Characterization of Human Breast Milk-Derived Limosilactobacillus reuteri MBHC 10138 with Respect to Purine Degradation, Anti-Biofilm, and Anti-Lipid Accumulation Activities
Abstract
1. Introduction
2. Results
2.1. L. reuteri MBHC 10138 Identification
2.2. Safety Assessment and Probiotic Characterization of L. reuteri MBHC 10138
2.2.1. L. reuteri MBHC 10138 Biogenic Amine Production
2.2.2. Hemolytic Activity
2.2.3. Bile Salt Deconjugation
2.2.4. D-Lactate Production
2.2.5. Antibiotic Susceptibility
2.2.6. Tolerance to Oral–Gastric–Intestinal Transit Stress
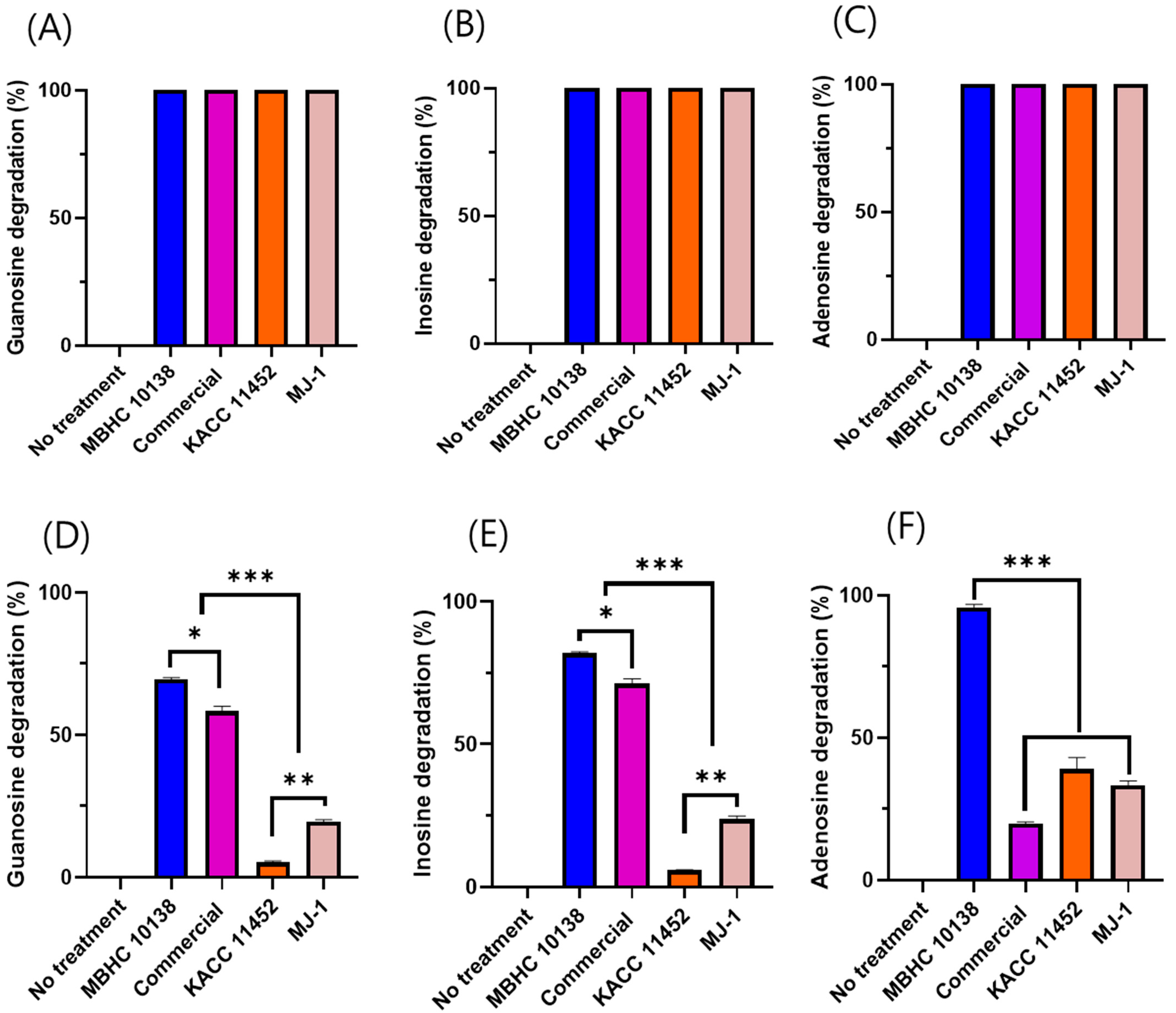
2.3. Nucleoside Degradation of L. reuteri Strains
2.4. Streptococcus mutans KCTC3065 Biofilm Inhibition
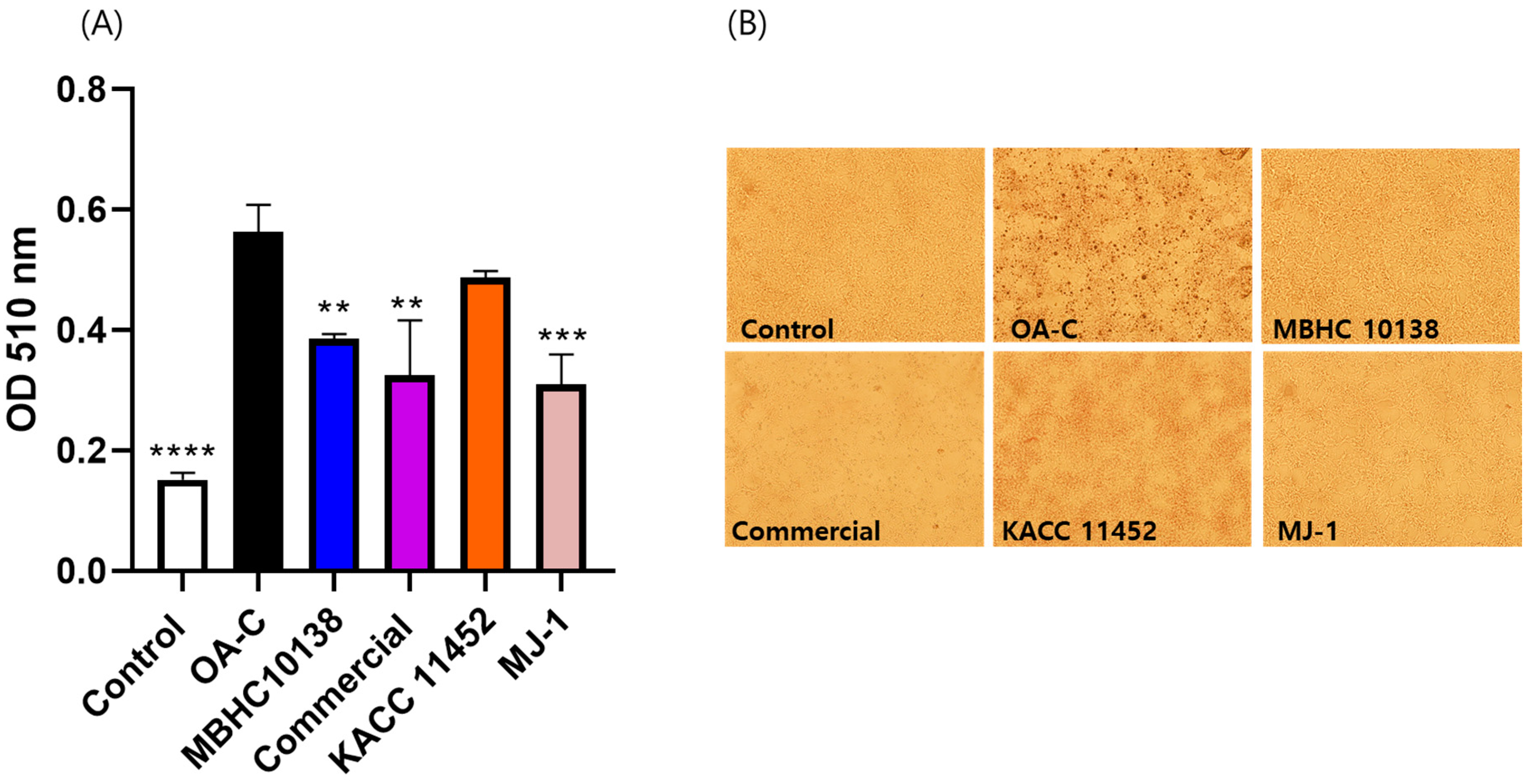
2.5. Effect of L. reuteri Strains on Lipid Accumulation in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Strains and Reagents
4.2. Identification of MBHC 10138 Strain by 16S rDNA Sequencing and Phylogenetic Analysis
4.3. Safety Assessment and Probiotic Characterization
4.3.1. Biogenic Amine Production Analysis
4.3.2. Hemolytic Activity Analysis
4.3.3. D-Lactic Acid Production Test
4.3.4. Bile Salt Deconjugation Test
4.3.5. Antibiotic Susceptibility Test
4.3.6. Oral–Gastric–Intestinal Transit Assay
4.4. Purine Degradation by L. reuteri MBHC 10138 and Related Strains
4.4.1. Purine Degradation Assay
4.4.2. HPLC Analysis
4.5. Analysis of Inhibitory Activity against Streptococcus Mutans KCTC3065 Biofilm
4.6. Assay of L. reuteri Strains on Lipid Accumulation in HepG2 Cells
4.6.1. Cell Culture
4.6.2. Sample Treatment
4.6.3. Staining and Quantification of Lipid Accumulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Alessandro, M.; Parolin, C.; Patrignani, S.; Sottile, G.; Antonazzo, P.; Vitali, B.; Lanciotti, R.; Patrignani, F. Human Breast Milk: A Source of Potential Probiotic Candidates. Microorganisms 2022, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, N.; Wang, C.; Zhao, Y.; Cao, J.; Li, X.; Zhang, Z.; Li, Y.; Yang, X.; Wang, X.; et al. Gut Microbiota and Immune Modulatory Properties of Human Breast Milk Streptococcus salivarius and S. Parasanguinis Strains. Front. Nutr. 2022, 9, 798403. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Yun, S.-I.; Park, M.-H.; Park, J.-H.; Jeong, S.-Y.; Park, H.-O. Anti-Obesity Effect of Lactobacillus gasseri BNR17 in High-Sucrose Diet-Induced Obese Mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef]
- Keddar, K.; Ziar, H.; Belmadani, N.; Monnoye, M.; Gérard, P.; Riazi, A. Probiotic Bacteria from Human Milk Can Alleviate Oral Bovine Casein Sensitization in Juvenile Wistar Rats. Microorganisms 2023, 11, 1030. [Google Scholar] [CrossRef]
- Notarbartolo, V.; Giuffrè, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194–210. [Google Scholar] [CrossRef]
- Indrio, F.; Di Mauro, A.; Riezzo, G.; Civardi, E.; Intini, C.; Corvaglia, L.; Ballardini, E.; Bisceglia, M.; Cinquetti, M.; Brazzoduro, E.; et al. Prophylactic Use of a Probiotic in the Prevention of Colic, Regurgitation, and Functional Constipation: A Randomized Clinical Trial. JAMA Pediatr. 2014, 168, 228–233. [Google Scholar] [CrossRef]
- Maldonado, J.; Cañabate, F.; Sempere, L.; Vela, F.; Sánchez, A.R.; Narbona, E.; López-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M.; et al. Human Milk Probiotic Lactobacillus fermentum CECT5716 Reduces the Incidence of Gastrointestinal and Upper Respiratory Tract Infections in Infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.; Blaser, M. Breast Milk, Formula, the Microbiome and Overweight. Nat. Rev. Endocrinol. 2018, 14, 510–511. [Google Scholar] [CrossRef]
- Jeong, H.; Moon, J.E.; Jeon, C.H. Hyperuricemia Is Associated with an Increased Prevalence of Metabolic Syndrome in a General Population and a Decreased Prevalence of Diabetes in Men. J. Rheum. Dis. 2020, 27, 247–260. [Google Scholar] [CrossRef]
- Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients 2020, 12, 3835. [Google Scholar] [CrossRef]
- James, A.; Ke, H.; Yao, T.; Wang, Y. The Role of Probiotics in Purine Metabolism, Hyperuricemia and Gout: Mechanisms and Interventions. Food Rev. Int. 2023, 39, 261–277. [Google Scholar] [CrossRef]
- Näse, L.; Hatakka, K.; Savilahti, E.; Saxelin, M.; Pönkä, A.; Poussa, T.; Korpela, R.; Meurman, J.H. Effect of Long-Term Consumption of a Probiotic Bacterium, Lactobacillus rhamnosus GG, in Milk on Dental Caries and Caries Risk in Children. Caries Res. 2001, 36, 412–420. Available online: https://pubmed.ncbi.nlm.nih.gov/11799281/ (accessed on 2 October 2024).
- Panchbhai, A.S.; Khatib, M.N.; Borle, R.M.; Deolia, S.S.; Babar, V.M.; Vasistha, A.H.; Parida, R.P. Efficacy and Safety of Probiotics for Dental Caries in Preschool Children: A Systematic Review and Meta-Analysis. Contemp. Clin. Dent. 2024, 15, 10–16. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11068249/ (accessed on 2 October 2024).
- Meurman, J.H.; Stamatova, I. Probiotics: Contributions to Oral Health. Oral Dis. 2007, 13, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Chalita, M.; Kim, Y.O.; Park, S.; Oh, H.-S.; Cho, J.H.; Moon, J.; Baek, N.; Moon, C.; Lee, K.; Yang, J.; et al. EzBioCloud: A Genome-Driven Database and Platform for Microbiome Identification and Discovery. Int. J. Syst. Evol. Microbiol. 2024, 74, 006421. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Carneiro, B.M.; Todorov, S.D.; Nero, L.A.; Rahal, P.; Penna, A.L.B. In Vitro Assessment of Safety and Probiotic Potential Characteristics of Lactobacillus Strains Isolated from Water Buffalo Mozzarella Cheese. Ann. Microbiol. 2017, 67, 289–301. [Google Scholar] [CrossRef]
- Li, F.; Cheng, C.C.; Zheng, J.; Liu, J.; Quevedo, R.M.; Li, J.; Roos, S.; Gänzle, M.G.; Walter, J. Limosilactobacillus balticus sp. Nov., Limosilactobacillus agrestis sp. Nov., Limosilactobacillus albertensis sp. Nov., Limosilactobacillus rudii sp. Nov. and Limosilactobacillus fastidiosus sp. Nov., Five Novel Limosilactobacillus Species Isolated from the Vertebrate Gastrointestinal Tract, and Proposal of Six Subspecies of Limosilactobacillus reuteri Adapted to the Gastrointestinal Tract of Specific Vertebrate Hosts. Int. J. Syst. Evol. Microbiol. 2021, 71, 004644. [Google Scholar] [CrossRef]
- Sinkiewicz, G.; Ljunggren, L. Occurrence of Lactobacillus reuteri in Human Breast Milk. Microb. Ecol. Health Dis. 2008, 20, 122–126. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and Histamine Intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Ladero, V.; Calles-Enriquez, M.; Fernandez, M.; Alvarez, M.A. Toxicological Effects of Dietary Biogenic Amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhou, W.; Hu, D.; Xu, H.; Ji, G. Secondary Bile Acids and Tumorigenesis in Colorectal Cancer. Front. Oncol. 2022, 12, 813745. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic Resistance in Probiotic Bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-W.; Hsieh, S.-H.; Chen, J.-F.; Liu, C.-R.; Chen, C.-W.; Huang, Y.-F.; Ho, H.-H. Lactobacillus reuteri TSR332 and Lactobacillus fermentum TSF331 Stabilize Serum Uric Acid Levels and Prevent Hyperuricemia in Rats. PeerJ 2021, 9, e11209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular Mechanisms of Inhibiting Glucosyltransferases for Biofilm Formation in Streptococcus mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; El-Rahman, O.A.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillus sp. Inhibit Growth, Biofilm Formation and Gene Expression of Caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef]
- Burton, J.P.; Chilcott, C.N.; Moore, C.J.; Speiser, G.; Tagg, J.R. A Preliminary Study of the Effect of Probiotic Streptococcus salivarius K12 on Oral Malodour Parameters. J. Appl. Microbiol. 2006, 100, 754–764. [Google Scholar] [CrossRef]
- Allaker, R.P.; Stephen, A.S. Use of Probiotics and Oral Health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef]
- Lee, M.; Park, J.; Kim, O.-K.; Kim, D.; Han, M.J.; Kim, S.H.; Kim, T.H.; Lee, J. Lactobacillus reuteri NCIMB 30242 (LRC) Inhibits Cholesterol Synthesis and Stimulates Cholesterol Excretion in Animal and Cell Models. J. Med. Food 2023, 26, 529–539. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lin, C.-H.; Kuo, Y.-W.; Liao, C.-A.; Chen, J.-F.; Tsai, S.-Y.; Li, C.-M.; Hsu, Y.-C.; Huang, Y.-Y.; Hsia, K.-C.; et al. Probiotic Lactobacillus fermentum TSF331, Lactobacillus reuteri TSR332, and Lactobacillus plantarum TSP05 Improved Liver Function and Uric Acid Management—A Pilot Study. PLoS ONE 2024, 19, e0307181. [Google Scholar] [CrossRef]
- Jeong, H.W.; Cho, D.; Roh, J.; Kim, W. Heat-Killed Lactiplantibacillus plantarum APSulloc 331261 Enhances Lipid Catabolism Through AMPK Activation in HepG2 Cells. Food Suppl. Biomater. Health 2022, 2, e30. [Google Scholar] [CrossRef]
- Kang, S.G.; Choi, Y.Y.; Mo, S.J.; Kim, T.H.; Ha, J.H.; Hong, D.K.; Lee, H.; Park, S.D.; Shim, J.-J.; Lee, J.-L.; et al. Effect of Gut Microbiome-Derived Metabolites and Extracellular Vesicles on Hepatocyte Functions in a Gut-Liver Axis Chip. Nano Converg. 2023, 10, 5. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Gu, M.; Werlinger, P.; Cho, J.-H.; Cheng, J.; Suh, J.-W. Lactobacillus sakei MJM60958 as a Potential Probiotic Alleviated Non-Alcoholic Fatty Liver Disease in Mice Fed a High-Fat Diet by Modulating Lipid Metabolism, Inflammation, and Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 13436. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Holzapfel, W.H. Improved Screening Procedure for Biogenic Amine Production by Lactic Acid Bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, S.A.; Damodharan, K.; Suh, J.-W.; Yang, S.H. In Vitro Characterization of Lactobacillus plantarum Strains with Inhibitory Activity on Enteropathogens for Use as Potential Animal Probiotics. Indian J. Microbiol. 2017, 57, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.B.; Viale, S.; Conti, S.; Fadda, M.E.; Deplano, M.; Melis, M.P.; Deiana, M.; Cosentino, S. Preliminary Evaluation of Probiotic Properties of Lactobacillus Strains Isolated from Sardinian Dairy Products. BioMed Res. Int. 2014, 2014, 286390. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Werlinger, P.; Nguyen, H.T.; Gu, M.; Cho, J.-H.; Cheng, J.; Suh, J.-W. Lactobacillus reuteri MJM60668 Prevent Progression of Non-Alcoholic Fatty Liver Disease through Anti-Adipogenesis and Anti-Inflammatory Pathway. Microorganisms 2022, 10, 2203. [Google Scholar] [CrossRef]
- Lee, Y.; Werlinger, P.; Suh, J.-W.; Cheng, J. Potential Probiotic Lacticaseibacillus paracasei MJM60396 Prevents Hyperuricemia in a Multiple Way by Absorbing Purine, Suppressing Xanthine Oxidase and Regulating Urate Excretion in Mice. Microorganisms 2022, 10, 851. [Google Scholar] [CrossRef]
- Gu, M.; Werlinger, P.; Cho, J.-H.; Jang, N.; Choi, S.S.; Suh, J.-W.; Cheng, J. Lactobacillus pentosus MJM60383 Inhibits Lipid Accumulation in Caenorhabditis Elegans Induced by Enterobacter Cloacae and Glucose. Int. J. Mol. Sci. 2022, 24, 280. [Google Scholar] [CrossRef]
- Sikkeland, J.; Jin, Y.; Saatcioglu, F. Methods to Assess Lipid Accumulation in Cancer Cells. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 542, pp. 407–423. ISBN 978-0-12-416618-9. [Google Scholar]


| Safety Test | L. reuteri MBHC 10138 | L. rhamnosus GG |
|---|---|---|
| Hemolytic activity | - | - |
| D-lactate production (g/L) | 18.39 | 23.11 |
| Bile salt deconjugation | - | - |
| Bioamine production | ||
| L-Histidine | - | - |
| L-Tyrosine | - | - |
| L-Phenylalanine | - | - |
| Arginine | - | - |
| Tryptophan | - | - |
| L-Ornithine | - | - |
| Antibiotic susceptibility to | ||
| Ampicillin | 32 (R) | 1 |
| Clindamycin | 0.25 | 1 |
| Chloramphenicol | 4 | 4 |
| Erythromycin | 1 | 1 |
| Gentamycin | 256 (R) | 32 (R) |
| Kanamycin | >256 (R) | R |
| Streptomycin | 128 (R) | 32 (R) |
| Tetracycline | 16 | 1 |
| OGI Transit (Log10 CFU/mL) | L. reuteri MBHC 10138 | L. rhamnosus GG |
|---|---|---|
| Initial | 9.16 ± 0.021 | 9.21 ± 0.023 |
| Oral stress | 9.14 ± 0.048 | 9.21 ± 0.006 |
| Gastric stress (pH3) | 9.11 ± 0.04 | 9.17 ± 0.103 |
| Gastric stress (pH2) | 9.04 ± 0.030 | 9.01 ± 0.035 * |
| Intestinal stress | 8.58 ± 0.050 **** | 7.98 ± 0.045 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Cho, J.-H.; Suh, J.-W. Characterization of Human Breast Milk-Derived Limosilactobacillus reuteri MBHC 10138 with Respect to Purine Degradation, Anti-Biofilm, and Anti-Lipid Accumulation Activities. Antibiotics 2024, 13, 964. https://doi.org/10.3390/antibiotics13100964
Cheng J, Cho J-H, Suh J-W. Characterization of Human Breast Milk-Derived Limosilactobacillus reuteri MBHC 10138 with Respect to Purine Degradation, Anti-Biofilm, and Anti-Lipid Accumulation Activities. Antibiotics. 2024; 13(10):964. https://doi.org/10.3390/antibiotics13100964
Chicago/Turabian StyleCheng, Jinhua, Joo-Hyung Cho, and Joo-Won Suh. 2024. "Characterization of Human Breast Milk-Derived Limosilactobacillus reuteri MBHC 10138 with Respect to Purine Degradation, Anti-Biofilm, and Anti-Lipid Accumulation Activities" Antibiotics 13, no. 10: 964. https://doi.org/10.3390/antibiotics13100964
APA StyleCheng, J., Cho, J.-H., & Suh, J.-W. (2024). Characterization of Human Breast Milk-Derived Limosilactobacillus reuteri MBHC 10138 with Respect to Purine Degradation, Anti-Biofilm, and Anti-Lipid Accumulation Activities. Antibiotics, 13(10), 964. https://doi.org/10.3390/antibiotics13100964






