Prevalence and Molecular Epidemiology of Intestinal Colonization by Multidrug-Resistant Bacteria among Hematopoietic Stem-Cell Transplantation Recipients: A Bulgarian Single-Center Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Characteristics
4.2. Fecal Screening
4.3. Species Identification
4.4. Antimicrobial Susceptibility Testing
4.5. Molecular-Genetic Experiments for Detection of Genes Associated with Antimicrobial Resistance
4.6. DNA Sequencing
4.7. Epidemiological Typing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henig, I.; Zuckerman, T. Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med. J. 2014, 5, e0028. [Google Scholar] [CrossRef] [PubMed]
- Worldwide Network for Blood & Marrow Transplantation. Available online: https://www.wbmt.org/wp-content/uploads/2023/07/2022-WBMT-Progress-Report.pdf (accessed on 13 March 2024).
- Jaing, T.H. Complications of haematopoietic stem cell transplantation. ISBT Sci. Ser. 2011, 6, 332–336. [Google Scholar] [CrossRef]
- Cao, W.; Guan, L.; Li, X.; Zhang, R.; Li, L.; Zhang, S.; Wang, C.; Xie, X.; Jiang, Z.; Wan, D.; et al. Clinical Analysis of Bloodstream Infections During Agranulocytosis After Allogeneic Hematopoietic Stem Cell Transplantation. Infect. Drug Resist. 2021, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Bertaina, A.; Piciocchi, A.; Perruccio, K.; Algarotti, A.; Busca, A.; Cattaneo, C.; Raiola, A.M.; Guidi, S.; Iori, A.P.; et al. Gruppo Italiano Trapianto di Midollo Osseo (GITMO) and Associazione Microbiologi Clinici Italiani (AMCLI). Incidence, Risk Factors and Outcome of Pre-engraftment Gram-Negative Bacteremia After Allogeneic and Autologous Hematopoietic Stem Cell Transplantation: An Italian Prospective Multicenter Survey. Clin. Infect. Dis. 2017, 65, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Moreira, F.; Guimaraes, T.; Spadão, F.; Ramos, J.F.; Batista, M.V.; Filho, J.S.; Costa, S.F.; Rocha, V. Epidemiology, risk factors and outcomes of multi-drug-resistant bloodstream infections in haematopoietic stem cell transplant recipients: Importance of previous gut colonization. J. Hosp. Infect. 2018, 100, 83–91. [Google Scholar] [CrossRef]
- Kelly, M.S.; Ward, D.V.; Severyn, C.J.; Arshad, M.; Heston, S.M.; Jenkins, K.; Martin, P.L.; McGill, L.; Stokhuyzen, A.; Bhattarai, S.K.; et al. Gut Colonization Preceding Mucosal Barrier Injury Bloodstream Infection in Pediatric Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. 2019, 25, 2274–2280. [Google Scholar] [CrossRef]
- Alrstom, A.; Alsuliman, T.; Daher, N.; Abouharb, R. The Impact of Modifying Empirical Antibiotic Therapy Based on Intestinal Colonization Status on Clinical Outcomes of Febrile Neutropenic Patients. Infect. Chemother. 2021, 53, 63–74. [Google Scholar] [CrossRef]
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef]
- Schinas, G.; Skintzi, K.; De Lastic, A.L.; Rodi, M.; Gogos, C.; Mouzaki, A.; Akinosoglou, K. Patterns, Cost, and Immunological Response of MDR vs. Non MDR-Bacteremia: A Prospective Cohort Study. Pathogens 2023, 12, 1044. [Google Scholar] [CrossRef]
- Krawiec, K.M.; Strzałka, P.; Stańczak, K.; Czemerska, M.; Szmigielska, A.; Grzybowska-Izydorczyk, O.; Wierzbowska, A.; Pluta, A. Evaluation of colonization and infection profile in allogeneic hematopoietic stem cell transplantation recipients. Acta Haematol. Pol. 2024, 55, 112–122. [Google Scholar] [CrossRef]
- Perez, P.; Patiño, J.; Estacio, M.; Pino, J.; Manzi, E.; Medina, D. Bacteremia in pediatric patients with hematopoietic stem cell transplantation. Hematol. Transfus. Cell Ther. 2020, 42, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, J.; Bian, Z.; Li, L.; Zhang, S.; Qin, Y.; Wan, D.; Jiang, Z.; Zhang, R. Active Screening of Intestinal Colonization of Carbapenem-Resistant Enterobacteriaceae for Subsequent Bloodstream Infection in Allogeneic Hematopoietic Stem Cell Transplantation. Infect. Drug Resist. 2022, 18, 5993–6006. [Google Scholar] [CrossRef] [PubMed]
- Niyazi, D.; Micheva, I.; Dokova, K.; Stoeva, T. Incidence, Risk Factors and Outcome of Bloodstream Infections in Patients After Hematopoietic Stem-Cell Transplantation: A Single Center Study. Indian J. Hematol. Blood Transfus. 2023, 39, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Scheich, S.; Lindner, S.; Koenig, R.; Reinheimer, C.; Wichelhaus, T.A.; Hogardt, M.; Besier, S.; Kempf, V.A.J.; Kessel, J.; Martin, H.; et al. Clinical impact of colonization with multidrug-resistant organisms on outcome after allogeneic stem cell transplantation in patients with acute myeloid leukemia. Cancer 2018, 124, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, J.; Robak, K.; Peric, Z.; Marchel, H.; Karakulska-Prystupiuk, E.; Halaburda, K.; Rusicka, P.; Swoboda-Kopec, E.; Wroblewska, M.; Wiktor-Jedrzejczak, W.; et al. Impact of Gut Colonization by Antibiotic-Resistant Bacteria on the Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective, Single-Center Study. Biol. Blood Marrow Transplant. 2016, 22, 1087–1093. [Google Scholar] [CrossRef]
- Ford, C.D.; Gazdik, M.A.; Lopansri, B.K.; Webb, B.; Mitchell, B.; Coombs, J.; Hoda, D.; Petersen, F.B. Vancomycin-Resistant Enterococcus Colonization and Bacteremia and Hematopoietic Stem Cell Transplantation Outcomes. Biol. Blood Marrow Transplant. 2017, 23, 340–346. [Google Scholar] [CrossRef]
- Scheich, S.; Reinheimer, C.; Brandt, C.; Wichelhaus, T.A.; Hogardt, M.; Kempf, V.A.J.; Brunnberg, U.; Brandts, C.; Ballo, O.; von Metzler, I.; et al. Clinical Impact of Colonization with Multidrug-Resistant Organisms on Outcome after Autologous Stem Cell Transplantation: A Retrospective Single-Center Study. Biol. Blood Marrow Transplant. 2017, 23, 1455–1462. [Google Scholar] [CrossRef]
- Giannella, M.; Bartoletti, M.; Campoli, C.; Rinaldi, M.; Coladonato, S.; Pascale, R.; Tedeschi, S.; Ambretti, S.; Cristini, F.; Tumietto, F.; et al. The impact of carbapenemase-producing Enterobacteriaceae colonization on infection risk after liver transplantation: A prospective observational cohort study. Clin. Microbiol. Infect. 2019, 25, 1525–1531. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Chevallier, P.; Montassier, E. Gut microbiome in allogeneic hematopoietic stem cell transplantation and specific changes associated with acute graft vs host disease. World J. Gastroenterol. 2021, 27, 7792–7800. [Google Scholar] [CrossRef]
- Arias Ramos, D.; Hoyos Pulgarín, J.A.; Moreno Gómez, G.A.; Alzate, J.A.; Olaya Gómez, J.C.; Cortés Bonilla, I.; Vargas Mosquera, C. Geographic mapping of Enterobacteriaceae with extended-spectrum β-lactamase (ESBL) phenotype in Pereira, Colombia. BMC Infect. Dis. 2020, 20, 540. [Google Scholar] [CrossRef]
- Kurittu, P.; Khakipoor, B.; Jalava, J.; Karhukorpi, J.; Heikinheimo, A. Whole-Genome Sequencing of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli From Human Infections in Finland Revealed Isolates Belonging to Internationally Successful ST131-C1-M27 Subclade but Distinct from Non-human Sources. Front. Microbiol. 2022, 12, 789280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Q.; Mao, X.; Li, L.; Dou, J.; He, Q.; Shao, H.; Luo, Q. Prevalence of Extended-Spectrum β-Lactamase-Resistant Genes in Escherichia coli Isolates from Central China during 2016–2019. Animals 2022, 12, 3191. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Chavda, K.D.; Baker, T.M.; Chen, L.; Shashkina, E.; Soave, R.; Small, C.B.; Jacobs, S.E.; Shore, T.B.; van Besien, K.; et al. Colonization with Levofloxacin-resistant Extended-spectrum β-Lactamase-producing Enterobacteriaceae and Risk of Bacteremia in Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. 2018, 67, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; McGregor, J.C.; Johnson, J.A.; Strauss, S.M.; Moore, A.C.; Standiford, H.C.; Hebden, J.N.; Morris, J.G., Jr. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg. Infect. Dis. 2007, 13, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Keuleyan, E.; Rasshofer, R.; Markovska, R.; Queenan, A.M.; Bauernfeind, A. VIM-15 and VIM-16, two new VIM-2-like metallo-beta-lactamases in Pseudomonas aeruginosa isolates from Bulgaria and Germany. Antimicrob. Agents Chemother. 2008, 52, 2977–2979. [Google Scholar] [CrossRef][Green Version]
- Strateva, T.; Setchanova, L.; Peykov, S. Characterization of a Bulgarian VIM-2 metallo-β-lactamase-producing Pseudomonas aeruginosa clinical isolate belonging to the high-risk sequence type 111. Infect. Dis. 2021, 53, 883–887. [Google Scholar] [CrossRef]
- Falcone, M.; Mezzatesta, M.L.; Perilli, M.; Forcella, C.; Giordano, A.; Cafiso, V.; Amicosante, G.; Stefani, S.; Venditti, M. Infections with VIM-1 metallo-{beta}-lactamase-producing enterobacter cloacae and their correlation with clinical outcome. J. Clin. Microbiol. 2009, 47, 3514–3519. [Google Scholar] [CrossRef]
- Heller, I.; Grif, K.; Orth, D. Emergence of VIM-1-carbapenemase-producing Enterobacter cloacae in Tyrol, Austria. J. Med. Microbiol. 2012, 61, 567–571. [Google Scholar] [CrossRef]
- Luo, H.; Chen, X.; Jiang, Z.; Yan, Q. Prevalence of and risk factors for intestinal colonisation by multidrug-resistant Gram-negative bacteria in patients with haematological malignancies: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2024, 63, 107043. [Google Scholar] [CrossRef]
- Stankova, P.; Markovska, R.; Boyanova, L.; Mitov, I. Antibiotic susceptibility of intestinal isolates suspected of esbl/carbapenemase production of the order of esbl/carbapenemase production of the order enterobacterales enterobacterales isolated from hospitalized patients isolated from hospitalized patients and healthy individuals for the period 2017–2018. Mod. Med. 2020, 64, 10–19. [Google Scholar]
- Sahitya, D.S.K.; Jandiyal, A.; Jain, A.; Senapati, J.; Nanda, S.; Aggarwal, M.; Kumar, P.; Mohapatra, S.; Ray, P.; Malhotra, P.; et al. Prevention and management of carbapenem-resistant Enterobacteriaceae in haematopoietic cell transplantation. Ther. Adv. Infect. Dis. 2021, 8, 20499361211053480. [Google Scholar] [CrossRef] [PubMed]
- Kömürcü, B.; Tigen, E.T.; Toptaş, T.; Tuğlular, F.T.; Korten, V. Rectal colonization with multidrug-resistant gram-negative bacteria in patients with hematological malignancies: A prospective study. Expert. Rev. Hematol. 2020, 13, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Baidya, A.; Kodan, P.; Fazal, F.; Tsering, S.; Menon, P.R.; Jorwal, P.; Chowdhury, U.K. Stenotrophomonas maltophilia: More than Just a Colonizer! Indian J. Crit. Care Med. 2019, 23, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Garrison, M.W.; Anderson, D.E.; Campbell, D.M.; Carroll, K.C.; Malone, C.L.; Anderson, J.D.; Hollis, R.J.; Pfaller, M.A. Stenotrophomonas maltophilia: Emergence of multidrug-resistant strains during therapy and in an in vitro pharmacodynamic chamber model. Antimicrob. Agents Chemother. 1996, 40, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.; Abou Haidar, M.; Ibrahim, R.; Rahal, R.; Abou Jaoude, J.; Harmouche, C.; Habr, B.; Ayoub, E.; Saliba, G.; Sleilaty, G.; et al. Stenotrophomonas maltophilia pneumonia in critical COVID-19 patients. Sci. Rep. 2023, 13, 3392. [Google Scholar] [CrossRef]
- Hitkova, H.Y.; Hristova, P.M. Enterococcus and enterococcus-like organisms recovered in faecal screening for vancomycin-resistance. J. IMAB 2019, 25, 2649–2654. [Google Scholar] [CrossRef]
- Strateva, T.; Sirakov, I.; Dimov, S.; Trifonova, A.; Savov, E.; Mitov, I. Clonal spread of vanA Enterococcus faecium sequence type 203 in Bulgarian hospitals. Infect. Dis. 2018, 50, 718–721. [Google Scholar] [CrossRef]
- Mullié, C.; Lemonnier, D.; Adjidé, C.C.; Maizel, J.; Mismacque, G.; Cappe, A.; Carles, T.; Pierson-Marchandise, M.; Zerbib, Y. Nosocomial outbreak of monoclonal VIM carbapenemase-producing Enterobacter cloacae complex in an intensive care unit during the COVID-19 pandemic: An integrated approach. J. Hosp. Infect. 2022, 120, 48–56. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2020 (accessed on 15 June 2024).
- Kharrat, M.; Chebbi, Y.; Ben Tanfous, F.; Lakhal, A.; Ladeb, S.; Othmen, T.B.; Achour, W. Extended spectrum beta-lactamase-producing Enterobacteriaceae infections in hematopoietic stem cell transplant recipients: Epidemiology and molecular characterization. Int. J. Antimicrob. Agents 2018, 52, 886–892. [Google Scholar] [CrossRef]
- Uemura, M.; Imataki, O.; Uchida, S.; Nakayama-Imaohji, H.; Ohue, Y.; Matsuka, H.; Mori, H.; Dobashi, H.; Kuwahara, T.; Kadowaki, N. Strain-specific transmission in an outbreak of ESBL-producing Enterobacteriaceae in the hemato-oncology care unit: A cohort study. BMC Infect. Dis. 2017, 17, 26. [Google Scholar] [CrossRef]
- Markovska, R.; Stankova, P.; Stoeva, T.; Murdjeva, M.; Marteva-Proevska, Y.; Ivanova, D.; Sredkova, M.; Petrova, A.; Mihova, K.; Boyanova, L. Dissemination of High-Risk Clones Enterobacterales among Bulgarian Fecal Carriage Isolates. Microorganisms 2022, 10, 2144. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Giron, M. Enterobacter Infections. [Updated 2023 Jun 26]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559296/ (accessed on 21 June 2024).
- Sanders, W.E., Jr.; Sanders, C.C. Enterobacter spp.: Pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 1997, 10, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lee, N.Y.; Yan, J.J.; Lee, H.C.; Chen, P.L.; Chang, C.M.; Wu, C.J.; Ko, N.Y.; Wang, L.R.; Chi, C.H.; et al. Bacteremia due to extended-spectrum-beta-lactamase-producing Enterobacter cloacae: Role of carbapenem therapy. Antimicrob. Agents Chemother. 2010, 54, 3551–3556. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, D.; Stoeva, T.; Markovska, R.; Stankova, P.; Mihova, K.; Kaneva, R.; Mitov, I. Molecular Epidemiology of Multidrug Resistant Enterobacter cloacae blood isolates from a University Hospital. J. IMAB 2019, 25, 2457–2464. [Google Scholar] [CrossRef]
- Sid Ahmed, M.A.; Hamid, J.M.; Hassan, A.M.M.; Abu Jarir, S.; Bashir Ibrahim, E.; Abdel Hadi, H. Phenotypic and Genotypic Characterization of Pan-Drug-Resistant Klebsiella pneumoniae Isolated in Qatar. Antibiotics 2024, 13, 275. [Google Scholar] [CrossRef]
- Sleiman, A.; Awada, B.; Mocadie, M.; Sherri, N.; Haraoui, L.P.; Baby, V.; Araj, G.F.; Kanj, S.S.; Rizk, N.; Matar, G.M.; et al. An unequivocal superbug: PDR Klebsiella pneumoniae with an arsenal of resistance and virulence factor genes. J. Infect. Dev. Ctries. 2021, 15, 404–414. [Google Scholar] [CrossRef]
- Fatima, S.; Liaqat, F.; Akbar, A.; Sahfee, M.; Samad, A.; Anwar, M.; Iqbal, S.; Khan, S.A.; Sadia, H.; Makai, G.; et al. Virulent and multidrug-resistant Klebsiella pneumoniae from clinical samples in Balochistan. Int. Wound J. 2021, 18, 510–518. [Google Scholar] [CrossRef]
- Weterings, V.; van Oosten, A.; Nieuwkoop, E.; Nelson, J.; Voss, A.; Wintermans, B.; van Lieshout, J.; Kluytmans, J.; Veenemans, J. Management of a hospital-wide vancomycin-resistant Enterococcus faecium outbreak in a Dutch general hospital, 2014-2017: Successful control using a restrictive screening strategy. Antimicrob. Resist. Infect. Control 2021, 10, 38. [Google Scholar] [CrossRef]
- Jabbari Shiadeh, S.M.; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef]
- Rangberg, A.; Larsen, A.L.; Kacelnik, O.; Sæther, H.S.; Bjørland, M.; Ringstad, J.; Jonassen, C.M. Molecular analysis and epidemiological typing of Vancomycin-resistant Enterococcus outbreak strains. Sci. Rep. 2019, 9, 11917. [Google Scholar] [CrossRef]
- García Martínez de Artola, D.; Castro, B.; Ramos, M.J.; Díaz Cuevas, Z.; Lakhwani, S.; Lecuona, M. Outbreak of vancomycin-resistant enterococcus on a haematology ward: Management and control. J. Infect. Prev. 2017, 18, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Ballard, S.; Sullivan, S.; Marshall, C. An outbreak of vanA vancomycin-resistant Enterococcus faecium in a hospital with endemic vanB VRE. Infect. Dis. Health 2019, 24, 82–91. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents (accessed on 13 February 2024).
- Markovska, R.; Schneider, I.; Keuleyan, E.; Sredkova, M.; Ivanova, D.; Markova, B.; Lazarova, G.; Dragijeva, E.; Savov, E.; Haydouchka, I.; et al. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in Bulgarian hospitals. Microb. Drug Resist. 2008, 14, 119–128. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Strateva, T.; Atanasova, D.; Mitov, I.; Sirakov, I.; Katrandjieva, A. Emergence of VanB phenotype-vanA genotype Enterococcus faecium clinical isolate in Bulgaria. Braz. J. Infect. Dis. 2014, 18, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Kariyama, R.; Mitsuhata, R.; Chow, J.W.; Clewell, D.B.; Kumon, H. Simple and reliable multiplex PCR assay for surveillance isolates of vancomycin-resistant enterococci. J. Clin. Microbiol. 2000, 38, 3092–3095. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, C.; Piché, L.; Larose, C.; Roy, P.H. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 1995, 39, 185–191. [Google Scholar] [CrossRef]
- Bakhshi, B.; Afshari, N.; Fallah, F. Enterobacterial repetitive intergenic consensus (ERIC)-PCR analysis as a reliable evidence for suspected Shigella spp. outbreaks. Braz. J. Microbiol. 2018, 49, 529–533. [Google Scholar] [CrossRef]
- Niyazi, D.S. Investigation of Bacteraemia and Invasive Fungal Infections in Patients Following Autologous and Allogeneic Hematopoietic Stem Cell Transplantation. Ph.D. Thesis, Medical University of Varna, Varna, Bulgaria, September 2022. [Google Scholar]
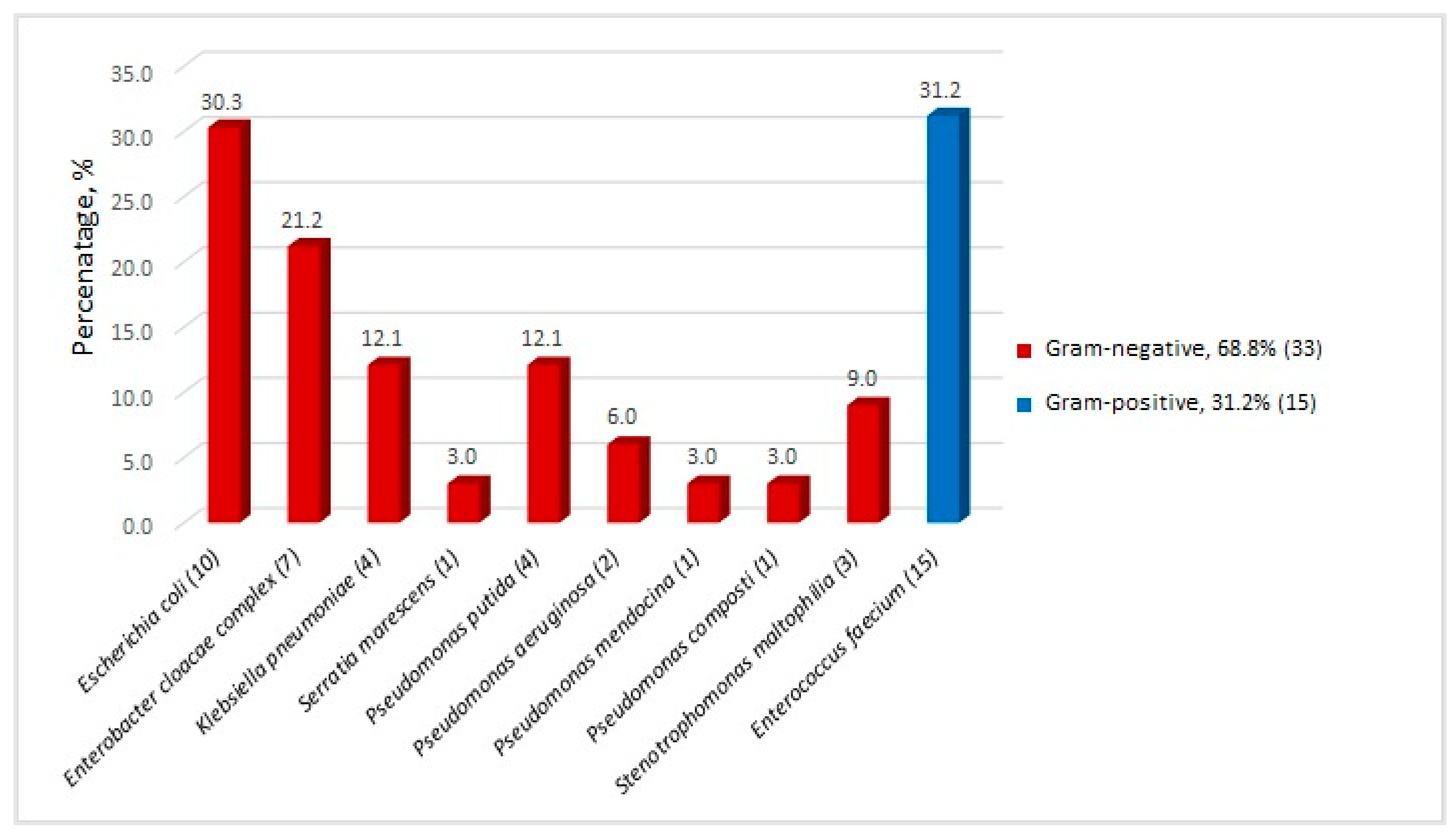
| Organism (n) | FEP | TZP | MEM | CIP | G | AK | TSM | COL |
|---|---|---|---|---|---|---|---|---|
| E. coli (10) | 10 (100) | 2 (20.0) | 0 (0.0) | 1 (10.0) | 4 (40.0) | 0 (0.0) | 4 (40.0) | 0 (0.0) |
| E. cloacae complex (7) | 7 (100) | 5 (71.4) | 1 (14.3) | 6 (85.7) | 7 (100) | 0 (0.0) | 2 (28.6) | 0 (0.0) |
| K. pneumoniae (4) | 4 (100) | 3 (75.0) | 0 (0.0) | 4 (100) | 3 (75.0) | 1 (25.0) | 3 (75.0) | 0 (0.0) |
| S. marcescens (1) | 1 (100) | 0 (0.0) | 0 (0.0) | 1 (100) | 1 (100) | 0 (0.0) | 0 (0.0) | NA |
| Pseudomonas spp. (8) | 8 (100) | 8 (100) | 8 (100) | 7 (87.5) | 5 (62.5) | 3 (37.5) | NA | 0 (0.0) |
| S. maltophilia (3) | NA | NA | NA | NA | NA | NA | 3 (100) | 0 (0.0) |
| Patient ID | Fecal Screening | ERIC/RAPD Type | |
|---|---|---|---|
| Isolate | Resistance Profile and Genetic Determinants | ||
| 2 | E. faecium | VRE (vanA) | RAPD Aa1 |
| 5 | E. faecium | VRE (vanA) | RAPD C |
| K. pneumoniae | ESBL (CTX-M-15, SHV-1, TEM) | Unique | |
| 6 | E. faecium | VRE (vanA) | RAPD Aa1 |
| 9 | P. putida | CR (VIM-2) | |
| 11 | E. cloacae | ESBL (CTX-M-15, TEM-1) | ERIC A |
| 14 | E. faecium | VRE (vanA) | Unique |
| E. coli | ESBL (TEM) | Unique | |
| P. putida | CR ** | ||
| 15 | E. coli | ESBL (CTX-M) | ERIC a |
| 16 | P. putida | CR (VIM-2) | |
| 18 | E. faecium | VRE (vanA) | Unique |
| E. coli | ESBL (CTX-M, TEM) | ERIC b | |
| 19 | E. cloacae | ESBL (CTX-M-15, TEM-1) | ERIC A |
| 20 | S. maltophilia | ||
| E. faecium | VRE (vanA) | RAPD Bb | |
| E. faecium | VRE (vanA) | RAPD Ba | |
| K. pneumoniae | ESBL (CTX-M, TEM, SHV) | Unique | |
| P. putida | CR (VIM-2) | ||
| E. coli | ESBL (CTX-M-15, TEM) | ERIC b | |
| 22 | E. coli | ESBL (CTX-M) | Unique |
| 26 | E. cloacae | ESBL + CR (CTX-M-15, TEM-1, VIM-1) | ERIC A |
| 28 | P. mendocina | CR (VIM-2) | |
| 32 | E. coli | ESBL (CTX-M) | ERIC a |
| 34 | E. faecium | VRE (vanA) | Unique |
| 35 | E. faecium | VRE (vanA) | RAPD Aa1 |
| 36 | S. maltophilia | ||
| 37 | E. faecium | VRE (vanA) | RAPD Aa2 |
| S. maltophilia | |||
| P. composti | CR (VIM-2) | ||
| 42 | K. pneumoniae | ESBL (SHV-12) | Unique |
| 45 | E. cloacae | ESBL (CTX-M-15, TEM-1) | ERIC A |
| 47 | E. faecium | VRE (vanA) | RAPD Ab |
| 48 | E. coli | ESBL (CTX-M) | Unique |
| 49 | P. aeruginosa | CR ** | |
| E. faecium | VRE (vanA) | Unique | |
| E. cloacae | ESBL (CTX-M-15, TEM-1) | ERIC A | |
| 50 | P. aeruginosa | CR (VIM-2) | |
| E. faecium | VRE (vanA) | Unique | |
| 53 | E. faecium | VRE (vanA) | RAPD Ab |
| E. coli | ESBL (CTX-M) | Unique | |
| 60 | E. cloacae | ESBL (CTX-M-3, TEM) | ERIC B |
| E. faecium | VRE (vanA) | RAPD Aa1 | |
| E. coli | ESBL (CTX-M-3, TEM) | Unique | |
| K. pneumoniae | ESBL (SHV, CTX-M) | Unique | |
| E. coli | ESBL (CTX-M-3) | Unique | |
| 67 | S. marcescens | ESBL (CTX-M-15, TEM) | |
| E. cloacae | ESBL (CTX-M-3, TEM) | ERIC B | |
| Target | Primer Sequence | T | Products Size | Reference |
|---|---|---|---|---|
| TEM | F: ATA AAA TTC TTG AAG AC R: TTA CCA ATG CTT AAT CA | 43 °C | 1075 bp | [57] |
| SHV | F: ACT GAA TGC GGC GCT TCC R: TCC CGC AGA TAA ATC A | 61 °C | 297 bp | [57] |
| CTX-M | F: CVA TGT GCA GYA CCA GTA A R: ARG TSA CCA GAA YMA GCG G | 61 °C | 585 bp | [57] |
| VIM | F: GAT GGT GTT TGG TCG CAT A R: CGA ATG CGC AGC ACC AG | 54 °C | 390 bp | [58] |
| IMP | F: GGA ATA GAG TGG CTT AAY TCT C R: GGT TTA AYA AAA CAA CCA CC | 54 °C | 270 bp | [58] |
| KPC | F: CGT CTA GTT CTG CTG TCT TG R: CTT GTC ATC CTT GTT AGG CG | 57 °C | 798 bp | [58] |
| NDM | F: GGT TTG GCG ATC TGG TTT TC R: CGG ATT GGC TCA TCA CGA TC | 57 °C | 621 bp | [58] |
| OXA-48 | F: GCG TGG TTA AGG ATG AAC AC R: CAT CAA GTT CAA CCC AAC CG | 57 °C | 438 bp | [58] |
| ERIC | ERIC 1R: ATG TAA GCT CCT GGG GAT TCA C ERIC 2: AAG TAA GTG ACT GGG GTG AGC | 54 °C | - | [62] |
| vanA/D | F: GAR GAY GGM WSC ATM CAR GGY R: MGT RAA WCC NGG CAK RGT RTT | 51.3 °C | 630 bp | [59] |
| vanA | F: CAT GAA TAG AAT AAA AGT TGC AAT A R: CCC CTT TAA CGC TAA TAC GAT CAA | 54 °C | 1030 bp | [60] |
| vanB | F: AAG CTA TGC AAG AAG CCA TG R: CCG ACA ATC AAA TCA TCC TC | 54 °C | 536 bp | [60] |
| RAPD | AB106: TGC TCT GCC C | 32 °C | - | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niyazi, D.; Vergiev, S.; Markovska, R.; Stoeva, T. Prevalence and Molecular Epidemiology of Intestinal Colonization by Multidrug-Resistant Bacteria among Hematopoietic Stem-Cell Transplantation Recipients: A Bulgarian Single-Center Study. Antibiotics 2024, 13, 920. https://doi.org/10.3390/antibiotics13100920
Niyazi D, Vergiev S, Markovska R, Stoeva T. Prevalence and Molecular Epidemiology of Intestinal Colonization by Multidrug-Resistant Bacteria among Hematopoietic Stem-Cell Transplantation Recipients: A Bulgarian Single-Center Study. Antibiotics. 2024; 13(10):920. https://doi.org/10.3390/antibiotics13100920
Chicago/Turabian StyleNiyazi, Denis, Stoyan Vergiev, Rumyana Markovska, and Temenuga Stoeva. 2024. "Prevalence and Molecular Epidemiology of Intestinal Colonization by Multidrug-Resistant Bacteria among Hematopoietic Stem-Cell Transplantation Recipients: A Bulgarian Single-Center Study" Antibiotics 13, no. 10: 920. https://doi.org/10.3390/antibiotics13100920
APA StyleNiyazi, D., Vergiev, S., Markovska, R., & Stoeva, T. (2024). Prevalence and Molecular Epidemiology of Intestinal Colonization by Multidrug-Resistant Bacteria among Hematopoietic Stem-Cell Transplantation Recipients: A Bulgarian Single-Center Study. Antibiotics, 13(10), 920. https://doi.org/10.3390/antibiotics13100920







