Point-Prevalence Survey of Antimicrobial Use and Healthcare-Associated Infections in Four Acute Care Hospitals in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Hospitals
2.2. Point-Prevalence Survey of Antimicrobial Use and Healthcare-Associated Infections
2.3. Antimicrobial Consumption in Hospitals
2.4. Statistical Analysis
3. Results
3.1. Study of Antimicrobial Use and Healthcare-Associated Infections in Acute Care Hospitals
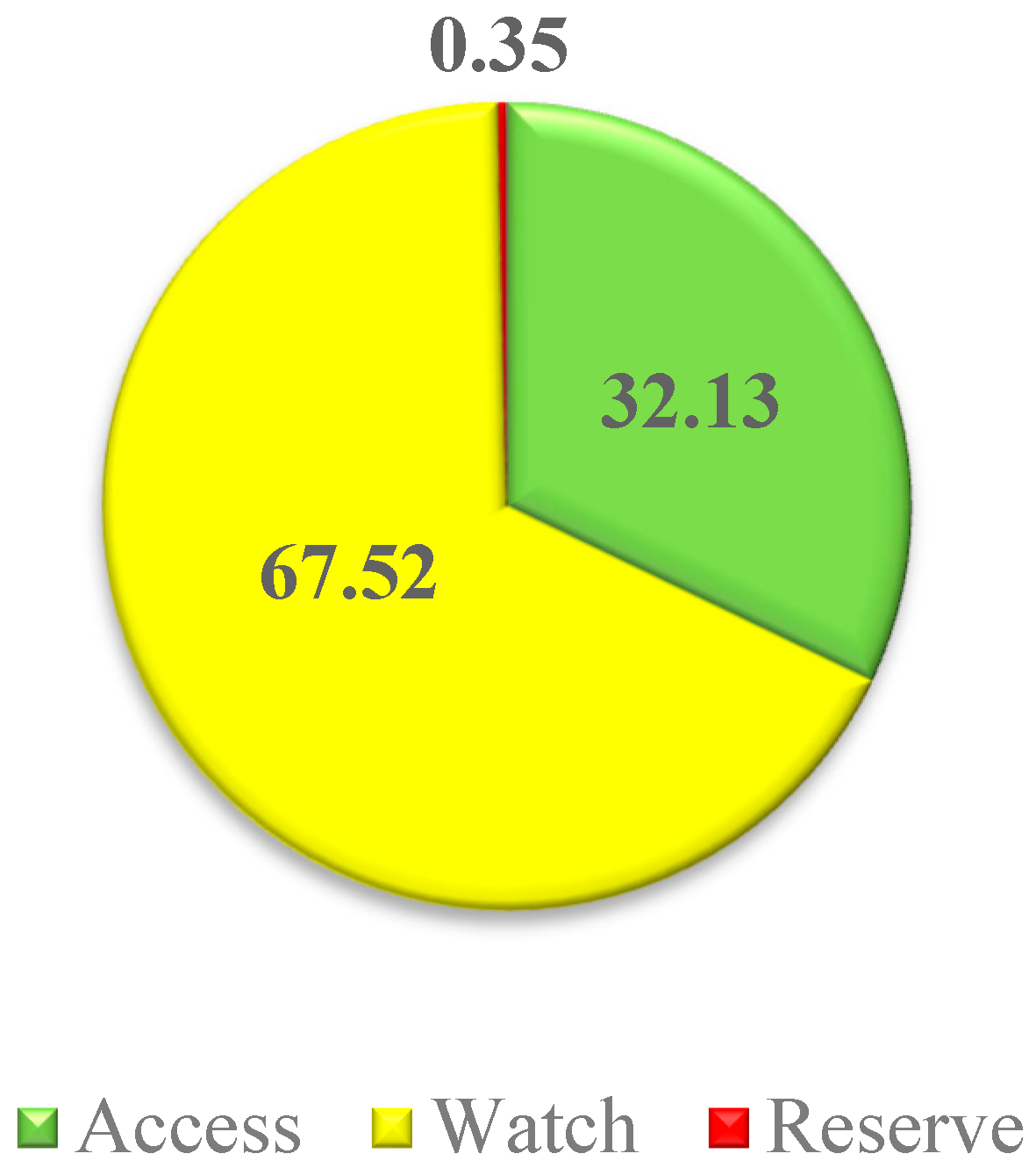
3.2. Study on Antibiotic Consumption in Acute Care Hospitals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Sadeq, A.A.; Hasan, S.S.; AbouKhater, N.; Conway, B.R.; Abdelsalam, A.E.; Shamseddine, J.M.; Babiker, Z.O.E.; Nsutebu, E.F.; Bond, S.E.; Aldeyab, M.A. Exploring Antimicrobial Stewardship Influential Interventions on Improving Antibiotic Utilization in Outpatient and Inpatient Settings: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Giamarellou, H.; Galani, L.; Karavasilis, T.; Ioannidis, K.; Karaiskos, I. Antimicrobial Stewardship in the Hospital Setting: A Narrative Review. Antibiotics 2023, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
- Grae, N.; Singh, A.; Jowitt, D.; Flynn, A.; Mountier, E.; Clendon, G.; Barratt, R.; Gibson, B.; Williams, C.; Roberts, S.A.; et al. Prevalence of healthcare-associated infections in public hospitals in New Zealand, 2021. J. Hosp. Infect. 2023, 131, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Emerging Infections Program Hospital Prevalence Survey Team. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Russo, P.L.; Stewardson, A.J.; Cheng, A.C.; Bucknall, T.; Mitchell, B.G. The prevalence of healthcare associated infections among adult inpatients at nineteen large Australian acute-care public hospitals: A point prevalence survey. Antimicrob. Resist. Infect. Control 2019, 8, 114. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/PPS-HAI-AMR-acute-care-europe-2022-2023 (accessed on 29 August 2024).
- Maki, G.; Zervos, M. Health Care-Acquired Infections in Low- and Middle-Income Countries and the Role of Infection Prevention and Control. Infect. Dis. Clin. N. Am. 2021, 35, 827–839. [Google Scholar] [CrossRef]
- Semenova, Y.; Lim, L.; Salpynov, Z.; Gaipov, A.; Jakovljevic, M. Historical evolution of healthcare systems of post-soviet Russia, Belarus, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, Armenia, and Azerbaijan: A scoping review. Heliyon 2024, 10, e29550. [Google Scholar] [CrossRef]
- Zhussupova, G.; Utepova, D.; Orazova, G.; Zhaldybayeva, S.; Skvirskaya, G.; Tossekbayev, K. Evaluation of Antibiotic Use in Kazakhstan for the Period 2017–2019 Based on WHO Access, Watch and Reserve Classification (AWaRe 2019). Antibiotics 2021, 10, 58. [Google Scholar] [CrossRef]
- Balapasheva, A.A.; Smagulova, G.A.; Mussina, A.Z.; Ziganshina, L.E.; Nurgaliyeva, Z.Z. Pharmacoepidemiological Analysis of Antibacterial Agents Used in a Provisional Hospital in Aktobe, Kazakhstan, in the Context of COVID-19: A Comparison with the Pre-Pandemic Period. Antibiotics 2023, 12, 1596. [Google Scholar] [CrossRef]
- World Health Organization. GLASS Guide for National Surveillance Systems for Monitoring Antimicrobial Consumption in Hospitals; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-000042-1. [Google Scholar]
- Vi-ORTIS. Market Research Company. Available online: https://base.viortis.kz/Account/LogOn?ReturnUrl=%2f (accessed on 29 August 2024).
- Semenova, Y.; Kussainova, A.; Kassym, L.; Aimurziyeva, A.; Semenov, D.; Lim, L. Consumption Trends of Antifungal and Antiprotozoal Agents for Human Systemic Use in Kazakhstan from 2017 to 2023. Antibiotics 2024, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Institute of Public Health. ATC/DDD Index. Available online: https://atcddd.fhi.no/atc_ddd_index/?code=J&showdescription=yes (accessed on 29 August 2024).
- World Health Organization. AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 3 August 2024).
- Viderman, D.; Khamzina, Y.; Kaligozhin, Z.; Khudaibergenova, M.; Zhumadilov, A.; Crape, B.; Azizan, A. An observational case study of hospital associated infections in a critical care unit in Astana, Kazakhstan. Antimicrob. Resist. Infect. Control 2018, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Viderman, D.; Brotfain, E.; Khamzina, Y.; Kapanova, G.; Zhumadilov, A.; Poddighe, D. Bacterial resistance in the intensive care unit of developing countries: Report from a tertiary hospital in Kazakhstan. J. Glob. Antimicrob. Resist. 2019, 17, 35–38. [Google Scholar] [CrossRef]
- Antonelli, A.; Ales, M.E.; Chiecca, G.; Dalla Valle, Z.; De Ponti, E.; Cereda, D.; Crottogini, L.; Renzi, C.; Signorelli, C.; Moro, M. Healthcare-associated infections and antimicrobial use in acute care hospitals: A point prevalence survey in Lombardy, Italy, in 2022. BMC Infect. Dis. 2024, 24, 632. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Astrinaki, E.; Vitsaxaki, E.; Bolikas, E.; Christofaki, D.; Salvaraki, A.; Lagoudaki, E.; Ioannidou, E.; Karakonstantis, S.; Saplamidou, S.; et al. A Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in Public Acute Care Hospitals in Crete, Greece. Antibiotics 2022, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016, 4, VMBF-0022-2015. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Xavier, B.B.; Bielen, K.; Lammens, C.; Moons, P.; Schepens, T.; Ieven, M.; Jorens, P.G.; Goossens, H.; Kumar-Singh, S.; et al. The endotracheal tube microbiome associated with Pseudomonas aeruginosa or Staphylococcus epidermidis. Sci. Rep. 2016, 6, 36507. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018, 23, 1800516. [Google Scholar] [CrossRef]
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Abubakar, U.; Amir, O.; Rodríguez-Baño, J. Healthcare-associated infections in Africa: A systematic review and meta-analysis of point prevalence studies. J. Pharm. Policy Pract. 2022, 15, 99. [Google Scholar] [CrossRef]
- Abalkhail, A.; Alslamah, T. Institutional Factors Associated with Infection Prevention and Control Practices Globally during the Infectious Pandemics in Resource-Limited Settings. Vaccines 2022, 10, 1811. [Google Scholar] [CrossRef]
- Sydnor, E.R.; Perl, T.M. Hospital epidemiology and infection control in acute-care settings. Clin. Microbiol. Rev. 2011, 24, 141–173. [Google Scholar] [CrossRef]
- Makalkina, L.; Ikhambayeva, A.; Akhmadyar, N.; Kalieva, S.; Kuzekov, A. Analysis of consumption of system antimicrobial drugs in children’s hospitals for 2015–2017 in the Republic of Kazakhstan. Georgian Med. News 2020, 304–305, 111–116. [Google Scholar]
- Semenova, Y.; Kassym, L.; Kussainova, A.; Aimurziyeva, A.; Makalkina, L.; Avdeyev, A.; Yessmagambetova, A.; Smagul, M.; Aubakirova, B.; Akhmetova, Z.; et al. Knowledge, Attitudes, and Practices towards Antibiotics and Antimicrobial Resistance, and Antibiotics Consumption in the Population of Kazakhstan. Antibiotics 2024, 13, 718. [Google Scholar] [CrossRef]
- Semenova, Y.; Pivina, L.; Khismetova, Z.; Auyezova, A.; Nurbakyt, A.; Kauysheva, A.; Ospanova, D.; Kuziyeva, G.; Kushkarova, A.; Ivankov, A.; et al. Anticipating the Need for Healthcare Resources Following the Escalation of the COVID-19 Outbreak in the Republic of Kazakhstan. J. Prev. Med. Public Health 2020, 53, 387–396. [Google Scholar] [CrossRef]
- Ahmed, N.J.; Haseeb, A.; AlQarni, A.; AlGethamy, M.; Mahrous, A.J.; Alshehri, A.M.; Alahmari, A.K.; Almarzoky Abuhussain, S.S.; Mohammed Ashraf Bashawri, A.; Khan, A.H. Antibiotics for preventing infection at the surgical site: Single dose vs. multiple doses. Saudi Pharm. J. 2023, 31, 101800. [Google Scholar] [CrossRef]
- Porto, A.P.M.; Goossens, H.; Versporten, A.; Costa, S.F.; Brazilian Global-PPS Working Group. Global point prevalence survey of antimicrobial consumption in Brazilian hospitals. J. Hosp. Infect. 2020, 104, 165–171. [Google Scholar] [CrossRef]
- Panditrao, A.M.; Shafiq, N.; Chatterjee, S.; Pathak, A.; Trivedi, N.; Sadasivam, B.; Kshirsagar, N.; Kaul, R.; Biswal, M.; Kakkar, A.; et al. A multicentre point prevalence survey (PPS) of antimicrobial use amongst admitted patients in tertiary care centres in India. J. Antimicrob. Chemother. 2021, 76, 1094–1101. [Google Scholar] [CrossRef]
- Masterton, R.G. Antibiotic de-escalation. Crit. Care Clin. 2011, 27, 149–162. [Google Scholar] [CrossRef]
- National Center for Healthcare Development Named after Salidat Kairbekova. Statistical Compilation for 2022–2023. Available online: https://nrchd.kz/files/%D0%BD%D0%BE%D0%B2%D0%BE%D0%B5%202023/%D0%A1%D0%B1%D0%BE%D1%80%D0%BD%D0%B8%D0%BA_%D0%B7%D0%B0%202021%20-2022%20%D0%B3%D0%B3.%20%D0%BE%D0%BA..pdf (accessed on 4 October 2024).
- Jeon, C.Y.; Neidell, M.; Jia, H.; Sinisi, M.; Larson, E. On the role of length of stay in healthcare-associated bloodstream infection. Infect. Control Hosp. Epidemiol. 2012, 33, 1213–1218. [Google Scholar] [CrossRef]
| Characteristics | Antimicrobial Agents Not Used (n = 433) N (%) | Antimicrobial Agents Used (n = 268) N (%) | Test of Difference | |
|---|---|---|---|---|
| Age, years | Median (25th; 75th percentiles) | 32.0 (12.0; 61.0) | 42.0 (18.25; 61.0) | p = 0.038 * |
| Age group, years | <18 | 154 (35.6) | 64 (23.9) | p = 0.011 ** |
| 18–29 | 47 (10.9) | 26 (9.7) | ||
| 30–45 | 73 (16.9) | 60 (22.4) | ||
| 46–64 | 78 (18.0) | 67 (25.0) | ||
| 65–75 | 63 (14.5) | 36 (13.4) | ||
| >75 | 18 (4.2) | 15 (5.6) | ||
| Sex | Male | 172 (39.7) | 138 (51.5) | p = 0.03 ** |
| Female | 261 (60.3) | 130 (48.5) | ||
| Type of hospital ward | ICU | p < 0.001 ** | ||
| Mixed Intensive Care Ward | 3 (0.7) | 24 (9.0) | ||
| Other Intensive Care Ward | 3 (0.7) | 17 (6.3) | ||
| Medical Intensive Care Ward | 6 (1.4) | 16 (6.0) | ||
| Specialized Intensive Care Ward | 5 (1.2) | 6 (2.2) | ||
| Pediatric Intensive Care Ward | 0 (0.0) | 6 (2.2) | ||
| Medical | ||||
| Neonatology | 34 (7.9) | 12 (4.5) | ||
| Hematology | 18 (4.2) | 12 (4.5) | ||
| Other Medical Ward | 55 (12.7) | 12 (4.5) | ||
| Nephrology | 53 (12.2) | 7 (2.6) | ||
| Endocrinology | 32 (7.4) | 3 (1.1) | ||
| Rheumatology | 14 (3.2) | 1 (0.4) | ||
| Rehabilitation | 12 (2.8) | 0 (0.0) | ||
| Surgical | ||||
| Neurosurgery | 59 (13.7) | 27 (10.0) | ||
| Other Surgical Ward | 4 (0.9) | 27 (10.1) | ||
| Traumatology | 10 (2.3) | 23 (8.6) | ||
| Orthopedics | 33 (7.6) | 20 (7.4) | ||
| Gynecology | 17 (3.9) | 13 (4.9) | ||
| Urology | 17 (3.7) | 13 (4.9) | ||
| Burns | 6 (1.4) | 11 (4.1) | ||
| Vascular Surgery | 10 (2.3) | 9 (3.4) | ||
| Obstetrics/Maternity | 42 (9.7) | 9 (3.4) | ||
| Type of specialty | Medical | 218 (50.3) | 48 (17.9) | p < 0.001 ** |
| Surgical | 198 (45.7) | 151 (56.3) | ||
| Intensive Care | 17 (3.9) | 69 (25.7) | ||
| Characteristics | Children (n = 64) N (%) | Adults (n = 204) N (%) | Test of Difference | |
|---|---|---|---|---|
| Sex | Male | 33 (51.6) | 99 (48.5) | p = 0.672 * |
| Female | 31 (48.4) | 105 (51.5) | ||
| Type of specialty | Medical | 21 (32.8) | 27 (13.2) | p < 0.001 * |
| Surgical | 20 (31.3) | 131 (64.2) | ||
| Intensive Care | 23 (35.9) | 46 (22.5) | ||
| Antimicrobial agent (up to 3 agents were administered simultaneously) | Beta-lactams | p < 0.001 * | ||
| Piperacillin and tazobactam | 7 (8.0) | 5 (1.8) | ||
| Amoxicillin and clavulanic acid | 0 (0.0) | 5 (1.8) | ||
| Tazobactam | 4 (4.5) | 0 (0.0) | ||
| Aminoglycosides | ||||
| Gentamicin | 13 (14.8) | 3 (1.1) | ||
| Amikacin | 4 (4.5) | 1 (0.4) | ||
| Fluoroquinolons | ||||
| Ciprofloxacin | 6 (6.8) | 40 (14.3) | ||
| Levofloxacin | 0 (0.0) | 18 (6.5) | ||
| Ofloxacin | 0 (0.0) | 2 (0.7) | ||
| Moxifloxacin | 0 (0.0) | 1 (0.4) | ||
| Penicillins | ||||
| Ampicillin | 12 (13.7) | 3 (1.1) | ||
| Amoxicillin | 1 (1.1) | 1 (0.4) | ||
| Cephalosporins | ||||
| Ceftriaxone | 9 (10.2) | 99 (35.6) | ||
| Cefazolin | 14 (16.0) | 40 (14.3) | ||
| Cefotaxime | 1 (1.1) | 8 (2.8) | ||
| Cefepime | 1 (1.1) | 4 (1.4) | ||
| Ceftazidime | 2 (2.3) | 3 (1.1) | ||
| Cefuroxime | 2 (2.3) | 3 (1.1) | ||
| Ceftriaxone, combinations | 1 (1.1) | 3 (1.1) | ||
| Cefatrizine | 1 (1.1) | 0 (0.0) | ||
| Cefazedone | 0 (0.0) | 1 (0.4) | ||
| Carbapenems | ||||
| Meropenem | 7 (8.0) | 0 (0.0) | ||
| Imipenem and cilastatin | 0 (0.0) | 3 (1.1) | ||
| Sulfonamides | ||||
| Sulfamethoxazole and trimethoprim | 1 (1.1) | 1 (0.4) | ||
| Sulfamethoxazole | 0 (0.0) | 1 (0.4) | ||
| Macrolides | ||||
| Spiramycin | 0 (0.0) | 1 (0.4) | ||
| Lincosamides | ||||
| Lincomycin | 0 (0.0) | 1 (0.4) | ||
| Glycopeptides | ||||
| Vancomycin (parenteral) | 0 (0.0) | 2 (0.7) | ||
| Polymyxins | ||||
| Colistin (injection, infusion) | 1 (1.1) | 2 (0.7) | ||
| Azoles | ||||
| Fluconazole | 1 (1.1) | 12 (4.3) | ||
| Metronidazole (oral, rectal) | 0 (0.0) | 1 (0.4) | ||
| Metronidazole (parenteral) | 0 (0.0) | 14 (5.0) | ||
| Route of administration | Oral | 6 (6.7) | 9 (3.2) | p = 0.403 ** |
| Parenteral | 83 (93.3) | 269 (96.4) | ||
| Rectal | 0 (0.0) | 1 (0.4) | ||
| Indication | Treatment intention for community-acquired infection | 11 (12.4) | 70 (25.1) | p < 0.001 * |
| Treatment intention for infection acquired in long-term care facility or chronic care hospital | 5 (5.6) | 0 (0.0) | ||
| Treatment intention for acute hospital-acquired infection | 10 (11.2) | 39 (14.0) | ||
| Surgical prophylaxis, single dose | 2 (2.2) | 12 (4.3) | ||
| Surgical prophylaxis, one day | 2 (2.2) | 2 (0.7) | ||
| Surgical prophylaxis, > 1 day | 21 (23.6) | 99 (35.5) | ||
| Medical prophylaxis | 32 (35.9) | 49 (17.6) | ||
| Unknown indication | 1 (1.1) | 8 (2.9) | ||
| Other indication | 5 (5.6) | 0 (0.0) | ||
| Diagnoses for which antimicrobial agents were administered | Not applicable; for antimicrobial use other than treatment | 63 (72.4) | 170 (61.6) | p < 0.001 * |
| Pneumonia | 7 (8.0) | 17 (6.2) | ||
| Surgical site infection involving skin or soft tissue but not bone | 0 (0.0) | 19 (6.9) | ||
| Intra-abdominal sepsis including hepatobiliary | 0 (0.0) | 16 (5.8) | ||
| Completely undefined, site with no systemic inflammation | 6 (6.9) | 10 (3.6) | ||
| Cellulitis, wound, deep soft tissue not involving bone, not related to surgery | 0 (0.0) | 14 (5.1) | ||
| Clinical sepsis | 6 (6.9) | 2 (0.7) | ||
| Gastrointestinal Infections (salmonellosis, antibiotic associated diarrhea) | 0 (0.0) | 6 (2.2) | ||
| Lab-confirmed bacteraemia | 0 (0.0) | 5 (1.8) | ||
| Acute bronchitis or exacerberations of chronic bronchitis | 0 (0.0) | 5 (1.8) | ||
| Symptomatic lower urinary tract infection | 2 (2.2) | 3 (1.1) | ||
| Systematic inflammatory response with no clear anatomic site | 2 (2.2) | 2 (0.7) | ||
| Asymptomatic bactreriuria | 0 (0.0) | 4 (1.4) | ||
| Septic arthritis, osteomyelitis, not related to surgery | 0 (0.0) | 2 (0.7) | ||
| Infections of ear, mouth, nose, throat or larynx | 1 (1.1) | 0 (0.0) | ||
| Infections of the central nervous system | 0 (0.0) | 1 (0.3) | ||
| Reasoning notes | No | 3 (3.4) | 166 (66.4) | p < 0.001 * |
| Yes | 85 (95.5) | 82 (32.8) | ||
| Unknown | 1 (1.1) | 2 (0.8) | ||
| Antimicrobial Changed | No | 60 (93.8) | 173 (85.2) | p = 0.013 * |
| Escalation | 2 (3.1) | 29 (14.3) | ||
| De-escalation | 2 (3.1) | 1 (0.5) | ||
| Characteristics | HAI * Is Absent (n = 674) N (%) | HAI * Is Present (n = 27) N (%) | Test of Difference | |
|---|---|---|---|---|
| Age, years | Median (25th; 75th percentiles) | 37.0 (14.0; 61.0) | 42.0 (18.0; 68.0) | p = 0.305 ** |
| Age group, years | <18 | 213 (31.6) | 5 (18.5) | p = 0.047 *** |
| 18–29 | 67 (9.9) | 6 (22.2) | ||
| 30–45 | 130 (19.3) | 3 (11.1) | ||
| 46–64 | 139 (20.6) | 6 (22.2) | ||
| 65–75 | 86 (12.8) | 7 (25.9) | ||
| >75 | 39 (5.8) | 0 (0.0) | ||
| Sex | Male | 295 (43.8) | 15 (55.6) | p = 0.220 *** |
| Female | 379 (56.2) | 12 (44.4) | ||
| Hospital ward | ICU | p < 0.001 *** | ||
| Other Intensive Care Ward | 15 (2.2) | 5 (18.6) | ||
| Mixed Intensive Care Ward | 23 (3.4) | 4 (14.8) | ||
| Pediatric Intensive Care Ward | 3 (0.4) | 3 (11.1) | ||
| Medical Intensive Care Ward | 21 (3.1) | 1 (3.7) | ||
| Specialized Intensive Care Ward | 11 (1.6) | 0 (0.0) | ||
| Medical | ||||
| Other Medical Ward | 64 (9.5) | 3 (11.1) | ||
| Endocrinology | 35 (5.2) | 0 (0.0) | ||
| Gynecology | 30 (4.5) | 0 (0.0) | ||
| Hematology | 30 (4.5) | 0 (0.0) | ||
| Neonatology | 46 (6.8) | 0 (0.0) | ||
| Nephrology | 60 (8.9) | 0 (0.0) | ||
| Rehabilitation | 12 (1.8) | 0 (0.0) | ||
| Rheumatology | 15 (2.2) | 0 (0.0) | ||
| Surgical | ||||
| Other surgical | 27 (4.0) | 4 (14.8) | ||
| Burns | 15 (2.2) | 2 (7.4) | ||
| Neurosurgery | 84 (12.4) | 2 (7.4) | ||
| Urology | 28 (4.2) | 2 (7.4) | ||
| Obstetrics/Maternity | 50 (7.4) | 1 (3.7) | ||
| Orthopedics | 53 (7.9) | 0 (0.0) | ||
| Traumatology | 33 (4.9) | 0 (0.0) | ||
| Vascular Surgery | 19 (2.8) | 0 (0.0) | ||
| Type of specialty | Medical | 263 (39.1) | 3 (11.1) | p < 0.001 *** |
| Surgical | 338 (50.1) | 11 (40.7) | ||
| Intensive Care | 73 (10.8) | 13 (48.1) | ||
| Characteristics | Type of Ward | Test of Difference | |||
|---|---|---|---|---|---|
| Medical (n = 3), N (%) | Surgical (n = 11), N (%) | Intensive Care (n = 13), N (%) | |||
| Sex | Male | 2 (66.7) | 6 (54.5) | 7 (53.8) | p = 0.919 * |
| Female | 1 (33.3) | 5 (45.5) | 6 (46.2) | ||
| Age group, years | <18 | 0 (0.0) | 1 (9.1) | 4 (30.8) | p = 0.571 * |
| 18–29 | 0 (0.0) | 3 (27.3) | 3 (23.1) | ||
| 30–45 | 0 (0.0) | 2 (18.2) | 1 (7.7) | ||
| 46–64 | 1 (33.3) | 3 (27.3) | 2 (15.4) | ||
| 65–74 | 2 (66.7) | 2 (18.2) | 3 (23.1) | ||
| Infection site | Surgical site infection | 0 (0.0) | 5 (45.5) | 3 (23.1) | p = 0.045 * |
| Pneumonia | 2 (66.7) | 0 (0.0) | 5 (38.5) | ||
| Mild/moderate COVID-19 | 0 (0.0) | 3 (27.3) | 0 (0.0) | ||
| Urinary tract infection | 0 (0.0) | 1 (9.1) | 2 (15.4) | ||
| Blood stream infection | 0 (0.0) | 0 (0.0) | 1 (7.7) | ||
| Sinusitis | 0 (0.0) | 0 (0.0) | 2 (15.4) | ||
| Gastroenteritis | 0 (0.0) | 1 (9.1) | 0 (0.0) | ||
| Reproductive tract infections | 1 (33.3) | 0 (0.0) | 0 (0.0) | ||
| Skin infections | 0 (0.0) | 1 (9.1) | 0 (0.0) | ||
| Infection present at admission | Yes | 0 (0.0) | 3 (27.3) | 3 (23.1) | p = 0.589 * |
| No | 3 (100.0) | 8 (72.7) | 10 (76.9) | ||
| Origin of the infection | Current hospital | 3 (100.0) | 10 (90.9) | 9 (69.2) | p = 0.521 * |
| Other acute care hospital | 0 (0.0) | 1 (9.1) | 2 (15.4) | ||
| Long-term care facility | 0 (0.0) | 0 (0.0) | 2 (15.4) | ||
| Microorganism | Acinetobacter baumannii | 0 (0.0) | 1 (9.1) | 0 (0.0) | p = 0.161 ** |
| Enterobacter aerogenes | 0 (0.0) | 1 (9.1) | 0 (0.0) | ||
| Enterococcus faecalis | 0 (0.0) | 0 (0.0) | 2 (15.4) | ||
| Escherichia coli | 0 (0.0) | 1 (9.1) | 0 (0.0) | ||
| Pseudomonas aeruginosa | 0 (0.0) | 1 (9.1) | 4 (30.8) | ||
| Staphylococcus aureus | 0 (0.0) | 1 (9.1) | 0 (0.0) | ||
| Staphylococcus epidermidis | 0 (0.0) | 0 (0.0) | 1 (7.7) | ||
| Other coagulase-negative staphylococci | 0 (0.0) | 0 (0.0) | 1 (7.7) | ||
| Streptococcus pyogenesis | 0 (0.0) | 1 (9.1) | 0 (0.0) | ||
| Not available | 3 (100.0) | 5 (45.5) | 5 (38.5) | ||
| Antimicrobial resistance phenotype | C3G (Third-generation cephalosporins) | 0 (0.0) | 2 (18.2) | 0 (0.0) | p = 0.715 ** |
| CAR (Carbapenems) | 0 (0.0) | 4 (36.4) | 4 (30.9) | ||
| GLY (Glycopeptides) | 0 (0.0) | 1 (9.1) | 2 (15.4) | ||
| OXA (Oxacillin) | 0 (0.0) | 1 (9.1) | 0 (0.0) | ||
| Not available | 3 (100.0) | 3 (27.3) | 7 (53.8) | ||
| ATC5 Code | Substance | Pharmacological Group | AWaRe * Category | Number of Packages | DDD ** | DDD/100 Patient-Days |
|---|---|---|---|---|---|---|
| J01AA02 | Doxycycline | Tetracyclines | Access | 19 | 190 | 0.13347 |
| J01CA01 | Ampicillin | Penicillins | 10,350 | 1279.16667 | 0.89855 | |
| J01CA04 | Amoxicillin | 86 | 624.666667 | 0.43880 | ||
| J01CE01 | Benzylpenicillin | 7100 | 1183.33333 | 0.83123 | ||
| J01CR02 | Amoxicillin and beta-lactamase inhibitor | Beta-lactam | 28,179 | 5317.66667 | 3.73539 | |
| J01CR05 | Piperacillin and beta-lactamase inhibitor | Watch | 14,514 | 4187.92857 | 2.68475 | |
| J01DB04 | Cefazolin | First-generation cephalosporins | Access | 79,540 | 2,6013.3333 | 18.27305 |
| J01DC02 | Cefuroxime | Second-generation cephalosporins | Watch | 11,360 | 4240 | 2.97839 |
| J01DD01 | Cefotaxime | Third-generation cephalosporins | 3700 | 925 | 0.64977 | |
| J01DD02 | Ceftazidime | 23,840 | 5497.5 | 3.86172 | ||
| J01DD04 | Ceftriaxone | 217,380 | 10,7940 | 75.82239 | ||
| J01DD08 | Cefixime | 50 | 12.5 | 0.00878 | ||
| J01DD12 | Cefoperazone | 1600 | 400 | 0.28098 | ||
| J01DD52 | Ceftazidime and beta-lactamase inhibitor | Reserve | 3 | 10 | 0.00007 | |
| J01DD62 | Cefoperazone and beta-lactamase inhibitor | 2900 | 725 | 0.50928 | ||
| J01DE01 | Cefepime | Fourth generation cephalosporins | Watch | 7200 | 1725 | 1.21173 |
| J01DH02 | Meropenem | Carbapenems | 12,528 | 3942.66667 | 2.76952 | |
| J01DH03 | Ertapenem | 630 | 630 | 0.44254 | ||
| J01DH04 | Doripenem | 2152 | 717.333333 | 0.50389 | ||
| J01DH51 | Imipenem and cilastatin | 3308 | 827 | 0.58093 | ||
| J01EE01 | Sulfamethoxazole and trimethoprim | Sulfonamide-trimethoprim combinations | Access | 2397 | 2313.9 | 1.62540 |
| J01FA09 | Clarithromycin | Macrolides | Watch | 78 | 896 | 0.62939 |
| J01FA10 | Azithromycin | 208 | 157.333333 | 0.11052 | ||
| J01GB01 | Tobramycin | Aminoglycosides | 17 | 85.6 | 0.06013 | |
| J01GB03 | Gentamicin | Access | 1378 | 22,966.6667 | 16.13292 | |
| J01GB04 | Kanamycin | Watch | 50 | 50 | 0.03512 | |
| J01GB06 | Amikacin | Access | 5815 | 4209 | 2.95661 | |
| J01MA01 | Ofloxacin | Fluoroquinolons | Watch | 1820 | 910 | 0.63923 |
| J01MA02 | Ciprofloxacin | 36,201 | 9501 | 6.67397 | ||
| J01MA12 | Levofloxacin | 10,703 | 10,737.5 | 7.54255 | ||
| J01MA14 | Moxifloxacin | 857 | 857 | 0.60200 | ||
| J01XA01 | Vancomycin | Glycopeptides | 4340 | 2170 | 1.52432 | |
| J01XB01 | Colistin | Polymyxins | Reserve | 495 | 550 | 0.38635 |
| J01XD01 | Metronidazole | Imidazoles | Access | 29,835 | 9977.08333 | 7.00840 |
| J01XE01 | Nitrofurantoin | Nitrofuran derivates | 70 | 175 | 0.12293 | |
| J01XX08 | Linezolid | Oxazolidinones | Reserve | 602 | 260.966667 | 0.18332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, Y.; Yessmagambetova, A.; Akhmetova, Z.; Smagul, M.; Zharylkassynova, A.; Aubakirova, B.; Soiak, K.; Kosherova, Z.; Aimurziyeva, A.; Makalkina, L.; et al. Point-Prevalence Survey of Antimicrobial Use and Healthcare-Associated Infections in Four Acute Care Hospitals in Kazakhstan. Antibiotics 2024, 13, 981. https://doi.org/10.3390/antibiotics13100981
Semenova Y, Yessmagambetova A, Akhmetova Z, Smagul M, Zharylkassynova A, Aubakirova B, Soiak K, Kosherova Z, Aimurziyeva A, Makalkina L, et al. Point-Prevalence Survey of Antimicrobial Use and Healthcare-Associated Infections in Four Acute Care Hospitals in Kazakhstan. Antibiotics. 2024; 13(10):981. https://doi.org/10.3390/antibiotics13100981
Chicago/Turabian StyleSemenova, Yuliya, Aizhan Yessmagambetova, Zaure Akhmetova, Manar Smagul, Akniyet Zharylkassynova, Bibigul Aubakirova, Kateryna Soiak, Zhanar Kosherova, Ainur Aimurziyeva, Larissa Makalkina, and et al. 2024. "Point-Prevalence Survey of Antimicrobial Use and Healthcare-Associated Infections in Four Acute Care Hospitals in Kazakhstan" Antibiotics 13, no. 10: 981. https://doi.org/10.3390/antibiotics13100981
APA StyleSemenova, Y., Yessmagambetova, A., Akhmetova, Z., Smagul, M., Zharylkassynova, A., Aubakirova, B., Soiak, K., Kosherova, Z., Aimurziyeva, A., Makalkina, L., Ikhambayeva, A., & Lim, L. (2024). Point-Prevalence Survey of Antimicrobial Use and Healthcare-Associated Infections in Four Acute Care Hospitals in Kazakhstan. Antibiotics, 13(10), 981. https://doi.org/10.3390/antibiotics13100981








