindo, F.; Motos, A.; Fernández-Barat, L.; Barbeta, E.; Gabarrús, A.; Ceccato, A.; Bermejo-Martin, J.F.; Ferrer, R.; Riera, J.; et al. Procalcitonin and C-reactive protein to rule out early bacterial coinfection in COVID-19 critically ill patients. Intensive Care Med. 2023, 49, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.-P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.; O’Hara, L.M.; Harris, A.D. Transmission Pathways of Multidrug-Resistant Organisms in the Hospital Setting: A Scoping Review. Infect. Control Hosp. Epidemiol. 2019, 40, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.; Edgeworth, J.; Bailey, S.L. Acquisition of MDR-GNB in Hospital Settings: A Systematic Review and Meta-Analysis Focusing on ESBL-E. J. Hosp. Infect. 2020, 106, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Mrvič, T.; Stevanoska, S.; Beović, B.; Logar, M.; Gregorčič, S.; Žnidaršič, B.; Seme, K.; Velimirović, I.; Švent Kučina, N.; Maver Vodičar, P.; et al. The impact of COVID-19 on multidrug-resistant bacteria at a Slovenian tertiary medical center. Antibiotics 2024, 13, 214. [Google Scholar] [CrossRef]
- Assefa, M. Multi-drug resistant Gram-negative bacterial pneumonia: Etiology, risk factors, and drug resistance patterns. Pneumonia 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2022; European Centre for Disease Prevention and Control: Stochkolm, Sweden, 2023.
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0, 2024; European Committee on Antimicrobial Susceptibility Testing: Stockholm, Sweden, 2024. [Google Scholar]
- Giske, C.; MartInez-Martinez, L.; Canton, R.; Stefani, S.; Skov, R.; Glupczynski, Y.; Nordmann, P.; Wootton, M.; Miriagou, V.; Simonsen, G.S.; et al. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Version 1.0, 2013; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2013. [Google Scholar]
- Taylor, S.J.; Letham, B. Forecasting at scale. Am. Stat. 2018, 72, 37–45. [Google Scholar] [CrossRef]


| Mann–Whitney U-Test | Interrupted Time Series Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-COVID Incidence Density Mean | COVID Incidence Density Mean | p-Value | Rank Biserial Correlation (RBC) 1 | 95% Confidence Interval of RBC | Pre-COVID Incidence Density Offset (m) | Pre-COVID Incidence Density Growth Rate (k) | COVID Incidence Density Growth Rate Change (δ) | |
| MDR Burden | ||||||||
| ALL MDR | 4.93 | 5.81 | 0.007 | −0.34 | (−0.54, −0.11) | 0.466 | 0.151 | −0.062 |
| MRSA | 1.45 | 1.21 | 0.067 | 0.23 | (−0.01, 0.45) | 0.485 | −0.124 | −0.005 |
| VRE-FA | 0.23 | 0.59 | 0.001 | −0.38 | (−0.57, −0.15) | 0.048 | 0.130 | −0.128 |
| ESBL-EC | 2.26 | 2.65 | 0.022 | −0.29 | (−0.50, −0.05) | 0.518 | 0.185 | −0.024 |
| ESBL-KP | 0.56 | 0.81 | 0.015 | −0.31 | (−0.52, −0.07) | 0.192 | 0.083 | 0.026 |
| CRE | 0.11 | 0.19 | 0.055 | −0.22 | (−0.44, 0.02) | 0.043 | 0.278 | 0.027 |
| CRPS | 0.11 | 0.20 | 0.023 | −0.27 | (−0.48, −0.02) | 0.083 | 0.307 | −0.006 |
| CRAB | 0.10 | 0.02 | 0.062 | 0.16 | (−0.09, 0.39) | 0.144 | −0.139 | 0.013 |
| MDR Infections | ||||||||
| ALL MDR | 1.61 | 1.29 | 0.019 | 0.30 | (0.06, 0.51) | 0.548 | −0.183 | 0.027 |
| MRSA | 0.32 | 0.30 | 0.384 | 0.11 | (−0.14, 0.35) | 0.262 | −0.011 | −0.014 |
| VRE-FA | 0.02 | 0.03 | 0.258 | −0.08 | (−0.32, 0.17) | 0.050 | 0.117 | −0.024 |
| ESBL-EC | 0.79 | 0.51 | <0.001 | 0.43 | (0.21, 0.61) | 0.531 | −0.281 | 0.038 |
| ESBL-KP | 0.27 | 0.28 | 0.462 | −0.09 | (−0.33, 0.16) | 0.217 | −0.043 | 0.036 |
| CRE | 0.06 | 0.02 | 0.039 | 0.16 | (−0.09, 0.39) | 0.088 | −0.056 | 0.010 |
| CRPS | 0.06 | 0.11 | 0.085 | −0.18 | (−0.41, 0.07) | 0.066 | 0.197 | −0.003 |
| CRAB | 0.05 | 0.01 | 0.008 | 0.18 | (−0.07, 0.41) | 0.120 | −0.121 | −0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeverica, S.; Maganja, D.B.; Dernič, J.; Golob, P.; Stepišnik, A.; Novak, B.; Papst, L.; Dodič, A.J.; Gasparini, M. The Influence of COVID-19 on Antimicrobial Resistance Trends at a Secondary Care Hospital in Slovenia: An Interrupted Time Series Analysis. Antibiotics 2024, 13, 1033. https://doi.org/10.3390/antibiotics13111033
Jeverica S, Maganja DB, Dernič J, Golob P, Stepišnik A, Novak B, Papst L, Dodič AJ, Gasparini M. The Influence of COVID-19 on Antimicrobial Resistance Trends at a Secondary Care Hospital in Slovenia: An Interrupted Time Series Analysis. Antibiotics. 2024; 13(11):1033. https://doi.org/10.3390/antibiotics13111033
Chicago/Turabian StyleJeverica, Samo, Darja Barlič Maganja, Jani Dernič, Peter Golob, Alenka Stepišnik, Bojan Novak, Lea Papst, Anamarija Juriševič Dodič, and Mladen Gasparini. 2024. "The Influence of COVID-19 on Antimicrobial Resistance Trends at a Secondary Care Hospital in Slovenia: An Interrupted Time Series Analysis" Antibiotics 13, no. 11: 1033. https://doi.org/10.3390/antibiotics13111033
APA StyleJeverica, S., Maganja, D. B., Dernič, J., Golob, P., Stepišnik, A., Novak, B., Papst, L., Dodič, A. J., & Gasparini, M. (2024). The Influence of COVID-19 on Antimicrobial Resistance Trends at a Secondary Care Hospital in Slovenia: An Interrupted Time Series Analysis. Antibiotics, 13(11), 1033. https://doi.org/10.3390/antibiotics13111033






