Behavioral Nudges to Encourage Appropriate Antimicrobial Use Among Health Professionals in Uganda
Abstract
1. Introduction
2. Results
2.1. Formative Research Phase Results
2.1.1. Behavioral Intervention Elements
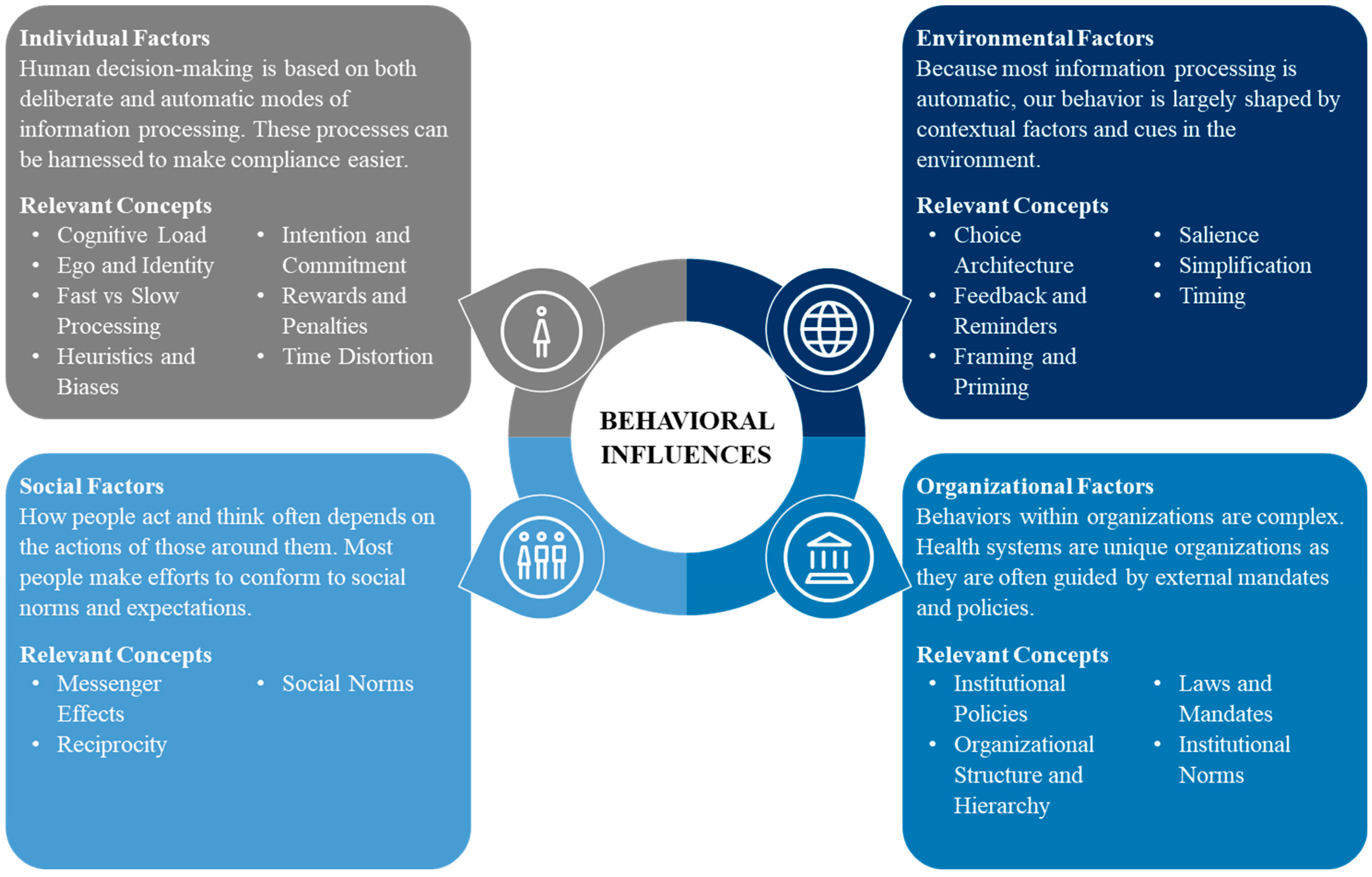
2.1.2. Individual Factors
2.1.3. Social Factors
2.1.4. Environmental Factors
2.1.5. Organizational Factors
2.2. Behavioral Intervention Implementation Phase Results
2.2.1. Participant Characteristics
2.2.2. Antimicrobial Prescription Prevalence
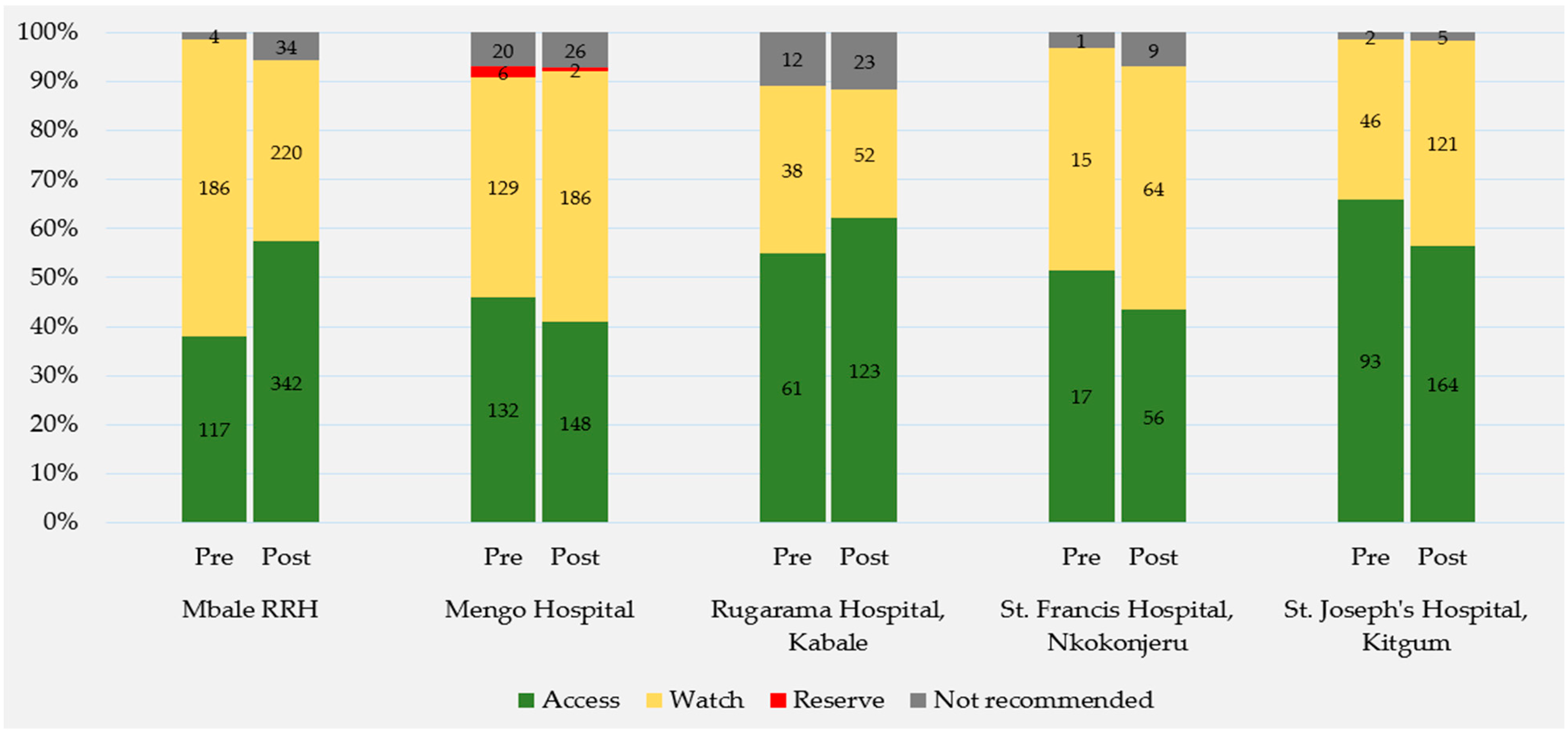
2.2.3. WHO AWaRe Classification
2.2.4. Evaluating Intervention Effects
3. Discussion
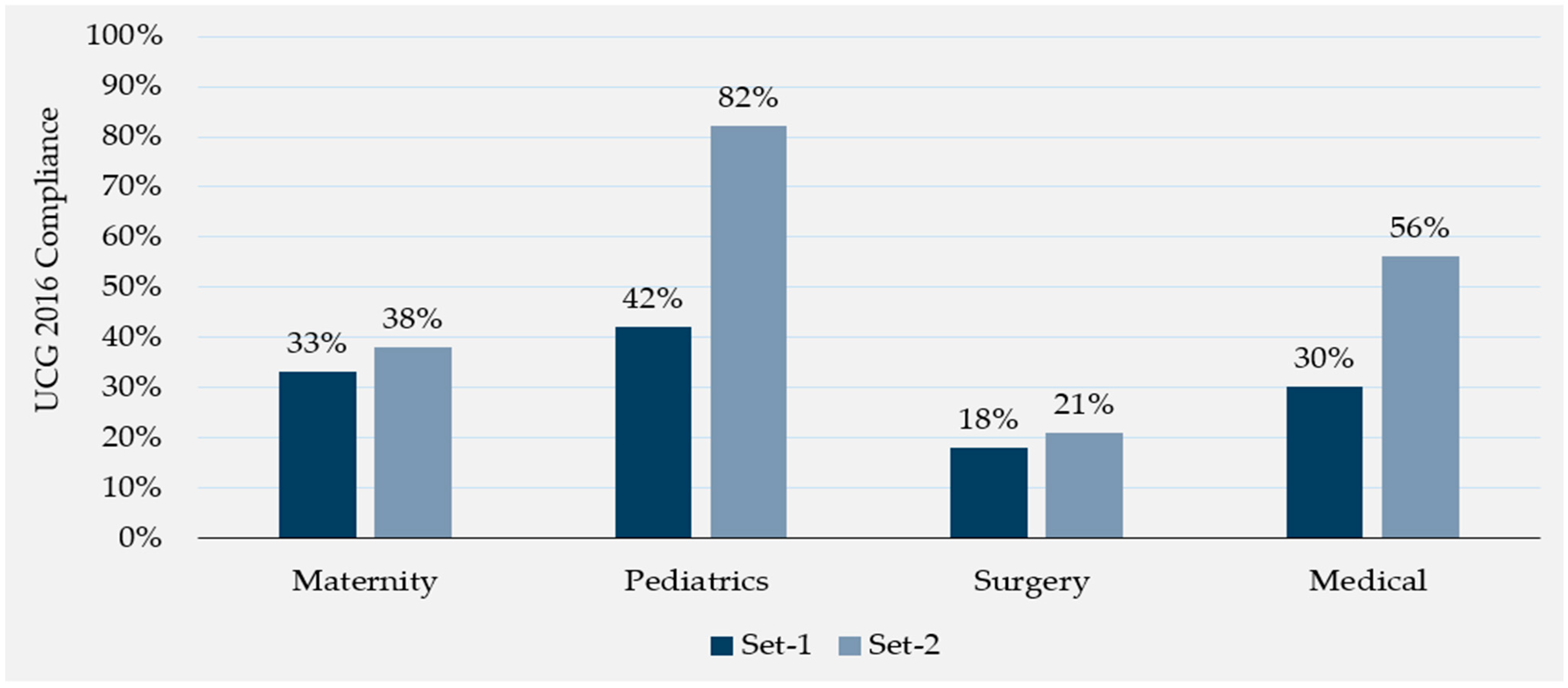
3.1. Uganda Clinical Guidelines Compliance
3.2. WHO AWaRe Classification of Antibiotics
3.3. Strengths and Limitations
3.4. Areas of Future Research
4. Materials and Methods
4.1. Formative Research Phase Methods
4.1.1. Study Setting
4.1.2. Behavioral Insights Framework
- Individual: People are faced with more information than they can process, so simplifying complex tasks, processes, and policies can guide behaviors.
- Social: Recognizing the importance we place on our connection to others, individuals can be motivated to behave better by comparing their behavior to the behavior of others.
- Environmental: Because behavior is shaped by our surroundings, prompts and cues can guide behavior so that the desired choice is the easy choice.
- Organizational: Individual decision-making within organizations is influenced by complex policies and rules, managerial controls, and chains of command, structures that can be shaped to encourage specific behaviors.
4.1.3. Formative Research Design
4.1.4. Formative Research Population and Data Collection
4.1.5. Formative Research Data Analysis
4.2. Behavioral Intervention Implementation Phase Methods
4.2.1. Behavioral Intervention Setting and Design
4.2.2. Behavioral Intervention Population and Data Collection
4.2.3. Behavioral Intervention Data Analysis
4.3. Study Ethics and Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Vaccines Could Avert Half a Million Deaths Associated with Anti-Microbial Resistance a Year. News. 2023. Available online: https://www.who.int/news/item/28-07-2023-vaccines-could-avert-half-a-million-deaths-associated-with-anti-microbial-resistance-a-year (accessed on 13 September 2024).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sánchez, E.; Moore, L.S.P.; Husson, F.; Holmes, A.H. What are the factors driving antimicrobial resistance? Perspectives from a public event in London, England. BMC Infect. Dis. 2016, 16, 465. [Google Scholar] [CrossRef] [PubMed]
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A.; Zhu, N. Differential Drivers of Antimicrobial Resistance across the World. Acc. Chem. Res. 2019, 52, 916–924. [Google Scholar] [CrossRef]
- Chatterjee, A.; Modarai, M.; Naylor, N.R.; Boyd, S.E.; Atun, R.; Barlow, J.; Holmes, A.H.; Johnson, A.; Robotham, J.V. Quantifying drivers of antibiotic resistance in humans: A systematic review. Lancet Infect. Dis. 2018, 18, e368–e378. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Smieszek, T.; Pouwels, K.B.; Dolk, F.C.K.; Smith, D.R.M.; Hopkins, S.; Sharland, M.; Hay, A.D.; Moore, M.V.; Robotham, J.V. Potential for reducing inappropriate antibiotic prescribing in English primary care. J. Antimicrob. Chemother. 2018, 73, ii36–ii43. [Google Scholar] [CrossRef]
- Grijalva, C.G.; Nuorti, J.P.; Griffin, M.R. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009, 302, 758–766. [Google Scholar] [CrossRef]
- World Health Organization (WHO). AWaRe. Available online: https://aware.essentialmeds.org/list (accessed on 13 September 2024).
- Ostini, R.; Hegney, D.; Jackson, C.; Williamson, M.; Mackson, J.M.; Gurman, K.; Hall, W.; Tett, S.E. Systematic review of interventions to improve prescribing. Ann. Pharmacother. 2009, 43, 502–513. [Google Scholar] [CrossRef]
- Borek, A.J.; Santillo, M.; Wanat, M.; Butler, C.C.; Tonkin-Crine, S. How can behavioural science contribute to qualitative research on antimicrobial stewardship in primary care? JAC-Antimicrob. Resist. 2022, 4, dlac007. [Google Scholar] [CrossRef]
- Martens, J.D.; Winkens, R.A.G.; Van Der Weijden, T.; De Bruyn, D.; Severens, J.L. Does a joint development and dissemination of multidisciplinary guidelines improve prescribing behaviour: A pre/post study with concurrent control group and a randomised trial. BMC Health Serv. Res. 2006, 6, 145. [Google Scholar] [CrossRef]
- Van Driel, M.L.; Coenen, S.; Dirven, K.; Lobbestael, J.; Janssens, I.; Van Royen, P.; Haaijer-Ruskamp, F.M.; De Meyere, M.; De Maeseneer, J.; Christiaens, T. What is the role of quality circles in strategies to optimise antibiotic prescribing? A pragmatic cluster-randomised controlled trial in primary care. Qual. Saf. Health Care 2007, 16, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, C.; Batura, N.; Wulandari, L.P.L.; Khan, M.; Wiseman, V. Improving antibiotic use through behaviour change: A systematic review of interventions evaluated in low- And middle-income countries. Health Policy Plan. 2021, 36, 754–773. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Marwick, C.; Scott, C.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.; Ramsay, C.; Michie, S. Interventions to Improve Antibiotic Prescribing Practices for Hospital Inpatients. Cochrane Database Syst Rev 2017, 2017, CD003543. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Simpson, S.A.; Dunstan, F.; Rollnick, S.; Cohen, D.; Gillespie, D.; Evans, M.R.; Alam, M.F.; Bekkers, M.J.; Evans, J.; et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: Practice based randomised controlled trial. BMJ 2012, 344, d8173. [Google Scholar] [CrossRef] [PubMed]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.L.; Melbye, H.; Santer, M.; Moore, M.; et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013, 382, 1175–1182. [Google Scholar] [CrossRef]
- Craig, J.; Sriram, A.; Sadoff, R.; Bennett, S.; Bahati, F.; Beauvais, W. Behavior-change interventions to improve antimicrobial stewardship in human health, animal health, and livestock agriculture: A systematic review. PLOS Glob. Public Health 2023, 4, e0002930. [Google Scholar] [CrossRef]
- Nair, M.M.; Mahajan, R.; Burza, S.; Zeegers, M.P. Behavioural interventions to address rational use of antibiotics in outpatient settings of low-income and lower-middle-income countries. Trop. Med. Int. Health 2021, 26, 504–517. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals. 2018. Available online: https://iris.who.int/bitstream/handle/10665/280063/WHO-EMP-IAU-2018.01-eng.pdf?sequence=1 (accessed on 14 October 2024).
- Republic of Uganda Ministry of Health (MOH). Uganda Clinical Guidelines 2016. 2017. Available online: https://health.go.ug/sites/default/files/Uganda Clinical Guidelines 2016_FINAL.pdf (accessed on 13 September 2024).
- Atuhaire, J.; Nakitto, D.; Kiguba, R.; Figueras, A.; Ndagije, H.B.; Serwanga, A.; Manirakiza, L.; Nambasa, V. Prescription of levofloxacin and moxifloxacin in select hospitals in Uganda: A pilot study to assess guideline concordance. Antibiotics 2020, 9, 439. [Google Scholar] [CrossRef]
- Kagoya, E.K.; Van Royen, K.; Waako, P.; Van Royen, P.; Iramiot, J.S.; Obakiro, S.B.; Kostyanev, T.; Anthierens, S. Experiences and views of healthcare professionals on the prescription of antibiotics in Eastern Uganda: A qualitative study. J. Glob. Antimicrob. Resist. 2021, 25, 66–71. [Google Scholar] [CrossRef]
- Kiggundu, R.; Wittenauer, R.; Waswa, J.P.; Nakambale, H.N.; Kitutu, F.E.; Murungi, M.; Okuna, N.; Morries, S.; Lawry, L.L.; Joshi, M.P.; et al. Point Prevalence Survey of Antibiotic Use across 13 Hospitals in Uganda. Antibiotics 2022, 11, 199. [Google Scholar] [CrossRef]
- Seni, J.; Mapunjo, S.G.; Wittenauer, R.; Valimba, R.; Stergachis, A.; Werth, B.J.; Saitoti, S.; Mhadu, N.H.; Lusaya, E.; Konduri, N. Antimicrobial use across six referral hospitals in Tanzania: A point prevalence survey. BMJ Open 2020, 10, e042819. [Google Scholar] [CrossRef] [PubMed]
- Skosana, P.P.; Schellack, N.; Godman, B.; Kurdi, A.; Bennie, M.; Kruger, D.; Meyer, J.C. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev. Anti. Infect. Ther. 2021, 19, 1353–1366. [Google Scholar] [CrossRef]
- Government of Uganda Antimicrobial Resistance National Action Plan 2018–2023. 2018. Available online: https://www.who.int/publications/m/item/uganda-antimicrobial-resistance-national-action-plan-2018-2023 (accessed on 30 September 2023).
- Kiggundu, R.; Waswa, J.P.; Nakambale, H.N.; Kakooza, F.; Kassuja, H.; Murungi, M.; Akello, H.; Morries, S.; Joshi, M.P.; Stergachis, A. Development and evaluation of a continuous quality improvement programme for antimicrobial stewardship in six hospitals in Uganda. BMJ Open Qual. 2023, 12, e002293. [Google Scholar] [CrossRef]
- Waswa, J.P.; Kiggundu, R.; Konduri, N.; Kasujja, H.; Lawry, L.L.; Joshi, M.P. What is the appropriate antimicrobial use surveillance tool at the health facility level for Uganda and other low- and middle-income countries? J. Glob. Antimicrob. Resist. 2023, 34, 145–149. [Google Scholar] [CrossRef]
- Kahneman, D. Thinking, Fast and Slow; Farrar, Straus and Giroux (FSG): New York, NY, USA, 2011. [Google Scholar]
- Bicchieri, C.; Dimant, E. Norm-Nudging: Harnessing Social Expectations for Behavior Change. SSRN Electron. J. 2023; published online first. [Google Scholar] [CrossRef]
- Mayora, C.; Kitutu, F.E.; Kandala, N.B.; Ekirapa-Kiracho, E.; Peterson, S.S.; Wamani, H. Private retail drug shops: What they are, how they operate, and implications for health care delivery in rural Uganda. BMC Health Serv. Res. 2018, 18, 532. [Google Scholar] [CrossRef]
- Ludwig, T.; Houmanfar, R. Understanding Complexity in Organizations: Behavioral Systems; Routledge: London, UK, 2015; ISBN 1317986946. [Google Scholar]
- Tayler, E.; Gregory, R.; Bloom, G.; Salama, P.; Balkhy, H. Universal health coverage: An opportunity to address antimicrobial resistance? Lancet Glob. Health 2019, 7, e1480–e1481. [Google Scholar] [CrossRef]
- Republic of Uganda Ministry of Health (MOH). A Roadmap Towards Universal Health Coverage in Uganda 2020/21 to 2029/30. 2020. Available online: https://www.uhfug.com/wp-content/uploads/2022/08/Universal-Health-Coverage-Roadmap-for-Uganda.pdf (accessed on 14 October 2024).
- Kwasnicka, D.; Dombrowski, S.U.; White, M.; Sniehotta, F. Theoretical explanations for maintenance of behaviour change: A systematic review of behaviour theories. Health Psychol. Rev. 2016, 10, 277–296. [Google Scholar] [CrossRef]
- Cash, P.; Wrobel, A.; Maier, A.; Hansen, J.P. Understanding long-term behaviour change techniques: A mixed methods study. J. Eng. Des. 2023, 34, 383–410. [Google Scholar] [CrossRef]
- Frey, E.; Rogers, T. Persistence: How Treatment Effects Persist After Interventions Stop. Policy Insights Behav. Brain Sci. 2014, 1, 172–179. [Google Scholar] [CrossRef]
- Hobbs, N.; Godfrey, A.; Lara, J.; Errington, L.; Meyer, T.D.; Rochester, L.; White, M.; Mathers, J.C.; Sniehotta, F.F. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med. 2013, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Cahill, K.; Perera, R. Competitions and incentives for smoking cessation. Cochrane Database Syst. Rev. 2011; published online first. [Google Scholar] [CrossRef]
- Rothman, A.J. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000, 19, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Cico, D.; Guyton, J.; LoPresti, E.; Miller, A.; Schafer, B.; Godby, S.; Greene, M.; Guszcza, J.; Kovar, S.; Lidrbauch, G.; et al. Behavioral Insights Toolkit. 2017. Available online: https://www.irs.gov/pub/irs-soi/17rpirsbehavioralinsights.pdf (accessed on 13 September 2024).
- Harun, M.G.D.; Sumon, S.A.; Hasan, I.; Akther, F.M.; Islam, M.S.; Anwar, M.M.U. Barriers, facilitators, perceptions and impact of interventions in implementing antimicrobial stewardship programs in hospitals of low-middle and middle countries: A scoping review. Antimicrob. Resist. Infect. Control 2024, 13, 8. [Google Scholar] [CrossRef]
- Akpan, M.R.; Isemin, N.U.; Udoh, A.E.; Ashiru-Oredope, D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020, 22, 317–324. [Google Scholar] [CrossRef]
- Wallace, L.J.; Kapiriri, L. Priority setting for maternal, newborn and child health in Uganda: A qualitative study evaluating actual practice. BMC Health Serv. Res. 2019, 19, 465. [Google Scholar] [CrossRef]
- World Health Organization (WHO). AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use, 2023. 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 5 April 2024).
- Murungi, M.; Ndagije, H.B.; Kiggundu, R.; Kesi, D.N.; Waswa, J.P.; Rajab, K.; Barigye, M.; Serwanga, A.; Manirakiza, L.; Kasujja, H. Antimicrobial consumption surveillance in Uganda: Results from an analysis of national import data for the human health sector, 2018–2021. J. Infect. Public Health 2023, 16, 45–51. [Google Scholar] [CrossRef]
- Namugambe, J.S.; Delamou, A.; Moses, F.; Ali, E.; Hermans, V.; Takarinda, K.; Thekkur, P.; Nanyonga, S.M.; Koroma, Z.; Mwoga, J.N.; et al. National antimicrobial consumption: Analysis of central warehouses supplies to in-patient care health facilities from 2017 to 2019 in Uganda. Trop. Med. Infect. Dis. 2021, 6, 83. [Google Scholar] [CrossRef]
- Anand Paramadhas, B.D.; Tiroyakgosi, C.; Mpinda-Joseph, P.; Morokotso, M.; Matome, M.; Sinkala, F.; Gaolebe, M.; Malone, B.; Molosiwa, E.; Shanmugam, M.G.; et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev. Anti. Infect. Ther. 2019, 17, 535–546. [Google Scholar] [CrossRef]
- Okoth, C.; Opanga, S.; Okalebo, F.; Oluka, M.; Baker Kurdi, A.; Godman, B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: Findings and implications. Hosp. Pract. 2018, 46, 128–136. [Google Scholar] [CrossRef]
- Amponsah, O.K.O.; Buabeng, K.O.; Owusu-Ofori, A.; Ayisi-Boateng, N.K.; Hämeen-Anttila, K.; Enlund, H. Point prevalence survey of antibiotic consumption across three hospitals in Ghana. JAC-Antimicrob. Resist. 2021, 3, dlab008. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.K.; Obeng-Nkrumah, N.; Nartey, E.T.; Bjerrum, S.; Adu-Aryee, N.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Antibiotic use in a tertiary healthcare facility in Ghana: A point prevalence survey. Antimicrob. Resist. Infect. Control 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Maina, M.; Mwaniki, P.; Odira, E.; Kiko, N.; McKnight, J.; Schultsz, C.; English, M.; Tosas-Auguet, O. Antibiotic use in Kenyan public hospitals: Prevalence, appropriateness and link to guideline availability. Int. J. Infect. Dis. 2020, 99, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Horumpende, P.G.; Mshana, S.E.; Mouw, E.F.; Mmbaga, B.T.; Chilongola, J.O.; De Mast, Q. Point prevalence survey of antimicrobial use in three hospitals in North-Eastern Tanzania. Antimicrob. Resist. Infect. Control 2020, 9, 149. [Google Scholar] [CrossRef]
- Kajumbula, H.; Fujita, A.W.; Mbabazi, O.; Najjuka, C.; Izale, C.; Akampurira, A.; Aisu, S.; Lamorde, M.; Walwema, R.; Bahr, N.C.; et al. Antimicrobial drug resistance in blood culture isolates at a tertiary hospital, Uganda. Emerg. Infect. Dis. 2018, 24, 174–175. [Google Scholar] [CrossRef]
- Kizito, M.; Lalitha, R.; Kajumbula, H.; Ssenyonga, R.; Muyanja, D.; Byakika-Kibwika, P. Antibiotic prevalence study and factors influencing prescription of who watch category antibiotic ceftriaxone in a tertiary care private not for profit hospital in uganda. Antibiotics 2021, 10, 1167. [Google Scholar] [CrossRef]
- Guz, D.; Bracha, M.; Steinberg, Y.; Kozlovsky, D.; Gafter-Gvili, A.; Avni, T. Ceftriaxone versus ampicillin for the treatment of community-acquired pneumonia. A propensity matched cohort study. Clin. Microbiol. Infect. 2023, 29, 70–76. [Google Scholar] [CrossRef]
- Meeker, D.; Linder, J.A.; Fox, C.R.; Friedberg, M.W.; Persell, S.D.; Goldstein, N.J.; Knight, T.K.; Hay, J.W.; Doctor, J.N. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices a randomized clinical trial. JAMA—J. Am. Med. Assoc. 2016, 315, 562–570. [Google Scholar] [CrossRef]
- Rowe, T.A.; Linder, J.A. Novel approaches to decrease inappropriate ambulatory antibiotic use. Expert Rev. Anti. Infect. Ther. 2019, 17, 511–521. [Google Scholar] [CrossRef]
- Republic of Uganda Ministry of Health (MOH). Uganda Clinical Guidelines 2023. 2024. Available online: http://library.health.go.ug/sites/default/files/resources/Uganda Clinical Guidelines 2023.pdf (accessed on 13 September 2024).
- Gittelsohn, J.; Steckler, A.; Johnson, C.C.; Pratt, C.; Grieser, M.; Pickrel, J.; Stone, E.J.; Conway, T.; Coombs, D.; Staten, L.K. Formative research in school and community-based health programs and studies: “State of the art” and the TAAG approach. Health Educ. Behav. 2006, 33, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Kaawa-Mafigiri, D.; Ekusai-Sebatta, D.; Rutebemberwa, E.; Sserwanga, A.; Kitutu, F.E.; Kapisi, J.; Hopkins, H.; Salami, O.; Nkeramahame, J.; Olliaro, P.; et al. A Qualitative Assessment of a Training and Communication Intervention on Antibiotic Prescription Practices Among Health Workers and Outpatients at Public Health Facilities in Uganda. Clin. Infect. Dis. 2023, 77, S191–S198. [Google Scholar] [CrossRef] [PubMed]
- Dehn Lunn, A. Reducing inappropriate antibiotic prescribing in upper respiratory tract infection in a primary care setting in Kolkata, India. BMJ Open Qual. 2018, 7, e000217. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of inappropriate antibiotic prescriptions among us ambulatory care visits, 2010–2011. JAMA—J. Am. Med. Assoc. 2016, 315, 1864–1873. [Google Scholar] [CrossRef]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, 1120. [Google Scholar] [CrossRef]
- Center for Disease Dynamics Economics & Policy (CDDEP). State of the World’s Antibiotics, 2015. 2015. Available online: https://onehealthtrust.org/wp-content/uploads/2017/06/swa_edits_9.16.pdf (accessed on 13 September 2024).
- De Cremer, D.; Bakker, M. Accountability and Cooperation in Social Dilemmas: The Influence of Others’ Reputational Concerns. Curr. Psychol. 2003, 22, 155–163. [Google Scholar] [CrossRef]
- Kiefe, C.I.; Allison, J.J.; Williams, O.D.; Person, S.D.; Weaver, M.T.; Weissman, N.W. Improving quality improvement using achievable benchmarks for physician feedback: A randomized controlled trial. JAMA 2001, 285, 2871–2879. [Google Scholar] [CrossRef]
- Hoa, N.Q.; Lan, P.T.; Phuc, H.D.; Chuc, N.T.K.; Lundborg, C.S. Antibiotic prescribing and dispensing for acute respiratory infections in children: Effectiveness of a multi-faceted intervention for health-care providers in Vietnam. Glob. Health Action 2017, 10, 1327638. [Google Scholar] [CrossRef]


| BI Framework Element | Behavioral Themes of Key Informant Interview Feedback |
|---|---|
| Individual |
|
| Social |
|
| Environmental |
|
| Organizational |
|
| Variable | Pre-Intervention Time Point A (n = 356) | Pre-Intervention Time Point B (n = 382) | Total Pre-Intervention (n = 738) | Immediate (n = 738) | Follow-Up (n = 702) | Total Post-Intervention (n = 1440) |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Female | 215 (60%) | 204 (53%) | 419 (57%) | 458 (62%) | 442 (63%) | 900 (63%) + |
| Male | 141 (40%) | 178 (47%) | 319 (43%) | 280 (38%) | 260 (37%) | 540 (38%) + |
| Age, median (interquartile range (IQR)) | 25 (7–43) | 27 (10.25–45) | 26 (9–44) | 23 (5–38.75) | 28 (12–45) | 25 (8–41) |
| Hospital ownership | ||||||
| Public | 143 (40%) | 140 (37%) | 283 (38%) | 285 (39%) | 253 (36%) | 538 (37%) |
| Private not-for-profit | 213 (60%) | 242 (63%) | 455 (62%) | 453 (61%) | 449 (64%) | 902 (63%) |
| Hospital | ||||||
| Mbale Regional Referral Hospital (RRH) | 143 (40%) | 140 (37%) | 283 (38%) | 285 (39%) | 253 (36%) | 538 (37%) |
| Mengo Hospital | 115 (32%) | 97 (25%) | 212 (29%) * | 146 (20%) | 154 (22%) | 300 (21%) + |
| Rugarama Hospital, Kabale | 34 (10%) | 56 (15%) | 90 (12%) * | 92 (12%) | 91 (13%) | 183 (13%) |
| St. Francis Hospital, Nkokonjeru | 7 (2%) | 17 (4%) | 24 (3%) | 68 (9%) | 47 (7%) | 115 (8%) + |
| St. Joseph’s Hospital, Kitgum | 57 (16%) | 72 (19%) | 129 (17%) | 147 (20%) | 157 (22%) | 304 (21%) + |
| Ward | ||||||
| Maternal | 97 (27%) | 96 (25%) | 193 (26%) | 204 (28%) | 199 (28%) | 403 (28%) |
| Medical | 71 (20%) | 97 (25%) | 168 (23%) | 190 (26%) | 189 (27%) | 379 (26%) |
| Pediatric | 110 (31%) | 95 (25%) | 205 (28%) | 221 (30%) | 153 (22%) | 374 (26%) ^ |
| Surgical | 78 (22%) | 94 (25%) | 172 (23%) | 123 (17%) | 161 (23%) | 284 (20%) ^ |
| Underlying patient condition | ||||||
| Central catheter | 1 (0.3%) | 2 (0.5%) | 3 (0.4%) | 4 (0.5%) | 4 (0.6%) | 8 (0.6%) |
| Peripheral catheter | 337 (95%) | 358 (94%) | 695 (94%) | 694 (94%) | 689 (98%) | 1383 (96%) ^ |
| Urinary catheter | 9 (3%) | 25 (7%) | 34 (5%) * | 34 (5%) | 39 (6%) | 73 (5%) |
| Intubation | 2 (1%) | 4 (1%) | 6 (1%) | 8 (1%) | 15 (2%) | 23 (2%) |
| Malaria | 48 (13%) | 43 (11%) | 91 (12%) | 153 (21%) | 80 (11%) | 233 (16%) ^+ |
| Tuberculosis | 5 (1%) | 5 (1%) | 10 (1%) | 3 (0%) | 6 (1%) | 9 (0.6%) |
| Human immunodeficiency virus | 7 (2%) | 13 (3%) | 20 (3%) | 18 (2%) | 28 (4%) | 46 (3%) |
| Chronic obstructive pulmonary disease | 3 (1%) | 4 (1%) | 7 (1%) | 4 (1%) | 7 (1%) | 11 (0.8%) |
| Malnutrition | 14 (4%) | 12 (3%) | 26 (4%) | 45 (6%) | 17 (2%) | 62 (4%) ^ |
| Total Prescription at Baseline (n = 879) | Baseline Compliance (Time A + B) | Total Prescriptions Post-Intervention (n = 1575) | Post-Intervention Compliance (Immediate + Follow-Up) | |
|---|---|---|---|---|
| Antimicrobial | ||||
| Ceftriaxone | 281 | 64 (23%) | 504 | 238 (47%) *** |
| Metronidazole | 203 | 48 (24%) | 415 | 131 (32%) * |
| Gentamicin | 70 | 33 (47%) | 122 | 61 (50%) |
| Ampicillin | 57 | 27 (47%) | 131 | 64 (49%) |
| Levofloxacin | 34 | 4 (12%) | 28 | 2 (7%) |
| Amoxicillin | 30 | 8 (27%) | 79 | 33 (42%) |
| Cefixime | 20 | 2 (10%) | 17 | 0 (0%) |
| Cloxacillin | 20 | 11 (55%) | 26 | 25 (96%) *** |
| Azithromycin | 19 | 4 (21%) | 22 | 5 (23%) |
| Flucamox (flucloxacillin and amoxicillin) | 18 | 3 (17%) | 14 | 2 (14%) |
| Ampiclox (ampicillin and cloxacillin) | 17 | 0 (0%) | 23 | 1 (4%) |
| Ciprofloxacin | 17 | 6 (35%) | 34 | 16 (47%) |
| Nitrofurantoin | 12 | 11 (92%) | 10 | 9 (90%) |
| Bacqure (imipenem and cilastatin) | 11 | 0 (0%) | 9 | 0 (0%) |
| Amoxiclav (amoxicillin and clavulanic acid) | 9 | 4 (44%) | 17 | 6 (35%) |
| Other ^ | 61 | 9 (15%) | 124 | 40 (32%) * |
| Total Prescriptions at Baseline (n = 879) | Baseline Compliance (Time A + B) | Total Prescriptions Post-Intervention (n = 1575) | Post-Intervention Compliance (Immediate + Follow-Up) | |
|---|---|---|---|---|
| Hospital | ||||
| Mbale RRH | 307 | 74 (24%) | 596 | 196 (33%) ** |
| Mengo Hospital | 287 | 58 (20%) | 362 | 128 (35%) *** |
| Rugarama Hospital, Kabale | 111 | 30 (27%) | 198 | 99 (50%) *** |
| St. Francis Hospital, Nkokonjeru | 33 | 10 (30%) | 129 | 54 (42%) |
| St. Joseph’s Hospital, Kitgum | 141 | 62 (44%) | 290 | 156 (54%) * |
| Total Prescriptions at Baseline (n = 879) | Baseline Compliance (Time A + B) | Total Prescriptions Post-Intervention (n = 1575) | Post-Intervention Compliance (Immediate + Follow-Up) | |
|---|---|---|---|---|
| Ward | ||||
| Maternal | 297 | 34 (11%) | 577 | 132 (23%) *** |
| Medical | 165 | 62 (38%) | 299 | 167 (56%) *** |
| Pediatric | 223 | 93 (42%) | 370 | 226 (61%) *** |
| Surgical | 194 | 45 (23%) | 329 | 108 (33%) * |
| Hospital and ward | ||||
| Mbale RRH | 307 | 596 | ||
| Maternal | 117 | 16 (14%) | 270 | 53 (20%) |
| Medical | 51 | 16 (31%) | 103 | 54 (52%) * |
| Pediatric | 57 | 23 (40%) | 118 | 57 (48%) |
| Surgical | 82 | 19 (23%) | 105 | 32 (31%) |
| Mengo Hospital | 287 | 362 | ||
| Maternal | 114 | 9 (8%) | 111 | 16 (14%) |
| Medical | 42 | 5 (12%) | 53 | 27 (51%) *** |
| Pediatric | 77 | 36 (47%) | 111 | 67 (60%) |
| Surgical | 54 | 8 (15%) | 87 | 18 (21%) |
| Rugarama Hospital, Kabale | 111 | 198 | ||
| Maternal | 19 | 1 (5%) | 63 | 17 (27%) * |
| Medical | 39 | 17 (44%) | 63 | 35 (56%) |
| Pediatric | 34 | 8 (24%) | 40 | 29 (73%) *** |
| Surgical | 19 | 4 (21%) | 32 | 18 (56%) * |
| St. Francis Hospital, Nkokonjeru | 33 | 129 | ||
| Maternal | 19 | 2 (11%) | 26 | 9 (35%) |
| Medical | 9 | 6 (67%) | 22 | 10 (46%) |
| Pediatric | 5 | 2 (40%) | 37 | 23 (62%) |
| Surgical | 0 | N/A | 44 | 12 (27%) |
| St. Joseph’s Hospital, Kitgum | 141 | 290 | ||
| Maternal | 28 | 6 (21%) | 107 | 37 (35%) |
| Medical | 24 | 18 (75%) | 58 | 41 (71%) |
| Pediatric | 50 | 24 (48%) | 64 | 50 (78%) *** |
| Surgical | 39 | 14 (36%) | 61 | 28 (46%) |
| Hospital Name | Region | Size | Public/Private |
|---|---|---|---|
| Mbale RRH | Eastern | 450 beds | Public |
| Mengo Hospital | Central | 300 beds | Private |
| Rugarama Hospital, Kabale | Western | 150 beds | Private |
| St. Francis Hospital, Nkokonjeru | Central | 60 beds | Private |
| St. Joseph’s Hospital, Kitgum | Northern | 280 beds | Private |
| Interviewee Number | Position Title | Hospital or National Level | Interview Round |
|---|---|---|---|
| 1 | Epidemiologist | National | Round 1 |
| 2 | Product Safety Director | National | Round 1 |
| 3 | Allied Health Professionals Council Member | National | Round 1 |
| 4 | Clinical Pharmacist and AMR Committee Member | National | Round 1 |
| 5 | Physician | National | Round 1 |
| 6 | Department of Microbiology Head | National | Round 1 |
| 7 | Department of Microbiology Member | National | Round 1 |
| 8 | Physician | National | Round 1, 2 |
| 9 | Medical Director | Hospital | Round 1, 2 |
| 10 | Pharmacist | Hospital | Round 1 |
| 11 | Medical Director | Hospital | Round 1, 2 |
| 12 | Pharmacist | Hospital | Round 1 |
| 13 | Medical Director | Hospital | Round 1, 2 |
| 14 | MTC Chairperson | Hospital | Round 1 |
| 15 | Medical Officer | Hospital | Round 1, 2 |
| 16 | MTC Chairperson | Hospital | Round 1 |
| 17 | MTC Vice Secretary | Hospital | Round 1, 2 |
| 18 | Head of Pharmacy | Hospital | Round 1 |
| 19 | Hospital Director | Hospital | Round 1, 2 |
| Hospital | Hospital-Specific Educational Workshop Topics |
|---|---|
| Mbale RRH |
|
| Mengo Hospital |
|
| Rugarama Hospital, Kabale |
|
| St. Francis Hospital, Nkokonjeru |
|
| St. Joseph’s Hospital, Kitgum |
|
| Time Point | Round | Data Collection Dates * | Patients (N) |
|---|---|---|---|
| Pre-intervention baseline | Time A | 12–21 December 2022; 25–26 March 2023 for Mengo | 356 |
| Pre-intervention baseline | Time B | 13–19 February 2023; 10–11 May 2023 for Mengo | 382 |
| Pre-intervention baseline | Total | 738 | |
| Immediate post-intervention | Time A | 28 August–8 September 2023 | 402 |
| Immediate post-intervention | Time B | 11–22 September 2023 | 336 |
| Immediate post-intervention | Total | 738 | |
| One-month follow-up | Time A | 25 September–20 October 2023 | 339 |
| One-month follow-up | Time B | 23 October–8 December 2023 | 363 |
| One-month follow-up | Total | 702 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, A.; Meacham, P.J.; Waswa, J.P.; Joshi, M.P.; Hafner, T.; Godby, S.; Johnson, C.; Londhe, S.; Aibo, D.; Kwikiriza, G.; et al. Behavioral Nudges to Encourage Appropriate Antimicrobial Use Among Health Professionals in Uganda. Antibiotics 2024, 13, 1016. https://doi.org/10.3390/antibiotics13111016
Ross A, Meacham PJ, Waswa JP, Joshi MP, Hafner T, Godby S, Johnson C, Londhe S, Aibo D, Kwikiriza G, et al. Behavioral Nudges to Encourage Appropriate Antimicrobial Use Among Health Professionals in Uganda. Antibiotics. 2024; 13(11):1016. https://doi.org/10.3390/antibiotics13111016
Chicago/Turabian StyleRoss, Allison, Philip J. Meacham, J. P. Waswa, Mohan P. Joshi, Tamara Hafner, Sarah Godby, Courtney Johnson, Shilpa Londhe, Dorothy Aibo, Grace Kwikiriza, and et al. 2024. "Behavioral Nudges to Encourage Appropriate Antimicrobial Use Among Health Professionals in Uganda" Antibiotics 13, no. 11: 1016. https://doi.org/10.3390/antibiotics13111016
APA StyleRoss, A., Meacham, P. J., Waswa, J. P., Joshi, M. P., Hafner, T., Godby, S., Johnson, C., Londhe, S., Aibo, D., Kwikiriza, G., Kasujja, H., Kiggundu, R., Cho, M., Kovar, S., & Kitutu, F. E. (2024). Behavioral Nudges to Encourage Appropriate Antimicrobial Use Among Health Professionals in Uganda. Antibiotics, 13(11), 1016. https://doi.org/10.3390/antibiotics13111016







