Beyond Antibiotics: What the Future Holds
Abstract
1. Introduction
2. Targeting Type III Secretion Systems (T3SS)
3. Targeting Quorum Sensing
3.1. AHL Inhibitors
3.2. AIP Inhibitors
4. Targeting Biofilm Formation
4.1. Anti-Second Messengers
4.2. Antiadhesion
4.2.1. Gram-Negative Bacteria Anti-Adhesins
4.2.2. Gram-Positive Bacteria Anti-Adhesins
5. Targeting Secreted Toxins
6. Bacteriophage Therapy
7. Fecal Microflora Transplants
8. Future Perspectives
Funding
Conflicts of Interest
References
- Schanzenbach, D.W.; Nunn, R.; Bauer, L. The Changing Landscape of American Life Expectancy; The Hamilton Project: Washington, DC, USA, 2016. [Google Scholar]
- Hauser, A.R.; Mecsas, J.; Moir, D.T. Beyond antibiotics: New therapeutic approaches for bacterial infections. Clin. Infect. Dis. 2016, 63, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Chetia, P. Antibiotics: From Mechanism of Action to Resistance and Beyond. Indian J. Microbiol. 2024, 64, 821–845. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 1929, 10, 226. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2018, 71, 153–184. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef] [PubMed]
- Akhil, D.; Lakshmi, D.; Senthil Kumar, P.; Vo, D.-V.N.; Kartik, A. Occurrence and removal of antibiotics from industrial wastewater. Environ. Chem. Lett. 2021, 19, 1477–1507. [Google Scholar] [CrossRef]
- Lopez Romo, A.; Quirós, R. Appropriate use of antibiotics: An unmet need. Ther. Adv. Urol. 2019, 11, 1756287219832174. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Adedeji, W. The treasure called antibiotics. Ann. Ib. Postgrad. Med. 2016, 14, 56. [Google Scholar]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef]
- Jacopin, E.; Lehtinen, S.; Débarre, F.; Blanquart, F. Factors favouring the evolution of multidrug resistance in bacteria. J. R. Soc. Interface 2020, 17, 20200105. [Google Scholar] [CrossRef]
- Arzanlou, M.; Chai, W.C.; Venter, H. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem. 2017, 61, 49–59. [Google Scholar] [PubMed]
- Okeke, I.N.; Lamikanra, A.; Edelman, R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg. Infect. Dis. 1999, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Allel, K.; García, P.; Labarca, J.; Munita, J.M.; Rendic, M.; de Resistencia Bacteriana, G.C.; Undurraga, E.A. Socioeconomic factors associated with antimicrobial resistance of Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli in Chilean hospitals (2008–2017). Rev. Panam. Salud Pública 2020, 44, 30. [Google Scholar] [CrossRef] [PubMed]
- Böhm, R.; Holtmann-Klenner, C.; Korn, L.; Santana, A.P.; Betsch, C. Behavioral determinants of antibiotic resistance: The role of social information. Appl. Psychol. Health Well-Being 2022, 14, 757–775. [Google Scholar] [CrossRef]
- Khan, M.; Stapleton, F.; Willcox, M.D.P. Susceptibility of contact lens-related Pseudomonas aeruginosa keratitis isolates to multipurpose disinfecting solutions, disinfectants, and antibiotics. Transl. Vis. Sci. Technol. 2020, 9, 2. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Bokhary, H.; Pangesti, K.N.; Rashid, H.; Abd El Ghany, M.; Hill-Cawthorne, G.A. Travel-related antimicrobial resistance: A systematic review. Trop. Med. Infect. Dis. 2021, 6, 11. [Google Scholar] [CrossRef]
- Banerjee, D.; Das, M.; Chatterjee, A.; Tank, S.; Aghera, N. Prevalence of antimicrobial resistance in Saurashtra, Gujarat and implications toward sustainable healthcare. Indian J. Microbiol. 2024, 1–8. [Google Scholar] [CrossRef]
- Shlaes, D.M.; Sahm, D.; Opiela, C.; Spellberg, B. The FDA reboot of antibiotic development. Antimicrob. Agents Chemother. 2013, 57, 4605–4607. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, J.; Tian, L.; Xin, L.; Liang, C.; Ren, X.; Li, M. A comprehensive overview of the antibiotics approved in the last two decades: Retrospects and prospects. Molecules 2023, 28, 1762. [Google Scholar] [CrossRef]
- Verma, T.; Aggarwal, A.; Singh, S.; Sharma, S.; Sarma, S.J. Current challenges and advancements towards discovery and resistance of antibiotics. J. Mol. Struct. 2022, 1248, 131380. [Google Scholar] [CrossRef]
- Iskandar, K.; Murugaiyan, J.; Hammoudi Halat, D.; Hage, S.E.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic discovery and resistance: The chase and the race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Martens, E. Antibiotics in late clinical development. Biochem. Pharmacol. 2017, 133, 152–163. [Google Scholar] [CrossRef]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E. Analysis of the clinical pipeline of treatments for drug-resistant bacterial infections: Despite progress, more action is needed. Antimicrob. Agents Chemother. 2022, 66, e01991-21. [Google Scholar] [CrossRef]
- Butler, M.S.; Henderson, I.R.; Capon, R.J.; Blaskovich, M.A. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 2023, 76, 431–473. [Google Scholar] [CrossRef]
- Cooper, K.; Bennett, W.M. Nephrotoxicity of common drugs used in clinical practice. Arch. Intern. Med. 1987, 147, 1213–1218. [Google Scholar] [CrossRef]
- David, S.; Hamilton, J.P. Drug-induced liver injury. US Gastroenterol. Hepatol. Rev. 2010, 6, 73. [Google Scholar]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H.; European Association for the Study of the Liver. EASL clinical practice guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed]
- Plackett, B. Why big pharma has abandoned antibiotics. Nature 2020, 586, S50. [Google Scholar] [CrossRef]
- Dutescu, I.A.; Hillier, S.A. Encouraging the development of new antibiotics: Are financial incentives the right way forward? A systematic review and case study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. In Review on Antimicrobial Resistance; Wellcome Trust and the UK Government: London, UK, 2014. [Google Scholar]
- Price, R. O’Neill report on antimicrobial resistance: Funding for antimicrobial specialists should be improved. Eur. J. Hosp. Pharm. 2016, 23, 245–247. [Google Scholar] [CrossRef]
- Bernatová, S.; Samek, O.; Pilát, Z.; Šerý, M.; Ježek, J.; Jákl, P.; Šiler, M.; Krzyžánek, V.; Zemánek, P.; Holá, V. Following the mechanisms of bacteriostatic versus bactericidal action using Raman spectroscopy. Molecules 2013, 18, 13188–13199. [Google Scholar] [CrossRef]
- Silver, L.L. Appropriate targets for antibacterial drugs. Cold Spring Harb. Perspect. Med. 2016, 6, a030239. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- John, P.; Whatley, F. Paracoccus denitrificans and the evolutionary origin of the mitochondrion. Nature 1975, 254, 495–498. [Google Scholar] [CrossRef]
- Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The similarities between human mitochondria and bacteria in the context of structure, genome, and base excision repair system. Molecules 2020, 25, 2857. [Google Scholar] [CrossRef]
- Yadav, S.; Shah, D.; Dalai, P.; Agrawal-Rajput, R. The tale of antibiotics beyond antimicrobials: Expanding horizons. Cytokine 2023, 169, 156285. [Google Scholar] [CrossRef] [PubMed]
- Chatzispyrou, I.A.; Held, N.M.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 2015, 75, 4446–4449. [Google Scholar] [CrossRef] [PubMed]
- Protasoni, M.; Kroon, A.M.; Taanman, J.-W. Mitochondria as oncotarget: A comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget 2018, 9, 33818. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Spina, C.S.; Costello, J.C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai, O.S.; Collins, J.J. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci. Transl. Med. 2013, 5, 192ra185. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, H.; Wu, S.; Ge, T.; Wang, G.; Zhou, Y.; Sheng, S.; Jiang, J. Antibiotic drug levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damage. Biomed. Pharmacother. 2016, 84, 1137–1143. [Google Scholar] [CrossRef]
- Yu, M.; Li, R.; Zhang, J. Repositioning of antibiotic levofloxacin as a mitochondrial biogenesis inhibitor to target breast cancer. Biochem. Biophys. Res. Commun. 2016, 471, 639–645. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Sharma, A.K.; Dhasmana, N.; Dubey, N.; Kumar, N.; Gangwal, A.; Gupta, M.; Singh, Y. Bacterial virulence factors: Secreted for survival. Indian J. Microbiol. 2017, 57, 1–10. [Google Scholar] [CrossRef]
- Peterson, J.W. Bacterial pathogenesis. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Nikolic, P.; Mudgil, P. The cell wall, cell membrane and virulence factors of Staphylococcus aureus and their role in antibiotic resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef]
- Pugazhendhi, A.S.; Wei, F.; Hughes, M.; Coathup, M. Bacterial adhesion, virulence, and biofilm formation. In Musculoskeletal Infection; Springer: Berlin/Heidelberg, Germany, 2022; pp. 19–64. [Google Scholar]
- Coburn, B.; Sekirov, I.; Finlay, B.B. Type III secretion systems and disease. Clin. Microbiol. Rev. 2007, 20, 535–549. [Google Scholar] [CrossRef]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; McCoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.; Finlay, B.B. Assembly, structure, function and regulation of type III secretion systems. Nat. Rev. Microbiol. 2017, 15, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Green, E.R.; Mecsas, J. Bacterial secretion systems: An overview. Virulence Mech. Bact. Pathog. 2016, 4, 213–239. [Google Scholar]
- Mariano, G.; Trunk, K.; Williams, D.J.; Monlezun, L.; Strahl, H.; Pitt, S.J.; Coulthurst, S.J. A family of Type VI secretion system effector proteins that form ion-selective pores. Nat. Commun. 2019, 10, 5484. [Google Scholar] [CrossRef] [PubMed]
- Filloux, A. Bacterial protein secretion systems: Game of types. Microbiology 2022, 168, 001193. [Google Scholar] [CrossRef]
- Dean, P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol. Rev. 2011, 35, 1100–1125. [Google Scholar] [CrossRef]
- Sanchez-Garrido, J.; Ruano-Gallego, D.; Choudhary, J.S.; Frankel, G. The type III secretion system effector network hypothesis. Trends Microbiol. 2022, 30, 524–533. [Google Scholar] [CrossRef]
- Rivera, I.; Chowdhury, M.; Sanchez, P.; Sato, M.; Huq, A.; Colwell, R.; Martins, M. Detection of cholera (ctx) and zonula occludens (zot) toxin genes in Vibrio cholerae O1, O139 and non-O1 strains. World J. Microbiol. Biotechnol. 1995, 11, 572–577. [Google Scholar] [CrossRef]
- Karasawa, T.; Mihara, T.; Kurazono, H.; Nair, G.B.; Garg, S.; Ramamurthy, T.; Takeda, Y. Distribution of the zot (zonula occludens toxin) gene among strains of Vibrio cholerae 01 and non-01. FEMS Microbiol. Lett. 1993, 106, 143–145. [Google Scholar] [CrossRef]
- Newman, J.W.; Floyd, R.V.; Fothergill, J.L. The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections. FEMS Microbiol. Lett. 2017, 364, fnx124. [Google Scholar] [CrossRef]
- Negrea, A.; Bjur, E.; Ygberg, S.E.; Elofsson, M.; Wolf-Watz, H.; Rhen, M. Salicylidene acylhydrazides that affect type III protein secretion in Salmonella enterica serovar typhimurium. Antimicrob. Agents Chemother. 2007, 51, 2867–2876. [Google Scholar] [CrossRef]
- Uusitalo, P.; Hägglund, U.; Rhöös, E.; Scherman Norberg, H.; Elofsson, M.; Sundin, C. The salicylidene acylhydrazide INP0341 attenuates Pseudomonas aeruginosa virulence in vitro and in vivo. J. Antibiot. 2017, 70, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Elofsson, M.; Roy, S. Attenuation of Pseudomonas aeruginosa infection by INP0341, a salicylidene acylhydrazide, in a murine model of keratitis. Virulence 2020, 11, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Mühlen, S.; Zapol’skii, V.A.; Bilitewski, U.; Dersch, P. Identification of translocation inhibitors targeting the type III secretion system of enteropathogenic Escherichia coli. Antimicrob. Agents Chemother. 2021, 65, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- Veenendaal, A.K.; Sundin, C.; Blocker, A.J. Small-molecule type III secretion system inhibitors block assembly of the Shigella type III secreton. J. Bacteriol. 2009, 191, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Muschiol, S.; Normark, S.; Henriques-Normark, B.; Subtil, A. Small molecule inhibitors of the Yersinia type III secretion system impair the development of Chlamydia after entry into host cells. BMC Microbiol. 2009, 9, 75. [Google Scholar] [CrossRef]
- Muschiol, S.; Bailey, L.; Gylfe, Å.; Sundin, C.; Hultenby, K.; Bergström, S.; Elofsson, M.; Wolf-Watz, H.; Normark, S.; Henriques-Normark, B. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 2006, 103, 14566–14571. [Google Scholar] [CrossRef]
- Hudson, D.L.; Layton, A.N.; Field, T.R.; Bowen, A.J.; Wolf-Watz, H.; Elofsson, M.; Stevens, M.P.; Galyov, E.E. Inhibition of type III secretion in Salmonella enterica serovar Typhimurium by small-molecule inhibitors. Antimicrob. Agents Chemother. 2007, 51, 2631–2635. [Google Scholar] [CrossRef]
- Duncan, M.C.; Linington, R.G.; Auerbuch, V. Chemical inhibitors of the type three secretion system: Disarming bacterial pathogens. Antimicrob. Agents Chemother. 2012, 56, 5433–5441. [Google Scholar] [CrossRef]
- Vareechon, C.; Zmina, S.E.; Karmakar, M.; Pearlman, E.; Rietsch, A. Pseudomonas aeruginosa effector ExoS inhibits ROS production in human neutrophils. Cell Host Microbe 2017, 21, 611–618.e615. [Google Scholar] [CrossRef]
- Heuck, A.P.; Brovedan, M.A. Evolutionary conservation, variability, and adaptation of Type III secretion systems. J. Membr. Biol. 2022, 255, 599–612. [Google Scholar] [CrossRef]
- Plé, S.; Job, V.; Dessen, A.; Attree, I. Cochaperone interactions in export of the type III needle component PscF of Pseudomonas aeruginosa. J. Bacteriol. 2010, 192, 3801–3808. [Google Scholar] [CrossRef] [PubMed]
- Zarivach, R.; Vuckovic, M.; Deng, W.; Finlay, B.B.; Strynadka, N.C. Structural analysis of a prototypical ATPase from the type III secretion system. Nat. Struct. Mol. Biol. 2007, 14, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Miki, T.; Ono, S.; Haneda, T.; Ito, M.; Okada, N. Functional characterization of the type III secretion ATPase SsaN encoded by Salmonella pathogenicity island 2. PLoS ONE 2014, 9, e94347. [Google Scholar] [CrossRef] [PubMed]
- Diepold, A.; Wagner, S. Assembly of the bacterial type III secretion machinery. FEMS Microbiol. Rev. 2014, 38, 802–822. [Google Scholar] [CrossRef]
- Veenendaal, A.K.; Hodgkinson, J.L.; Schwarzer, L.; Stabat, D.; Zenk, S.F.; Blocker, A.J. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol. Microbiol. 2007, 63, 1719–1730. [Google Scholar] [CrossRef]
- Soto, J.E.; Galán, J.E.; Lara-Tejero, M. Assembly and architecture of the type III secretion sorting platform. Proc. Natl. Acad. Sci. USA 2022, 119, e2218010119. [Google Scholar] [CrossRef]
- Abrusci, P.; Vergara-Irigaray, M.; Johnson, S.; Beeby, M.D.; Hendrixson, D.R.; Roversi, P.; Friede, M.E.; Deane, J.E.; Jensen, G.J.; Tang, C.M. Architecture of the major component of the type III secretion system export apparatus. Nat. Struct. Mol. Biol. 2013, 20, 99–104. [Google Scholar] [CrossRef]
- Chaudhury, S.; de Azevedo Souza, C.; Plano, G.V.; De Guzman, R.N. The LcrG tip chaperone protein of the Yersinia pestis type III secretion system is partially folded. J. Mol. Biol. 2015, 427, 3096–3109. [Google Scholar] [CrossRef]
- Takaya, A.; Takeda, H.; Tashiro, S.; Kawashima, H.; Yamamoto, T. Chaperone-mediated secretion switching from early to middle substrates in the type III secretion system encoded by Salmonella pathogenicity island 2. J. Biol. Chem. 2019, 294, 3783–3793. [Google Scholar] [CrossRef]
- Spreter, T.; Yip, C.K.; Sanowar, S.; André, I.; Kimbrough, T.G.; Vuckovic, M.; Pfuetzner, R.A.; Deng, W.; Yu, A.C.; Finlay, B.B. A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nat. Struct. Mol. Biol. 2009, 16, 468–476. [Google Scholar] [CrossRef]
- Demers, J.-P.; Sgourakis, N.G.; Gupta, R.; Loquet, A.; Giller, K.; Riedel, D.; Laube, B.; Kolbe, M.; Baker, D.; Becker, S. The common structural architecture of Shigella flexneri and Salmonella typhimurium type three secretion needles. PLoS Pathog. 2013, 9, e1003245. [Google Scholar] [CrossRef] [PubMed]
- Nordfelth, R.; Kauppi, A.M.; Norberg, H.; Wolf-Watz, H.; Elofsson, M. Small-molecule inhibitors specifically targeting type III secretion. Infect. Immun. 2005, 73, 3104–3114. [Google Scholar] [CrossRef] [PubMed]
- Tree, J.J.; Wang, D.; McInally, C.; Mahajan, A.; Layton, A.; Houghton, I.; Elofsson, M.; Stevens, M.P.; Gally, D.L.; Roe, A.J. Characterization of the effects of salicylidene acylhydrazide compounds on type III secretion in Escherichia coli O157: H7. Infect. Immun. 2009, 77, 4209–4220. [Google Scholar] [CrossRef] [PubMed]
- Hotinger, J.A.; Pendergrass, H.A.; May, A.E. Molecular targets and strategies for inhibition of the bacterial type III secretion system (T3SS); inhibitors directly binding to T3SS components. Biomolecules 2021, 11, 316. [Google Scholar] [CrossRef]
- Slepenkin, A.; Chu, H.; Elofsson, M.; Keyser, P.; Peterson, E.M. Protection of mice from a Chlamydia trachomatis vaginal infection using a salicylidene acylhydrazide, a potential microbicide. J. Infect. Dis. 2011, 204, 1313–1320. [Google Scholar] [CrossRef][Green Version]
- Bailey, L.; Gylfe, Å.; Sundin, C.; Muschiol, S.; Elofsson, M.; Nordström, P.; Henriques-Normark, B.; Lugert, R.; Waldenström, A.; Wolf-Watz, H. Small molecule inhibitors of type III secretion in Yersinia block the Chlamydia pneumoniae infection cycle. FEBS Lett. 2007, 581, 587–595. [Google Scholar] [CrossRef]
- Felise, H.B.; Nguyen, H.V.; Pfuetzner, R.A.; Barry, K.C.; Jackson, S.R.; Blanc, M.-P.; Bronstein, P.A.; Kline, T.; Miller, S.I. An inhibitor of gram-negative bacterial virulence protein secretion. Cell Host Microbe 2008, 4, 325–336. [Google Scholar] [CrossRef]
- Wolf, K.; Betts, H.; Chellas-Géry, B.; Hower, S.; Linton, C.; Fields, K. Treatment of Chlamydia trachomatis with a small molecule inhibitor of the Yersinia type III secretion system disrupts progression of the chlamydial developmental cycle. Mol. Microbiol. 2006, 61, 1543–1555. [Google Scholar] [CrossRef]
- Kauppi, A.M.; Nordfelth, R.; Uvell, H.; Wolf-Watz, H.; Elofsson, M. Targeting bacterial virulence: Inhibitors of type III secretion in Yersinia. Chem. Biol. 2003, 10, 241–249. [Google Scholar] [CrossRef]
- Pan, N.; Goguen, J.; Lee, C. High throughput screening for small-molecule inhibitors of type III secretion in Yersinia pestis. Genus Yersinia Genom. Funct. 2007, 603, 367–375. [Google Scholar]
- Kimura, K.; Iwatsuki, M.; Nagai, T.; Matsumoto, A.; Takahashi, Y.; Shiomi, K.; Ōmura, S.; Abe, A. A small-molecule inhibitor of the bacterial type III secretion system protects against in vivo infection with Citrobacter rodentium. J. Antibiot. 2011, 64, 197–203. [Google Scholar] [CrossRef]
- Larzabal, M.; Mercado, E.C.; Vilte, D.A.; Salazar-Gonzalez, H.; Cataldi, A.; Navarro-Garcia, F. Designed coiled-coil peptides inhibit the type three secretion system of enteropathogenic Escherichia coli. PLoS ONE 2010, 5, e9046. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, H.A.; May, A.E. Natural product type III secretion system inhibitors. Antibiotics 2019, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.K.; Garrity-Ryan, L.K.; Bartlett, V.J.; Grier, M.C.; Verma, A.K.; Medjanis, G.; Donatelli, J.E.; Macone, A.B.; Tanaka, S.K.; Levy, S.B. N-hydroxybenzimidazole inhibitors of the transcription factor LcrF in Yersinia: Novel antivirulence agents. J. Med. Chem. 2009, 52, 5626–5634. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.; Philipovskiy, A.V.; Wulff-Strobel, C.R.; Ye, Z.; Straley, S.C. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect. Immun. 2005, 73, 6127–6137. [Google Scholar] [CrossRef]
- Abramov, V.M.; Kosarev, I.V.; Motin, V.L.; Khlebnikov, V.S.; Vasilenko, R.N.; Sakulin, V.K.; Machulin, A.V.; Uversky, V.N.; Karlyshev, A.V. Binding of LcrV protein from Yersinia pestis to human T-cells induces apoptosis, which is completely blocked by specific antibodies. Int. J. Biol. Macromol. 2019, 122, 1062–1070. [Google Scholar] [CrossRef]
- Sittner, A.; Mechaly, A.; Vitner, E.; Aftalion, M.; Levy, Y.; Levy, H.; Mamroud, E.; Fisher, M. Improved production of monoclonal antibodies against the LcrV antigen of Yersinia pestis using FACS-aided hybridoma selection. J. Biol. Methods 2018, 5, e100. [Google Scholar] [CrossRef]
- Ali, S.O.; Yu, X.Q.; Robbie, G.J.; Wu, Y.; Shoemaker, K.; Yu, L.; DiGiandomenico, A.; Keller, A.E.; Anude, C.; Hernandez-Illas, M. Phase 1 study of MEDI3902, an investigational anti–Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin. Microbiol. Infect. 2019, 25, 629-e1. [Google Scholar] [CrossRef]
- Sawa, T.; Ito, E.; Nguyen, V.H.; Haight, M. Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa. Hum. Vaccines Immunother. 2014, 10, 2843–2852. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Keller, A.E.; Gao, C.; Rainey, G.J.; Warrener, P.; Camara, M.M.; Bonnell, J.; Fleming, R.; Bezabeh, B.; Dimasi, N. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014, 6, 262ra155. [Google Scholar] [CrossRef]
- Tabor, D.; Oganesyan, V.; Keller, A.; Yu, L.; McLaughlin, R.; Song, E.; Warrener, P.; Rosenthal, K.; Esser, M.; Qi, Y. Pseudomonas aeruginosa PcrV and Psl, the molecular targets of bispecific antibody MEDI3902, are conserved among diverse global clinical isolates. J. Infect. Dis. 2018, 218, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Le, H.N.; Tran, V.G.; Vu, T.T.; Gras, E.; Le, V.T.; Pinheiro, M.G.; Aguiar-Alves, F.; Schneider-Smith, E.; Carter, H.C.; Sellman, B.R. Treatment efficacy of MEDI3902 in Pseudomonas aeruginosa bloodstream infection and acute pneumonia rabbit models. Antimicrob. Agents Chemother. 2019, 63, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; François, B.; Bourgeois, M.; Komnos, A.; Ferrer, R.; Rahav, G.; De Schryver, N.; Lepape, A.; Koksal, I.; Luyt, C.-E. Safety, efficacy, and pharmacokinetics of gremubamab (MEDI3902), an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody, in P. aeruginosa-colonised, mechanically ventilated intensive care unit patients: A randomised controlled trial. Crit. Care 2022, 26, 355. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Iannuzzo, J.R.; Levine, D.R.; Saeid, K.K.; Schwartz, R.C.; Sucic, N.T.; Terleckyj, O.D.; Young, J.M. Bacterial communication (“quorum sensing”) via ligands and receptors: A novel pharmacologic target for the design of antibiotic drugs. J. Pharmacol. Exp. Ther. 2005, 312, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e10. [Google Scholar] [CrossRef]
- Chadha, J.; Harjai, K.; Chhibber, S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: A chronicle through the perspective of quorum sensing. Environ. Microbiol. 2022, 24, 2630–2656. [Google Scholar] [CrossRef]
- Prescott, R.D.; Decho, A.W. Flexibility and adaptability of quorum sensing in nature. Trends Microbiol. 2020, 28, 436–444. [Google Scholar] [CrossRef]
- Azimi, S.; Klementiev, A.D.; Whiteley, M.; Diggle, S.P. Bacterial quorum sensing during infection. Annu. Rev. Microbiol. 2020, 74, 201–219. [Google Scholar] [CrossRef]
- Li, Y.H.; Tian, X.L. Quorum sensing and bacterial social interactions in biofilms: Bacterial cooperation and competition. Stress Environ. Regul. Gene Expr. Adapt. Bact. 2016, 12, 1195–1205. [Google Scholar]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Kostylev, M.; Kim, D.Y.; Smalley, N.E.; Salukhe, I.; Greenberg, E.P.; Dandekar, A.A. Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc. Natl. Acad. Sci. USA 2019, 116, 7027–7032. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.S.; Calfee, M.W.; Rocha, E.R.; Ling, E.A.; Engstrom, E.; Coleman, J.P.; Pesci, E.C. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 4372–4380. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Li, M.; Wang, J.; Wang, B. Inhibitors and antagonists of bacterial quorum sensing. Med. Res. Rev. 2009, 29, 65–124. [Google Scholar] [CrossRef] [PubMed]
- Yada, S.; Kamalesh, B.; Sonwane, S.; Guptha, I.; Swetha, R. Quorum sensing inhibition, relevance to periodontics. J. Int. Oral Health JIOH 2015, 7, 67. [Google Scholar]
- Naga, N.G.; El-Badan, D.E.; Ghanem, K.M.; Shaaban, M.I. It is the time for quorum sensing inhibition as alternative strategy of antimicrobial therapy. Cell Commun. Signal. 2023, 21, 133. [Google Scholar] [CrossRef]
- Czajkowski, R.; Jafra, S. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 2009, 56, 1–16. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef]
- Srinivasarao, S.; Nizalapur, S.; Yu, T.T.; Wenholz, D.S.; Trivedi, P.; Ghosh, B.; Rangan, K.; Kumar, N.; Gowri Chandra Sekhar, K.V. Design, Synthesis and Biological Evaluation of Triazole-Containing 2-Phenylindole and Salicylic Acid as Quorum Sensing Inhibitors Against Pseudomonas aeruginosa. ChemistrySelect 2018, 3, 9170–9180. [Google Scholar] [CrossRef]
- Tung, T.T.; Quoc, T.N. 2-Difluoromethylpyridine as a bioisosteric replacement of pyridine-N-oxide: The case of quorum sensing inhibitors. RSC Med. Chem. 2021, 12, 2065–2070. [Google Scholar] [CrossRef]
- Lidor, O.; Al-Quntar, A.; Pesci, E.; Steinberg, D. Mechanistic analysis of a synthetic inhibitor of the Pseudomonas aeruginosa LasI quorum-sensing signal synthase. Sci. Rep. 2015, 5, 16569. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, S.; Fan, J.; Cao, Z.; Ouyang, W.; Tong, N.; Hu, X.; Hu, J.; Li, P.; Feng, Z. Anti-biofilm effect of novel thiazole acid analogs against Pseudomonas aeruginosa through IQS pathways. Eur. J. Med. Chem. 2018, 145, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Jakobsen, T.H.; Dao, T.T.; Fuglsang, A.T.; Givskov, M.; Christensen, S.B.; Nielsen, J. Fusaric acid and analogues as Gram-negative bacterial quorum sensing inhibitors. Eur. J. Med. Chem. 2017, 126, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, A.; Sharma, N.; Nanaji, Y.; Kumar, D.; Singh, G.; Barnwal, R.P.; Yadav, A.K. Quorum sensing inhibitors as Therapeutics: Bacterial biofilm inhibition. Bioorganic Chem. 2023, 136, 106551. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, N.S.; Shaaban, M.I. Synthesis, Antimicrobial, antiquorum-sensing, and cytotoxic activities of new series of isoindoline-1, 3-dione, pyrazolo [5, 1-a] isoindole, and pyridine derivatives. Arch. Der Pharm. 2015, 348, 666–680. [Google Scholar] [CrossRef]
- El-Gohary, N.; Shaaban, M. Antimicrobial and antiquorum-sensing studies. Part 2: Synthesis, antimicrobial, antiquorum-sensing and cytotoxic activities of new series of fused [1, 3, 4] thiadiazole and [1, 3] benzothiazole derivatives. Med. Chem. Res. 2014, 23, 287–299. [Google Scholar] [CrossRef]
- Hopa, C.; Kara, H.; Aybey, A. Synthesis, structural characterization and biological evaluation of novel mixed-ligand Co (II) complexes as quorum sensing inhibitory agent. J. Mol. Struct. 2020, 1202, 127322. [Google Scholar] [CrossRef]
- Yang, S.; Abdel-Razek, O.A.; Cheng, F.; Bandyopadhyay, D.; Shetye, G.S.; Wang, G.; Luk, Y.-Y. Bicyclic brominated furanones: A new class of quorum sensing modulators that inhibit bacterial biofilm formation. Bioorganic Med. Chem. 2014, 22, 1313–1317. [Google Scholar] [CrossRef]
- Biswas, N.N.; Kutty, S.K.; Barraud, N.; Iskander, G.M.; Griffith, R.; Rice, S.A.; Willcox, M.; Black, D.S.; Kumar, N. Indole-based novel small molecules for the modulation of bacterial signalling pathways. Org. Biomol. Chem. 2015, 13, 925–937. [Google Scholar] [CrossRef]
- Park, J.; Jagasia, R.; Kaufmann, G.F.; Mathison, J.C.; Ruiz, D.I.; Moss, J.A.; Meijler, M.M.; Ulevitch, R.J.; Janda, K.D. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem. Biol. 2007, 14, 1119–1127. [Google Scholar] [CrossRef]
- Amara, N.; Krom, B.P.; Kaufmann, G.F.; Meijler, M.M. Macromolecular inhibition of quorum sensing: Enzymes, antibodies, and beyond. Chem. Rev. 2011, 111, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, G.F.; Park, J.; Mayorov, A.V.; Kubitz, D.M.; Janda, K.D. Generation of quorum quenching antibodies. Quor. Sens. Methods Protoc. 2011, 692, 299–311. [Google Scholar]
- Manefield, M.; de Nys, R.; Naresh, K.; Roger, R.; Givskov, M.; Peter, S.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Givskov, M.; de Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Joshi, J.R.; Khazanov, N.; Charkowski, A.; Faigenboim, A.; Senderowitz, H.; Yedidia, I. Interkingdom signaling interference: The effect of plant-derived small molecules on quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2021, 59, 153–190. [Google Scholar] [CrossRef]
- Bernabè, G.; Marzaro, G.; Di Pietra, G.; Otero, A.; Bellato, M.; Pauletto, A.; Scarpa, M.; Sut, S.; Chilin, A.; Dall’Acqua, S. A novel phenolic derivative inhibits AHL-dependent quorum sensing signaling in Pseudomonas aeruginosa. Front. Pharmacol. 2022, 13, 996871. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial activity of naturally occurring phenols and derivatives against biofilm and planktonic bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef]
- Ugurlu, A.; Yagci, A.K.; Ulusoy, S.; Aksu, B.; Bosgelmez-Tinaz, G. Phenolic compounds affect production of pyocyanin, swarming motility and biofilm formation of Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2016, 6, 698–701. [Google Scholar] [CrossRef]
- Yang, D.; Hao, S.; Zhao, L.; Shi, F.; Ye, G.; Zou, Y.; Song, X.; Li, L.; Yin, Z.; He, X. Paeonol attenuates quorum-sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 692474. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.M.F.; Winans, S.C.; Pinto, U.M. Quorum sensing interference by phenolic compounds—A matter of bacterial misunderstanding. Heliyon 2023, 9, e17657. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.-P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; Alcalde-Rico, M.; Gil-Gil, T.; Valverde, J.R.; Martínez, J.L. Naringenin inhibition of the Pseudomonas aeruginosa quorum sensing response is based on its time-dependent competition with N-(3-Oxo-dodecanoyl)-L-homoserine lactone for LasR binding. Front. Mol. Biosci. 2020, 7, 25. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Gusti, A.R.; Zhang, Q.; Xu, J.-L.; Zhang, L.-H. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl. Environ. Microbiol. 2002, 68, 1754–1759. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.-Y.; Lee, J.-J.; Yum, D.-Y.; Koo, B.-T.; Lee, J.-K. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol. 2002, 68, 3919–3924. [Google Scholar] [CrossRef]
- Zhao, C.; Zeng, H.; Yu, Z.; Sun, M. N-Acyl homoserine lactonase promotes prevention of Erwinia virulence with Zwittermicin A-producing strain Bacillus cereus. Biotechnol. Bioeng. 2008, 100, 599–603. [Google Scholar] [CrossRef]
- Zhu, C.; Yu, Z.; Sun, M. Restraining Erwinia virulence by expression of N-acyl homoserine lactonase gene pro3A-aiiA in Bacillus thuringiensis subsp leesis. Biotechnol. Bioeng. 2006, 95, 526–532. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Xu, J.-L.; Li, X.-Z.; Zhang, L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Wang, L.-H.; Xu, J.-L.; Zhang, H.-B.; Zhang, X.-F.; Zhang, L.-H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Momb, J.; Thomas, P.W.; Moulin, A.; Petsko, G.A.; Fast, W.; Ringe, D. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 1. Product-bound structures. Biochemistry 2008, 47, 7706–7714. [Google Scholar] [CrossRef]
- Reimmann, C.; Ginet, N.; Michel, L.; Keel, C.; Michaux, P.; Krishnapillai, V.; Zala, M.; Heurlier, K.; Triandafillu, K.; Harms, H. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 2002, 148, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Augustine, N.; Kumar, P.; Thomas, S. Inhibition of Vibrio cholerae biofilm by AiiA enzyme produced from Bacillus spp. Arch. Microbiol. 2010, 192, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.; Constantinescu, F.; Michel, L.; Reimmann, C.; Duffy, B.; Défago, G. Degradation of pathogen quorum-sensing molecules by soil bacteria: A preventive and curative biological control mechanism. FEMS Microbiol. Ecol. 2003, 45, 71–81. [Google Scholar] [CrossRef]
- Ulrich, R.L. Quorum quenching: Enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis. Appl. Environ. Microbiol. 2004, 70, 6173–6180. [Google Scholar] [CrossRef]
- Yang, F.; Wang, L.-H.; Wang, J.; Dong, Y.-H.; Hu, J.Y.; Zhang, L.-H. Quorum quenching enzyme activity is widely conserved in the sera of mammalian species. FEBS Lett. 2005, 579, 3713–3717. [Google Scholar] [CrossRef]
- Ozer, E.A.; Pezzulo, A.; Shih, D.M.; Chun, C.; Furlong, C.; Lusis, A.J.; Greenberg, E.P.; Zabner, J. Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. FEMS Microbiol. Lett. 2005, 253, 29–37. [Google Scholar] [CrossRef]
- Draganov, D.I.; Teiber, J.F.; Speelman, A.; Osawa, Y.; Sunahara, R.; La Du, B.N. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005, 46, 1239–1247. [Google Scholar] [CrossRef]
- Khersonsky, O.; Tawfik, D.S. Structure− reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 2005, 44, 6371–6382. [Google Scholar] [CrossRef]
- Leadbetter, J.R.; Greenberg, E.P. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 2000, 182, 6921–6926. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Xu, J.L.; Hu, J.; Wang, L.H.; Ong, S.L.; Leadbetter, J.R.; Zhang, L.H. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 2003, 47, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Han, J.-I.; Zhang, L.-H.; Leadbetter, J.R. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2003, 69, 5941–5949. [Google Scholar] [CrossRef] [PubMed]
- Sio, C.F.; Otten, L.G.; Cool, R.H.; Diggle, S.P.; Braun, P.G.; Bos, R.; Daykin, M.; Cámara, M.; Williams, P.; Quax, W.J. Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1. Infect. Immun. 2006, 74, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Petersen, A.; Whiteley, M.; Leadbetter, J.R. Identification of QuiP, the product of gene PA1032, as the second acyl-homoserine lactone acylase of Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2006, 72, 1190–1197. [Google Scholar] [CrossRef]
- Chen, C.-N.; Chen, C.-J.; Liao, C.-T.; Lee, C.-Y. A probable aculeacin A acylase from the Ralstonia solanacearum GMI1000 is N-acyl-homoserine lactone acylase with quorum-quenching activity. BMC Microbiol. 2009, 9, 89. [Google Scholar] [CrossRef]
- Shepherd, R.W.; Lindow, S.E. Two dissimilar N-acyl-homoserine lactone acylases of Pseudomonas syringae influence colony and biofilm morphology. Appl. Environ. Microbiol. 2009, 75, 45–53. [Google Scholar] [CrossRef]
- Wahjudi, M.; Papaioannou, E.; Hendrawati, O.; van Assen, A.H.; van Merkerk, R.; Cool, R.H.; Poelarends, G.J.; Quax, W.J. PA0305 of Pseudomonas aeruginosa is a quorum quenching acylhomoserine lactone acylase belonging to the Ntn hydrolase superfamily. Microbiology 2011, 157, 2042–2055. [Google Scholar] [CrossRef]
- Bokhove, M.; Jimenez, P.N.; Quax, W.J.; Dijkstra, B.W. The quorum-quenching N-acyl homoserine lactone acylase PvdQ is an Ntn-hydrolase with an unusual substrate-binding pocket. Proc. Natl. Acad. Sci. USA 2010, 107, 686–691. [Google Scholar] [CrossRef]
- Uroz, S.; Chhabra, S.R.; Camara, M.; Williams, P.; Oger, P.; Dessaux, Y. N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 2005, 151, 3313–3322. [Google Scholar] [CrossRef]
- Kaufmann, G.F.; Sartorio, R.; Lee, S.-H.; Mee, J.M.; Altobell, L.J.; Kujawa, D.P.; Jeffries, E.; Clapham, B.; Meijler, M.M.; Janda, K.D. Antibody interference with N-acyl homoserine lactone-mediated bacterial quorum sensing. J. Am. Chem. Soc. 2006, 128, 2802–2803. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.D.L.; Xu, Y.; Meijler, M.M.; Janda, K.D. Antibody catalyzed hydrolysis of a quorum sensing signal found in Gram-negative bacteria. Bioorganic Med. Chem. Lett. 2007, 17, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, G.F.; Park, J.; Mee, J.M.; Ulevitch, R.J.; Janda, K.D. The quorum quenching antibody RS2-1G9 protects macrophages from the cytotoxic effects of the Pseudomonas aeruginosa quorum sensing signalling molecule N-3-oxo-dodecanoyl-homoserine lactone. Mol. Immunol. 2008, 45, 2710–2714. [Google Scholar] [CrossRef] [PubMed]
- Grebe, T.W.; Stock, J.B. The histidine protein kinase superfamily. Adv. Microb. Physiol. 1999, 41, 139–227. [Google Scholar]
- Parkinson, J.S. Genetic approaches for signaling pathways and proteins. Two-Compon. Signal Transduct. 1995, 2, 7–23. [Google Scholar]
- Matsushita, M.; Janda, K.D. Histidine kinases as targets for new antimicrobial agents. Bioorganic Med. Chem. 2002, 10, 855–867. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Zielinski, N.A.; Ninfa, A.J.; Allen, N.E.; Jungheim, L.N.; Nicas, T.I.; Chakrabarty, A. Inhibitors of two-component signal transduction systems: Inhibition of alginate gene activation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1993, 90, 965–969. [Google Scholar] [CrossRef]
- Barrett, J.; Goldschmidt, R.; Lawrence, L.; Foleno, B.; Chen, R.; Demers, J.; Johnson, S.; Kanojia, R.; Fernandez, J.; Bernstein, J. Antibacterial agents that inhibit two-component signal transduction systems. Proc. Natl. Acad. Sci. USA 1998, 95, 5317–5322. [Google Scholar] [CrossRef]
- Macielag, M.J.; Demers, J.P.; Fraga-Spano, S.A.; Hlasta, D.J.; Johnson, S.G.; Kanojia, R.M.; Russell, R.K.; Sui, Z.; Weidner-Wells, M.A.; Werblood, H. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J. Med. Chem. 1998, 41, 2939–2945. [Google Scholar] [CrossRef]
- Stephenson, K.; Yamaguchi, Y.; Hoch, J.A. The mechanism of action of inhibitors of bacterial two-component signal transduction systems. J. Biol. Chem. 2000, 275, 38900–38904. [Google Scholar] [CrossRef]
- Hilliard, J.J.; Goldschmidt, R.M.; Licata, L.; Baum, E.Z.; Bush, K. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob. Agents Chemother. 1999, 43, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Giacometti, A.; Cirioni, O.; Gov, Y.; Ghiselli, R.; Mocchegiani, F.; Viticchi, C.; Del Prete, M.S.; Saba, V.; Scalise, G. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 2003, 187, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, T.; Iwabuchi, R.; Minagawa, S.; Sawada, S.; Okumura, R.; Hoshino, K.; Cappiello, J.; Utsumi, R. Synthesis of a novel inhibitor against MRSA and VRE: Preparation from zerumbone ring opening material showing histidine-kinase inhibition. Bioorganic Med. Chem. Lett. 2007, 17, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Mayville, P.; Ji, G.; Beavis, R.; Yang, H.; Goger, M.; Novick, R.P.; Muir, T.W. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc. Natl. Acad. Sci. USA 1999, 96, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.J.; Mayville, P.; Muir, T.W.; Novick, R.P. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc. Natl. Acad. Sci. USA 2000, 97, 13330–13335. [Google Scholar] [CrossRef]
- Karathanasi, G.; Bojer, M.S.; Baldry, M.; Johannessen, B.A.; Wolff, S.; Greco, I.; Kilstrup, M.; Hansen, P.R.; Ingmer, H. Linear peptidomimetics as potent antagonists of Staphylococcus aureus agr quorum sensing. Sci. Rep. 2018, 8, 3562. [Google Scholar] [CrossRef]
- Scott, R.J.; Lian, L.-Y.; Muharram, S.H.; Cockayne, A.; Wood, S.J.; Bycroft, B.W.; Williams, P.; Chan, W.C. Side-chain-to-tail thiolactone peptide inhibitors of the staphylococcal quorum-sensing system. Bioorganic Med. Chem. Lett. 2003, 13, 2449–2453. [Google Scholar] [CrossRef]
- Wright III, J.S.; Lyon, G.J.; George, E.A.; Muir, T.W.; Novick, R.P. Hydrophobic interactions drive ligand-receptor recognition for activation and inhibition of staphylococcal quorum sensing. Proc. Natl. Acad. Sci. USA 2004, 101, 16168–16173. [Google Scholar] [CrossRef]
- Vasquez, J.K.; Blackwell, H.E. Simplified autoinducing peptide mimetics with single-nanomolar activity against the Staphylococcus aureus AgrC quorum sensing receptor. ACS Infect. Dis. 2019, 5, 484–492. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef]
- Park, J.S.; Ryu, E.-J.; Li, L.; Choi, B.-K.; Kim, B.M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017, 137, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, W.; Zhu, X.; Zhao, H.; Lu, Y.; Zhang, C.; Lu, Z. Surfactin effectively inhibits Staphylococcus aureus adhesion and biofilm formation on surfaces. Appl. Microbiol. Biot. 2019, 103, 4565–4574. [Google Scholar] [CrossRef] [PubMed]
- Hume, E.; Baveja, J.; Muir, B.; Schubert, T.; Kumar, N.; Kjelleberg, S.; Griesser, H.; Thissen, H.; Read, R.; Poole-Warren, L. The control of Staphylococcus epidermidis biofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials 2004, 25, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Walz, J.M.; Avelar, R.L.; Longtine, K.J.; Carter, K.L.; Mermel, L.A.; Heard, S.O.; FCCM for the 5-FU Catheter Study Group. Anti-infective external coating of central venous catheters: A randomized, noninferiority trial comparing 5-fluorouracil with chlorhexidine/silver sulfadiazine in preventing catheter colonization. Crit. Care Med. 2010, 38, 2095–2102. [Google Scholar] [CrossRef]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in bacterial quorum sensing: A biopharmaceutical perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Smyth, A.R.; Cifelli, P.M.; Ortori, C.A.; Righetti, K.; Lewis, S.; Erskine, P.; Holland, E.D.; Givskov, M.; Williams, P.; Cámara, M. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis—A pilot randomized controlled trial. Pediatr. Pulmonol. 2010, 45, 356–362. [Google Scholar] [CrossRef]
- van Delden, C.; Köhler, T.; Brunner-Ferber, F.; François, B.; Carlet, J.; Pechère, J.-C. Azithromycin to prevent Pseudomonas aeruginosa ventilator-associated pneumonia by inhibition of quorum sensing: A randomized controlled trial. Intensive Care Med. 2012, 38, 1118–1125. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Floyd, K.; Eberly, A.; Hadjifrangiskou, M. Adhesion of bacteria to surfaces and biofilm formation on medical devices. In Biofilms and Implantable Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–95. [Google Scholar]
- Hector, A.; Frey, N.; Hartl, D. Update on host-pathogen interactions in cystic fibrosis lung disease. Mol. Cell. Pediatr. 2016, 3, 12. [Google Scholar] [CrossRef]
- Starner, T.D.; Zhang, N.; Kim, G.; Apicella, M.A.; McCray, P.B., Jr. Haemophilus influenzae forms biofilms on airway epithelia: Implications in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Chan, R.; Lam, K.; Costerton, J. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 1980, 28, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Pham, D.T.N.; Tabassum, N.; Khan, M.S.A.; Kim, Y.-M. Mixed biofilms of pathogenic Candida-bacteria: Regulation mechanisms and treatment strategies. Crit. Rev. Microbiol. 2021, 47, 699–727. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Al-Bayati, M.; Samarasinghe, S. Biofilm and gene expression characteristics of the carbapenem-resistant enterobacterales, Escherichia coli IMP, and Klebsiella pneumoniae NDM-1 associated with common bacterial infections. Int. J. Environ. Res. Public Health 2022, 19, 4788. [Google Scholar] [CrossRef]
- Niba, E.T.E.; Naka, Y.; Nagase, M.; Mori, H.; Kitakawa, M. A genome-wide approach to identify the genes involved in biofilm formation in E. coli. DNA Res. 2007, 14, 237–246. [Google Scholar] [CrossRef]
- González, J.F.; Hahn, M.M.; Gunn, J.S. Chronic biofilm-based infections: Skewing of the immune response. Pathog. Dis. 2018, 76, fty023. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef]
- Skerker, J.M.; Berg, H.C. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. USA 2001, 98, 6901–6904. [Google Scholar] [CrossRef]
- Merz, A.J.; So, M.; Sheetz, M.P. Pilus retraction powers bacterial twitching motility. Nature 2000, 407, 98–102. [Google Scholar] [CrossRef]
- Pandey, G.; Jain, R.K. Bacterial chemotaxis toward environmental pollutants: Role in bioremediation. Appl. Environ. Microbiol. 2002, 68, 5789–5795. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.M.; Foster, K.R.; Durham, W.M. Single-cell twitching chemotaxis in developing biofilms. Proc. Natl. Acad. Sci. USA 2016, 113, 6532–6537. [Google Scholar] [CrossRef] [PubMed]
- Dunne, W.M., Jr. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, G.; Kurz, S.; Hubner, C.; Aepinus, C.; Theiss, S.; Guckenberger, M.; Panzner, U.; Weber, J.; Frosch, M. Transcriptome analysis of Neisseria meningitidis during infection. J. Bacteriol. 2003, 185, 155–164. [Google Scholar] [CrossRef]
- Siryaporn, A.; Kuchma, S.L.; O’Toole, G.A.; Gitai, Z. Surface attachment induces Pseudomonas aeruginosa virulence. Proc. Natl. Acad. Sci. USA 2014, 111, 16860–16865. [Google Scholar] [CrossRef]
- Kansal, R.; Rasko, D.A.; Sahl, J.W.; Munson, G.P.; Roy, K.; Luo, Q.; Sheikh, A.; Kuhne, K.J.; Fleckenstein, J.M. Transcriptional modulation of enterotoxigenic Escherichia coli virulence genes in response to epithelial cell interactions. Infect. Immun. 2013, 81, 259–270. [Google Scholar] [CrossRef]
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella biofilm formation, chronic infection, and immunity within the intestine and hepatobiliary tract. Front. Cell. Infect. Microbiol. 2021, 10, 624622. [Google Scholar] [CrossRef]
- Paula, A.J.; Hwang, G.; Koo, H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat. Commun. 2020, 11, 1354. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Bumm, C.V.; Folwaczny, M. Infective endocarditis and oral health—A Narrative Review. Cardiovasc. Diagn. Ther. 2021, 11, 1403. [Google Scholar] [CrossRef]
- Del Giudice, C.; Vaia, E.; Liccardo, D.; Marzano, F.; Valletta, A.; Spagnuolo, G.; Ferrara, N.; Rengo, C.; Cannavo, A.; Rengo, G. Infective endocarditis: A focus on oral microbiota. Microorganisms 2021, 9, 1218. [Google Scholar] [CrossRef] [PubMed]
- Purificação, A.D.d.; Azevedo, N.M.d.; Araujo, G.G.d.; Souza, R.F.d.; Guzzo, C.R. The world of cyclic dinucleotides in bacterial behavior. Molecules 2020, 25, 2462. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Lau, P.C.; Tang, N.; Svensäter, G.; Ellen, R.P.; Cvitkovitch, D.G. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 2002, 184, 6333–6342. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Gomelsky, M.; Galperin, M.Y. C-di-GMP: The dawning of a novel bacterial signalling system. Mol. Microbiol. 2005, 57, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Simm, R.; Morr, M.; Kader, A.; Nimtz, M.; Römling, U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004, 53, 1123–1134. [Google Scholar] [CrossRef]
- Cotter, P.A.; Stibitz, S. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr. Opin. Microbiol. 2007, 10, 17–23. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Römling, U. Cyclic di-GMP, an established secondary messenger still speeding up. Environ. Microbiol. 2012, 14, 1817–1829. [Google Scholar] [CrossRef]
- Purcell, E.B.; McKee, R.W.; McBride, S.M.; Waters, C.M.; Tamayo, R. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J. Bacteriol. 2012, 194, 3307–3316. [Google Scholar] [CrossRef]
- An, S.; Wu, J.e.; Zhang, L.-H. Modulation of Pseudomonas aeruginosa biofilm dispersal by a cyclic-Di-GMP phosphodiesterase with a putative hypoxia-sensing domain. Appl. Environ. Microbiol. 2010, 76, 8160–8173. [Google Scholar] [CrossRef]
- Roy, A.B.; Petrova, O.E.; Sauer, K. The phosphodiesterase DipA (PA5017) is essential for Pseudomonas aeruginosa biofilm dispersion. J. Bacteriol. 2012, 194, 2904–2915. [Google Scholar] [CrossRef] [PubMed]
- Monds, R.D.; Newell, P.D.; Gross, R.H.; O’Toole, G.A. Phosphate-dependent modulation of c-di-GMP levels regulates Pseudomonas fluorescens Pf0-1 biofilm formation by controlling secretion of the adhesin LapA. Mol. Microbiol. 2007, 63, 656–679. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Yang, Z.; Pu, M.; Peti, W.; Wood, T.K. Engineering a novel c-di-GMP-binding protein for biofilm dispersal. Environ. Microbiol. 2011, 13, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, G.; Wood, T.K. Escherichia coli BdcA controls biofilm dispersal in Pseudomonas aeruginosa and Rhizobium meliloti. BMC Res. Notes 2011, 4, 447. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.-S.; Choi, H.-Y.; Yoon, S.S.; Kim, W.-G. Terrein is an inhibitor of quorum sensing and c-di-GMP in Pseudomonas aeruginosa: A connection between quorum sensing and c-di-GMP. Sci. Rep. 2018, 8, 8617. [Google Scholar] [CrossRef]
- Andersen, J.B.; Hultqvist, L.D.; Jansen, C.U.; Jakobsen, T.H.; Nilsson, M.; Rybtke, M.; Uhd, J.; Fritz, B.G.; Seifert, R.; Berthelsen, J. Identification of small molecules that interfere with c-di-GMP signaling and induce dispersal of Pseudomonas aeruginosa biofilms. NPJ Biofilms Microbiomes 2021, 7, 59. [Google Scholar] [CrossRef]
- Xuan, T.-F.; Wang, Z.-Q.; Liu, J.; Yu, H.-T.; Lin, Q.-W.; Chen, W.-M.; Lin, J. Design and synthesis of novel c-di-GMP G-quadruplex inducers as bacterial biofilm inhibitors. J. Med. Chem. 2021, 64, 11074–11089. [Google Scholar] [CrossRef]
- Lieberman, O.J.; Orr, M.W.; Wang, Y.; Lee, V.T. High-throughput screening using the differential radial capillary action of ligand assay identifies ebselen as an inhibitor of diguanylate cyclases. ACS Chem. Biol. 2014, 9, 183–192. [Google Scholar] [CrossRef]
- Wiggers, H.J.; Crusca, E.; Silva, É.E.; Cheleski, J.; Torres, N.U.; Navarro, M.V. Identification of anti-inflammatory and anti-hypertensive drugs as inhibitors of bacterial diguanylate cyclases. J. Braz. Chem. Soc. 2018, 29, 297–309. [Google Scholar] [CrossRef]
- Fernicola, S.; Paiardini, A.; Giardina, G.; Rampioni, G.; Leoni, L.; Cutruzzolà, F.; Rinaldo, S. In silico discovery and in vitro validation of catechol-containing sulfonohydrazide compounds as potent inhibitors of the diguanylate cyclase PleD. J. Bacteriol. 2016, 198, 147–156. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Viljoen, A. Binding strength of gram-positive bacterial adhesins. Front. Microbiol. 2020, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.A.; Foster, T.J.; Speziale, P.; Dufrêne, Y.F. Live-cell nanoscopy in antiadhesion therapy. Trends Microbiol. 2017, 25, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N.A.; Ton-That, H. Bacterial pili and fimbriae. eLS 2020, 1–13. [Google Scholar] [CrossRef]
- Cascioferro, S.; Cusimano, M.G.; Schillaci, D. Antiadhesion agents against Gram-positive pathogens. Future Microbiol. 2014, 9, 1209–1220. [Google Scholar] [CrossRef]
- Totsika, M.; Kostakioti, M.; Hannan, T.J.; Upton, M.; Beatson, S.A.; Janetka, J.W.; Hultgren, S.J.; Schembri, M.A. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J. Infect. Dis. 2013, 208, 921–928. [Google Scholar] [CrossRef]
- Shanmugasundarasamy, T.; Govindarajan, D.K.; Kandaswamy, K. A review on pilus assembly mechanisms in Gram-positive and Gram-negative bacteria. Cell Surf. 2022, 8, 100077. [Google Scholar] [CrossRef]
- Connell, I.; Agace, W.; Klemm, P.; Schembri, M.; Mărild, S.; Svanborg, C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 1996, 93, 9827–9832. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Kalas, V.; Pinkner, J.S.; Chen, S.L.; Spaulding, C.N.; Dodson, K.W.; Hultgren, S.J. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc. Natl. Acad. Sci. USA 2013, 110, 15530–15537. [Google Scholar] [CrossRef]
- Mulvey, M.A.; Schilling, J.D.; Hultgren, S.J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 2001, 69, 4572–4579. [Google Scholar] [CrossRef]
- Clyne, M. Oral FimH inhibitors effective against UTI. Nat. Rev. Urol. 2012, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Mousavifar, L.; Sarshar, M.; Bridot, C.; Scribano, D.; Ambrosi, C.; Palamara, A.T.; Vergoten, G.; Roubinet, B.; Landemarre, L.; Bouckaert, J. Insightful Improvement in the design of potent uropathogenic E. coli FimH antagonists. Pharmaceutics 2023, 15, 527. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and anti-adhesive therapeutics: A disarming strategy against uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef]
- Sattigeri, J.A.; Garg, M.; Bhateja, P.; Soni, A.; Rauf, A.R.A.; Gupta, M.; Deshmukh, M.S.; Jain, T.; Alekar, N.; Barman, T.K. Synthesis and evaluation of thiomannosides, potent and orally active FimH inhibitors. Bioorganic Med. Chem. Lett. 2018, 28, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Laveissière, A.; Desachy, G.; Barnich, N.; Sivignon, A.; Maresca, M.; Nicoletti, C.; Di Pasquale, E.; Martinez-Medina, M.; Simpson, K.W. Blockage of bacterial FimH prevents mucosal inflammation associated with Crohn’s disease. Microbiome 2021, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, T.; Brissonnet, Y.; Sivignon, A.; Deniaud, D.; Cremet, L.; Barnich, N.; Bouckaert, J.; Gouin, S. Inhibition profiles of mono-and polyvalent FimH antagonists against 10 different Escherichia coli strains. Org. Biomol. Chem. 2015, 13, 11369–11375. [Google Scholar] [CrossRef]
- Cusumano, C.K.; Pinkner, J.S.; Han, Z.; Greene, S.E.; Ford, B.A.; Crowley, J.R.; Henderson, J.P.; Janetka, J.W.; Hultgren, S.J. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci. Transl. Med. 2011, 3, 109ra115. [Google Scholar] [CrossRef]
- Wang, T.; Jimmidi, R.; Roubinet, B.; Landemarre, L.; Vincent, S.P. Glycofullerene–AuNPs as multivalent ligands of DC-SIGN and bacterial lectin FimH: Tuning nanoparticle size and ligand density. Nanoscale 2023, 15, 11657–11666. [Google Scholar] [CrossRef]
- Durka, M.; Buffet, K.; Iehl, J.; Holler, M.; Nierengarten, J.-F.; Taganna, J.; Bouckaert, J.; Vincent, S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH—Towards novel bacterial antiadhesives. Chem. Commun. 2011, 47, 1321–1323. [Google Scholar] [CrossRef]
- Denis, K.; Le Bris, M.; Le Guennec, L.; Barnier, J.-P.; Faure, C.; Gouge, A.; Bouzinba-Ségard, H.; Jamet, A.; Euphrasie, D.; Durel, B. Targeting Type IV pili as an antivirulence strategy against invasive meningococcal disease. Nat. Microbiol. 2019, 4, 972–984. [Google Scholar] [CrossRef]
- Dye, K.J.; Vogelaar, N.J.; O’Hara, M.; Sobrado, P.; Santos, W.; Carlier, P.R.; Yang, Z. Discovery of two inhibitors of the type IV pilus assembly ATPase PilB as potential antivirulence compounds. Microbiol. Spectr. 2022, 10, e03877-22. [Google Scholar] [CrossRef] [PubMed]
- Kisiela, D.I.; Avagyan, H.; Friend, D.; Jalan, A.; Gupta, S.; Interlandi, G.; Liu, Y.; Tchesnokova, V.; Rodriguez, V.B.; Sumida, J.P. Inhibition and reversal of microbial attachment by an antibody with parasteric activity against the FimH adhesin of uropathogenic E. coli. PLoS Pathog. 2015, 11, e1004857. [Google Scholar] [CrossRef] [PubMed]
- Sokurenko, E.V.; Tchesnokova, V.; Interlandi, G.; Klevit, R.; Thomas, W.E. Neutralizing antibodies against allosteric proteins: Insights from a bacterial adhesin. J. Mol. Biol. 2022, 434, 167717. [Google Scholar] [CrossRef] [PubMed]
- Tursi, S.A.; Puligedda, R.D.; Szabo, P.; Nicastro, L.K.; Miller, A.L.; Qiu, C.; Gallucci, S.; Relkin, N.R.; Buttaro, B.A.; Dessain, S.K. Salmonella Typhimurium biofilm disruption by a human antibody that binds a pan-amyloid epitope on curli. Nat. Commun. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Svensson, A.; Larsson, A.; Emtenäs, H.; Hedenström, M.; Fex, T.; Hultgren, S.J.; Pinker, J.S.; Almqvist, F.; Kihlberg, J. Design and evaluation of pilicides: Potential novel antibacterial agents directed against uropathogenic Escherichia coli. ChemBioChem (Print) 2001, 2, 915–918. [Google Scholar] [CrossRef]
- Åberg, V.; Fällman, E.; Axner, O.; Uhlin, B.E.; Hultgren, S.J.; Almqvist, F. Pilicides regulate pili expression in E. coli without affecting the functional properties of the pilus rod. Mol. BioSystems 2007, 3, 214–218. [Google Scholar] [CrossRef]
- Chorell, E.; Pinkner, J.S.; Phan, G.; Edvinsson, S.; Buelens, F.; Remaut, H.; Waksman, G.; Hultgren, S.J.; Almqvist, F. Design and synthesis of C-2 substituted thiazolo and dihydrothiazolo ring-fused 2-pyridones: Pilicides with increased antivirulence activity. J. Med. Chem. 2010, 53, 5690–5695. [Google Scholar] [CrossRef]
- Chorell, E.; Pinkner, J.S.; Bengtsson, C.; Banchelin, T.S.-L.; Edvinsson, S.; Linusson, A.; Hultgren, S.J.; Almqvist, F. Mapping pilicide anti-virulence effect in Escherichia coli, a comprehensive structure–activity study. Bioorganic Med. Chem. 2012, 20, 3128–3142. [Google Scholar] [CrossRef]
- Greene, S.E.; Pinkner, J.S.; Chorell, E.; Dodson, K.W.; Shaffer, C.L.; Conover, M.S.; Livny, J.; Hadjifrangiskou, M.; Almqvist, F.; Hultgren, S.J. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 2014, 5, e02038-14. [Google Scholar] [CrossRef]
- Åberg, V.; Almqvist, F. Pilicides—Small molecules targeting bacterial virulence. Org. Biomol. Chem. 2007, 5, 1827–1834. [Google Scholar] [CrossRef]
- Chorell–, E.; Pinkner, J.S.; Bengtsson, C.; Edvinsson, S.; Cusumano, C.K.; Rosenbaum, E.; Johansson, L.B.; Hultgren, S.J.; Almqvist, F. Design and synthesis of fluorescent pilicides and curlicides: Bioactive tools to study bacterial virulence mechanisms. Chem.–A Eur. J. 2012, 18, 4522–4532. [Google Scholar] [CrossRef] [PubMed]
- Cegelski, L.; Pinkner, J.S.; Hammer, N.D.; Cusumano, C.K.; Hung, C.S.; Chorell, E.; Åberg, V.; Walker, J.N.; Seed, P.C.; Almqvist, F. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009, 5, 913–919. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—the’accidental’pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Immune evasion by staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Surface proteins of Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. The MSCRAMM family of cell-wall-anchored surface proteins of gram-positive cocci. Trends Microbiol. 2019, 27, 927–941. [Google Scholar] [CrossRef]
- Paharik, A.E.; Horswill, A.R. The staphylococcal biofilm: Adhesins, regulation, and host response. Virulence Mech. Bact. Pathog. 2016, 4, 529–566. [Google Scholar]
- Deivanayagam, C.C.; Perkins, S.; Danthuluri, S.; Owens, R.T.; Bice, T.; Nanavathy, T.; Foster, T.J.; Höök, M.; Narayana, S.V. Crystallization of ClfA and ClfB fragments: The fibrinogen-binding surface proteins of Staphylococcus aureus. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999, 55, 554–556. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbone, D.; Parrino, B.; Cascioferro, S.; Diana, P. Recent developments in the inhibition of bacterial adhesion as promising anti-virulence strategy. Int. J. Mol. Sci. 2023, 24, 4872. [Google Scholar] [CrossRef]
- Carbone, A.; Cascioferro, S.; Parrino, B.; Carbone, D.; Pecoraro, C.; Schillaci, D.; Cusimano, M.G.; Cirrincione, G.; Diana, P. Thiazole analogues of the marine alkaloid nortopsentin as inhibitors of bacterial biofilm formation. Molecules 2020, 26, 81. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, D.; Cascioferro, S.; Pecoraro, C.; Giovannetti, E.; Deng, D.; Di Sarno, V.; Musella, S.; Auriemma, G.; Cusimano, M.G. 1, 2, 4-Oxadiazole topsentin analogs as staphylococcal biofilm inhibitors targeting the bacterial transpeptidase sortase A. Eur. J. Med. Chem. 2021, 209, 112892. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Parrino, B.; Petri, G.L.; Cusimano, M.G.; Schillaci, D.; Di Sarno, V.; Musella, S.; Giovannetti, E.; Cirrincione, G.; Diana, P. 2, 6-Disubstituted imidazo [2, 1-b][1, 3, 4] thiadiazole derivatives as potent staphylococcal biofilm inhibitors. Eur. J. Med. Chem. 2019, 167, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luan, Y.; Hou, J.; Jiang, T.; Zhao, Y.; Song, W.; Wang, L.; Kong, X.; Guan, J.; Song, D. The protection effect of rhodionin against methicillin-resistant Staphylococcus aureus-induced pneumonia through sortase A inhibition. World J. Microbiol. Biotechnol. 2023, 39, 18. [Google Scholar] [CrossRef] [PubMed]
- Jaudzems, K.; Kurbatska, V.; Jëkabsons, A.; Bobrovs, R.; Rudevica, Z.; Leonchiks, A. Targeting bacterial Sortase A with covalent inhibitors: 27 new starting points for structure-based hit-to-lead optimization. ACS Infect. Dis. 2019, 6, 186–194. [Google Scholar] [CrossRef]
- Barthels, F.; Meyr, J.; Hammerschmidt, S.J.; Marciniak, T.; Räder, H.-J.; Ziebuhr, W.; Engels, B.; Schirmeister, T. 2-Sulfonylpyrimidines as Privileged Warheads for the Development of S. aureus Sortase A Inhibitors. Front. Mol. Biosci. 2022, 8, 804970. [Google Scholar] [CrossRef]
- Kim, S.-H.; Shin, D.-S.; Oh, M.-N.; Chung, S.-C.; Lee, J.-S.; Chang, I.-M.; Oh, K.-B. Inhibition of sortase, a bacterial surface protein anchoring transpeptidase, by β-sitosterol-3-O-glucopyranoside from Fritillaria verticillata. Biosci. Biotechnol. Biochem. 2003, 67, 2477–2479. [Google Scholar] [CrossRef]
- Kim, S.-H.; Shin, D.-S.; Oh, M.-N.; Chung, S.-C.; Lee, J.-S.; Oh, K.-B. Inhibition of the bacterial surface protein anchoring transpeptidase sortase by isoquinoline alkaloids. Biosci. Biotechnol. Biochem. 2004, 68, 421–424. [Google Scholar] [CrossRef]
- Oh, K.-B.; Mar, W.; Kim, S.; Kim, J.-Y.; Oh, M.-N.; Kim, J.-G.; Shin, D.; Sim, C.J.; Shin, J. Bis (indole) alkaloids as sortase A inhibitors from the sponge Spongosorites sp. Bioorganic Med. Chem. Lett. 2005, 15, 4927–4931. [Google Scholar] [CrossRef]
- Jang, K.H.; Chung, S.-C.; Shin, J.; Lee, S.-H.; Kim, T.-I.; Lee, H.-S.; Oh, K.-B. Aaptamines as sortase A inhibitors from the tropical sponge Aaptos aaptos. Bioorganic Med. Chem. Lett. 2007, 17, 5366–5369. [Google Scholar] [CrossRef]
- Oh, I.; Yang, W.-Y.; Chung, S.-C.; Kim, T.-Y.; Oh, K.-B.; Shin, J. In vitro sortase A inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Arch. Pharmacal Res. 2011, 34, 217–222. [Google Scholar] [CrossRef]
- Park, B.-S.; Kim, J.-G.; Kim, M.-R.; Lee, S.-E.; Takeoka, G.R.; Oh, K.-B.; Kim, J.-H. Curcuma longa L. constituents inhibit sortase A and Staphylococcus aureus cell adhesion to fibronectin. J. Agric. Food Chem. 2005, 53, 9005–9009. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-Y.; Won, T.H.; Ahn, C.-H.; Lee, S.-H.; Yang, H.-C.; Shin, J.; Oh, K.-B. Streptococcus mutans sortase A inhibitory metabolites from the flowers of Sophora japonica. Bioorganic Med. Chem. Lett. 2015, 25, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, I.-H.; Lee, J.-H.; Yang, W.-Y.; Oh, K.-B.; Shin, J. Sortase A inhibitory metabolites from the roots of Pulsatilla koreana. Bioorganic Med. Chem. Lett. 2014, 24, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.; Wereszczynski, J.; Amer, B.R.; Yi, S.W.; Jung, M.E.; McCammon, J.A.; Clubb, R.T. Discovery of s taphylococcus aureus sortase a inhibitors using virtual screening and the relaxed complex scheme. Chem. Biol. Drug Des. 2013, 82, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Zhu, K.; Gong, S.; Dramsi, S.; Wang, Y.-T.; Li, J.; Chen, F.; Zhang, R.; Zhou, L. Antiinfective therapy with a small molecule inhibitor of Staphylococcus aureus sortase. Proc. Natl. Acad. Sci. USA 2014, 111, 13517–13522. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Li, J.; Jing, S.; Jin, Y.; Yang, L.; Yu, H.; Wang, D.; Wang, T.; Wang, L. Eriodictyol as a potential candidate inhibitor of sortase A protects mice from methicillin-resistant Staphylococcus aureus-induced pneumonia. Front. Microbiol. 2021, 12, 635710. [Google Scholar] [CrossRef]
- Prencipe, F.; Alsibaee, A.; Khaddem, Z.; Norton, P.; Towell, A.M.; Ali, A.F.; Reid, G.; Fleury, O.M.; Foster, T.J.; Geoghegan, J.A. Allantodapsone is a Pan-inhibitor of Staphylococcus aureus adhesion to fibrinogen, Loricrin, and cytokeratin 10. Microbiol. Spectr. 2022, 10, e01175-21. [Google Scholar] [CrossRef]
- Hall, A.E.; Domanski, P.J.; Patel, P.R.; Vernachio, J.H.; Syribeys, P.J.; Gorovits, E.L.; Johnson, M.A.; Ross, J.M.; Hutchins, J.T.; Patti, J.M. Characterization of a Protective Monoclonal AntibodyRecognizing Staphylococcus aureus MSCRAMM ProteinClumping FactorA. Infect. Immun. 2003, 71, 6864–6870. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Doan, T.N.; Sato, K.; Tkaczyk, C.; Sellman, B.R.; Diep, B.A. Monoclonal antibodies neutralizing alpha-hemolysin, bicomponent leukocidins, and clumping factor A protected against Staphylococcus aureus-induced acute circulatory failure in a mechanically ventilated rabbit model of hyperdynamic septic shock. Front. Immunol. 2023, 14, 1260627. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Hamilton, M.; Sadowska, A.; Shi, Y.; Chang, C.; Chowdhury, P.; Buonapane, R.; Xiao, X.; Warrener, P.; Mediavilla, J. Targeting alpha toxin and ClfA with a multimechanistic monoclonal-antibody-based approach for prophylaxis of serious Staphylococcus aureus disease. mBio 2016, 7, e00528-16. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Kasturirangan, S.; Minola, A.; Jones-Nelson, O.; Gunter, V.; Shi, Y.; Rosenthal, K.; Aleti, V.; Semenova, E.; Warrener, P. Multimechanistic monoclonal antibodies (MAbs) targeting Staphylococcus aureus alpha-toxin and clumping factor A: Activity and efficacy comparisons of a MAb combination and an engineered bispecific antibody approach. Antimicrob. Agents Chemother. 2017, 61, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- Feuillie, C.; Formosa-Dague, C.; Hays, L.M.; Vervaeck, O.; Derclaye, S.; Brennan, M.P.; Foster, T.J.; Geoghegan, J.A.; Dufrêne, Y.F. Molecular interactions and inhibition of the staphylococcal biofilm-forming protein SdrC. Proc. Natl. Acad. Sci. USA 2017, 114, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Haataja, S.; Verma, P.; Fu, O.; Papageorgiou, A.C.; Pöysti, S.; Pieters, R.J.; Nilsson, U.J.; Finne, J. Rationally designed chemically modified glycodendrimer inhibits Streptococcus suis adhesin SadP at picomolar concentrations. Chem.-A Eur. J. 2018, 24, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Herman-Bausier, P.; Valotteau, C.; Pietrocola, G.; Rindi, S.; Alsteens, D.; Foster, T.J.; Speziale, P.; Dufrêne, Y.F. Mechanical strength and inhibition of the Staphylococcus aureus collagen-binding protein Cna. mBio 2016, 7, e01529-16. [Google Scholar] [CrossRef]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef]
- Sambanthamoorthy, K.; Gokhale, A.A.; Lao, W.; Parashar, V.; Neiditch, M.B.; Semmelhack, M.F.; Lee, I.; Waters, C.M. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 2011, 55, 4369–4378. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel approaches to combat medical device-associated biofilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Sheehan, J.; Sadlier, C.; O’Brien, B. Bacterial endotoxins and exotoxins in intensive care medicine. BJA Educ. 2022, 22, 224. [Google Scholar] [CrossRef]
- Fasano, A.; Baudry, B.; Pumplin, D.W.; Wasserman, S.S.; Tall, B.D.; Ketley, J.M.; Kaper, J. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 1991, 88, 5242–5246. [Google Scholar] [CrossRef]
- Benyamini, P. Phylogenetic Tracing of Evolutionarily Conserved Zonula Occludens Toxin Reveals a “High Value” Vaccine Candidate Specific for Treating Multi-Strain Pseudomonas aeruginosa Infections. Toxins 2024, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Lawn, J.; Vandelaer, J.; Roper, M.; Cousens, S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int. J. Epidemiol. 2010, 39, i102–i109. [Google Scholar] [CrossRef] [PubMed]
- Stratton, K.R.; Howe, C.J.; Johnston, R.B., Jr. Diphtheria and tetanus toxoids. In Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality; National Academies Press (US): New York, NY, USA, 1994. [Google Scholar]
- Rezzoagli, C.; Archetti, M.; Mignot, I.; Baumgartner, M.; Kümmerli, R. Combining antibiotics with antivirulence compounds can have synergistic effects and reverse selection for antibiotic resistance in Pseudomonas aeruginosa. PLoS Biol. 2020, 18, e3000805. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Pethe, K.; Chan-Park, M.B. Chemical basis of combination therapy to combat antibiotic resistance. JACS Au 2023, 3, 276–292. [Google Scholar] [CrossRef]
- Marinaro, M.; Fasano, A.; De Magistris, M.T. Zonula occludens toxin acts as an adjuvant through different mucosal routes and induces protective immune responses. Infect. Immun. 2003, 71, 1897–1902. [Google Scholar] [CrossRef]
- Jeon, S.; Kelly, M.; Yun, J.; Lee, B.; Park, M.; Whang, Y.; Lee, C.; Halvorsen, Y.-D.; Verma, S.; Charles, R.C. Scalable production and immunogenicity of a cholera conjugate vaccine. Vaccine 2021, 39, 6936–6946. [Google Scholar] [CrossRef]
- Stratton, K.; Ford, A.; Rusch, E.; Clayton, E.W.; Vaccines, C. Diphtheria Toxoid–, Tetanus Toxoid–, and Acellular Pertussis–Containing Vaccines. In Adverse Effects of Vaccines: Evidence and Causality; National Academies Press (US): New York, NY, USA, 2011. [Google Scholar]
- Germanier, R.; Fürer, E.; Varallya, S.; Inderbitzin, T. Antigenicity of cholera toxoid in humans. J. Infect. Dis. 1977, 135, 512–516. [Google Scholar] [CrossRef]
- Roper, M.H.; Wassilak, S.G.; Scobie, H.M.; Ridpath, A.D.; Orenstein, W.A. Tetanus toxoid. In Plotkin’s Vaccines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1052–1079.e1018. [Google Scholar]
- Mills, J.P.; Rao, K.; Young, V.B. Probiotics for prevention of Clostridium difficile infection. Curr. Opin. Gastroenterol. 2018, 34, 3–10. [Google Scholar] [CrossRef]
- Megighian, A.; Pirazzini, M.; Fabris, F.; Rossetto, O.; Montecucco, C. Tetanus and tetanus neurotoxin: From peripheral uptake to central nervous tissue targets. J. Neurochem. 2021, 158, 1244–1253. [Google Scholar] [CrossRef]
- Fishman, P.S.; Carrigan, D.R. Motoneuron uptake from the circulation of the binding fragment of tetanus toxin. Arch. Neurol. 1988, 45, 558–561. [Google Scholar] [CrossRef]
- Montecucco, C.; Schiavo, G. Structure and function of tetanus and botulinum neurotoxins. Q. Rev. Biophys. 1995, 28, 423–472. [Google Scholar] [CrossRef] [PubMed]
- Sakari, M.; Laisi, A.; Pulliainen, A.T. Exotoxin-targeted drug modalities as antibiotic alternatives. ACS Infect. Dis. 2022, 8, 433–456. [Google Scholar] [CrossRef]
- Pirazzini, M.; Grinzato, A.; Corti, D.; Barbieri, S.; Leka, O.; Vallese, F.; Tonellato, M.; Silacci-Fregni, C.; Piccoli, L.; Kandiah, E. Exceptionally potent human monoclonal antibodies are effective for prophylaxis and treatment of tetanus in mice. J. Clin. Investig. 2021, 131, e151676. [Google Scholar] [CrossRef] [PubMed]
- Aliprandini, E.; Takata, D.Y.; Lepique, A.; Kalil, J.; Boscardin, S.B.; Moro, A.M. An oligoclonal combination of human monoclonal antibodies able to neutralize tetanus toxin in vivo. Toxicon X 2019, 2, 100006. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Kamei, M.; Sugimoto, N.; Hashizume, S. Application of Anti-Tetanus Human Monoclonal Antibodies. In Proceedings of the Animal Cell Technology: Basic & Applied Aspects: Proceedings of the Fifth International Meeting of the Japanese Association for Animal Cell Technology, Omiya, Japan, 30 November–4 December 1992; pp. 617–623.
- Shinefield, H.; Black, S.; Fattom, A.; Horwith, G.; Rasgon, S.; Ordonez, J.; Yeoh, H.; Law, D.; Robbins, J.B.; Schneerson, R. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 2002, 346, 491–496. [Google Scholar] [CrossRef]
- Begier, E.; Seiden, D.J.; Patton, M.; Zito, E.; Severs, J.; Cooper, D.; Eiden, J.; Gruber, W.C.; Jansen, K.U.; Anderson, A.S. SA4Ag, a 4-antigen Staphylococcus aureus vaccine, rapidly induces high levels of bacteria-killing antibodies. Vaccine 2017, 35, 1132–1139. [Google Scholar] [CrossRef]
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220. [Google Scholar] [CrossRef]
- Ihssen, J.; Kowarik, M.; Dilettoso, S.; Tanner, C.; Wacker, M.; Thöny-Meyer, L. Production of glycoprotein vaccines in Escherichia coli. Microb. Cell Factories 2010, 9, 61. [Google Scholar] [CrossRef]
- Wacker, M.; Wang, L.; Kowarik, M.; Dowd, M.; Lipowsky, G.; Faridmoayer, A.; Shields, K.; Park, S.; Alaimo, C.; Kelley, K.A. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J. Infect. Dis. 2014, 209, 1551–1561. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Zhou, H.; Yang, Y.; Tong, Y.-N.; Peng, L.-S.; Zhu, B.-H.; Diao, W.-B.; Zeng, H.; Sun, H.-W.; Zou, Q.-M. Protective effects of a nanoemulsion adjuvant vaccine (2C-Staph/NE) administered intranasally against invasive Staphylococcus aureus pneumonia. RSC Adv. 2018, 8, 9996–10008. [Google Scholar] [CrossRef]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus vaccine research and development: The past, present and future, including novel therapeutic strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef] [PubMed]
- Cripps, A.W.; Peek, K.; Dunkley, M.; Vento, K.; Marjason, J.K.; McIntyre, M.E.; Sizer, P.; Croft, D.; Sedlak-Weinstein, L. Safety and immunogenicity of an oral inactivated whole-cell Pseudomonas aeruginosa vaccine administered to healthy human subjects. Infect. Immun. 2006, 74, 968–974. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamei, A.; Coutinho-Sledge, Y.S.; Goldberg, J.B.; Priebe, G.P.; Pier, G.B. Mucosal vaccination with a multivalent, live-attenuated vaccine induces multifactorial immunity against Pseudomonas aeruginosa acute lung infection. Infect. Immun. 2011, 79, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Meynet, E.; Laurin, D.; Lenormand, J.L.; Camara, B.; Toussaint, B.; Le Gouëllec, A. Killed but metabolically active Pseudomonas aeruginosa-based vaccine induces protective humoral-and cell-mediated immunity against Pseudomonas aeruginosa pulmonary infections. Vaccine 2018, 36, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, F.; Zou, J.; Wu, W.; Jing, H.; Gou, Q.; Li, H.; Gu, J.; Zou, Q.; Zhang, J. Immunization with Pseudomonas aeruginosa outer membrane vesicles stimulates protective immunity in mice. Vaccine 2018, 36, 1047–1054. [Google Scholar] [CrossRef]
- Chirani, A.S.; Majidzadeh, R.; Pouriran, R.; Heidary, M.; Nasiri, M.J.; Gholami, M.; Goudarzi, M.; Omrani, V.F. The effect of in silico targeting Pseudomonas aeruginosa patatin-like protein D, for immunogenic administration. Comput. Biol. Chem. 2018, 74, 12–19. [Google Scholar] [CrossRef]
- Saha, S.; Takeshita, F.; Matsuda, T.; Jounai, N.; Kobiyama, K.; Matsumoto, T.; Sasaki, S.; Yoshida, A.; Xin, K.-Q.; Klinman, D.M. Blocking of the TLR5 activation domain hampers protective potential of flagellin DNA vaccine. J. Immunol. 2007, 179, 1147–1154. [Google Scholar] [CrossRef]
- Solanki, V.; Tiwari, M.; Tiwari, V. Prioritization of potential vaccine targets using comparative proteomics and designing of the chimeric multi-epitope vaccine against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 5240. [Google Scholar] [CrossRef]
- Micoli, F.; Costantino, P.; Adamo, R. Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef]
- Fereshteh, S.; Haririzadeh Jouriani, F.; Noori Goodarzi, N.; Torkamaneh, M.; Khasheii, B.; Badmasti, F. Defeating a superbug: A breakthrough in vaccine design against multidrug-resistant Pseudomonas aeruginosa using reverse vaccinology. PLoS ONE 2023, 18, e0289609. [Google Scholar] [CrossRef]
- Killough, M.; Rodgers, A.M.; Ingram, R.J. Pseudomonas aeruginosa: Recent advances in vaccine development. Vaccines 2022, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Rungta, T.; Goyal, K.; Singh, M.P. Designing a multi-epitope based vaccine to combat Kaposi Sarcoma utilizing immunoinformatics approach. Sci. Rep. 2019, 9, 2517. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, M.; Bian, Y.; Zheng, X.; Tong, R.; Sun, X. Advanced subunit vaccine delivery technologies: From vaccine cascade obstacles to design strategies. Acta Pharm. Sin. B 2023, 13, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ullah, I.; Zhu, B.; Adnan, M.; Liaqat, R.; Kong, W.-B.; Niu, S. In silico analysis of epitope-based vaccine candidate against tuberculosis using reverse vaccinology. Sci. Rep. 2021, 11, 1249. [Google Scholar] [CrossRef]
- Jalal, K.; Abu-Izneid, T.; Khan, K.; Abbas, M.; Hayat, A.; Bawazeer, S.; Uddin, R. Identification of vaccine and drug targets in Shigella dysenteriae sd197 using reverse vaccinology approach. Sci. Rep. 2022, 12, 251. [Google Scholar] [CrossRef]
- Shahbazi, S.; Sabzi, S.; Goodarzi, N.N.; Fereshteh, S.; Bolourchi, N.; Mirzaie, B.; Badmasti, F. Identification of novel putative immunogenic targets and construction of a multi-epitope vaccine against multidrug-resistant Corynebacterium jeikeium using reverse vaccinology approach. Microb. Pathog. 2022, 164, 105425. [Google Scholar] [CrossRef]
- Bianconi, I.; Alcalá-Franco, B.; Scarselli, M.; Dalsass, M.; Buccato, S.; Colaprico, A.; Marchi, S.; Masignani, V.; Bragonzi, A. Genome-based approach delivers vaccine candidates against Pseudomonas aeruginosa. Front. Immunol. 2019, 9, 3021. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Hendrix, R.W. Bacteriophages and their genomes. Curr. Opin. Virol. 2011, 1, 298–303. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Devoto, A.E.; Santini, J.M.; Olm, M.R.; Anantharaman, K.; Munk, P.; Tung, J.; Archie, E.A.; Turnbaugh, P.J.; Seed, K.D.; Blekhman, R. Megaphages infect Prevotella and variants are widespread in gut microbiomes. Nat. Microbiol. 2019, 4, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Doore, S.M.; Fane, B.A. The microviridae: Diversity, assembly, and experimental evolution. Virology 2016, 491, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S. Communication between viruses guides lysis–lysogeny decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S.; Datta, S.; Prasad, R.; Dubey, D.; Prasad, R.K.; Vairale, M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2017, 62, 17–55. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Marshall, M.S. Observations on d’Herelle’s bacteriophage. J. Infect. Dis. 1925, 37, 126–160. [Google Scholar] [CrossRef]
- Düzgüneş, N.; Sessevmez, M.; Yildirim, M. Bacteriophage therapy of bacterial infections: The rediscovered frontier. Pharmaceuticals 2021, 14, 34. [Google Scholar] [CrossRef]
- Chanishvili, N.; Myelnikov, D.; Blauvelt, T.K. Professor Giorgi Eliava and the Eliava Institute of Bacteriophage. Ther. Appl. Res. 2022, 3, 71–80. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Jończyk-Matysiak, E.; Kniotek, M.; Letkiewicz, S. Therapeutic Phages as Modulators of the Immune Response: Practical Implications. Clin. Infect. Dis. 2023, 77, S433–S439. [Google Scholar] [CrossRef] [PubMed]
- Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Górski, A. Phage therapy in Poland–a centennial journey to the first ethically approved treatment facility in Europe. Front. Microbiol. 2020, 11, 531093. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; De Vos, D.; Verbeken, G.; Merabishvili, M.; Chanishvili, N.; Vaneechoutte, M.; Zizi, M.; Laire, G.; Lavigne, R.; Huys, I. The phage therapy paradigm: Prêt-à-porter or sur-mesure? Pharm. Res. 2011, 28, 934–937. [Google Scholar] [CrossRef]
- Mattila, S.; Ruotsalainen, P.; Jalasvuori, M. On-demand isolation of bacteriophages against drug-resistant bacteria for personalized phage therapy. Front. Microbiol. 2015, 6, 1271. [Google Scholar] [CrossRef]
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic engineering of bacteriophages against infectious diseases. Front. Microbiol. 2019, 10, 954. [Google Scholar] [CrossRef]
- Gibson, S.B.; Green, S.I.; Liu, C.G.; Salazar, K.C.; Clark, J.R.; Terwilliger, A.L.; Kaplan, H.B.; Maresso, A.W.; Trautner, B.W.; Ramig, R.F. Constructing and characterizing bacteriophage libraries for phage therapy of human infections. Front. Microbiol. 2019, 10, 2537. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered bacteriophage therapeutics: Rationale, challenges and future. BioDrugs 2021, 35, 255–280. [Google Scholar] [CrossRef]
- Tommasi, R.; Brown, D.G.; Walkup, G.K.; Manchester, J.I.; Miller, A.A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 2015, 14, 529–542. [Google Scholar] [CrossRef]
- Hatfull, G. Science Education Alliance Phage Hunters Advancing Genomics and Evolutionary Science Program, KwaZulu-Natal Research Institute for Tuberculosis and HIV Mycobacterial Genetics Course Students, Phage Hunters Integrating Research and Education Program. 2012. Complet. Genome Seq. 2012, 138, 2382–2384. [Google Scholar]
- Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaró, R. Current clinical landscape and global potential of bacteriophage therapy. Viruses 2023, 15, 1020. [Google Scholar] [CrossRef] [PubMed]
- Sakandelidze, V. The combined use of specific phages and antibiotics in different infectious allergoses. Vrachebnoe Delo 1991, 3, 60–63. [Google Scholar]
- Rice, T.B. Use of bacteriophage filtrates in treatment of suppurative conditions: Report of 300 cases. Am. J. Med. Sci. 1930, 179, 345–360. [Google Scholar] [CrossRef]
- Schless, R.A. Staphylococcus aureus meningitis: Treatment with specific bacteriophage. Am. J. Dis. Child. 1932, 44, 813–822. [Google Scholar] [CrossRef]
- Djordjevic, G.; O’sullivan, D.; Walker, S.; Conkling, M.; Klaenhammer, T. A triggered-suicide system designed as a defense against bacteriophages. J. Bacteriol. 1997, 179, 6741–6748. [Google Scholar] [CrossRef]
- Hibstu, Z.; Belew, H.; Akelew, Y.; Mengist, H.M. Phage therapy: A different approach to fight bacterial infections. Biol. Targets Ther. 2022, 16, 173–186. [Google Scholar] [CrossRef]
- Fujiki, J.; Schnabl, B. Phage Therapy: Targeting Intestinal Bacterial Microbiota for Treatment of Liver Diseases. JHEP Rep. 2023, 5, 100909. [Google Scholar] [CrossRef]
- Ali, Y.; Inusa, I.; Sanghvi, G.; Mandaliya, V.B.; Bishoyi, A.K. The current status of phage therapy and its advancement towards establishing standard antimicrobials for combating multi drug-resistant bacterial pathogens. Microb. Pathog. 2023, 181, 106199. [Google Scholar] [CrossRef]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of phage therapy across the world. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar] [PubMed]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.; Campbell, J.L. Considerations for the use of phage therapy in clinical practice. Antimicrob. Agents Chemother. 2022, 66, e02071-21. [Google Scholar] [CrossRef] [PubMed]
- Pelak, K.; Goldstein, D.; Walley, N.; Fellay, J.; Ge, D.; Shianna, K.; Gumbs, C.; Gao, X.; Maia, J.; Cronin, K. National Institute of Allergy and Infectious Diseases Center for HIV. AIDS Vaccine Immunology (CHAVI). Host determinants of HIV-1 control in African Americans. J Infect Dis 2010, 201, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef]
- LaFee, S.; Buschman, H. Novel phage therapy saves patient with multidrug-resistant bacterial infection; UC San Diego Today: La Jolla, CA, USA, 2017. [Google Scholar]
- Little, J.S.; Dedrick, R.M.; Freeman, K.G.; Cristinziano, M.; Smith, B.E.; Benson, C.A.; Jhaveri, T.A.; Baden, L.R.; Solomon, D.A.; Hatfull, G.F. Bacteriophage treatment of disseminated cutaneous Mycobacterium chelonae infection. Nat. Commun. 2022, 13, 2313. [Google Scholar] [CrossRef]
- Wu, N.; Chen, L.-K.; Zhu, T. Phage therapy for secondary bacterial infections with COVID-19. Curr. Opin. Virol. 2022, 52, 9–14. [Google Scholar] [CrossRef]
- Rostkowska, O.M.; Międzybrodzki, R.; Miszewska-Szyszkowska, D.; Górski, A.; Durlik, M. Treatment of recurrent urinary tract infections in a 60-year-old kidney transplant recipient. The use of phage therapy. Transpl. Infect. Dis. 2021, 23, e13391. [Google Scholar] [CrossRef]
- Lebeaux, D.; Merabishvili, M.; Caudron, E.; Lannoy, D.; Van Simaey, L.; Duyvejonck, H.; Guillemain, R.; Thumerelle, C.; Podglajen, I.; Compain, F. A case of phage therapy against pandrug-resistant Achromobacter xylosoxidans in a 12-year-old lung-transplanted cystic fibrosis patient. Viruses 2021, 13, 60. [Google Scholar] [CrossRef]
- Stratton, C.W. Dead bugs don’t mutate: Susceptibility issues in the emergence of bacterial resistance. Emerg. Infect. Dis. 2003, 9, 10. [Google Scholar] [CrossRef]
- Abedon, S.T.; Thomas-Abedon, C. Phage therapy pharmacology. Curr. Pharm. Biotechnol. 2010, 11, 28–47. [Google Scholar] [CrossRef]
- Skurnik, M.; Pajunen, M.; Kiljunen, S. Biotechnological challenges of phage therapy. Biotechnol. Lett. 2007, 29, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Borysowski, J.; Międzybrodzki, R.; Beata-Weber-Dąbrowska, B.-W.-D. Bacteriophages in Medicine; Caister Academic Press: Norfolk, UK, 2007. [Google Scholar]
- Skurnik, M.; Strauch, E. Phage therapy: Facts and fiction. Int. J. Med. Microbiol. 2006, 296, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar]
- Gupta, R.; Prasad, Y. Efficacy of polyvalent bacteriophage P-27/HP to control multidrug resistant Staphylococcus aureus associated with human infections. Curr. Microbiol. 2011, 62, 255–260. [Google Scholar] [CrossRef]
- Carlton, R.M. Phage therapy: Past history and future prospects. Arch. Immunol. Ther. Exp.-Engl. Ed. 1999, 47, 267–274. [Google Scholar]
- Alisky, J.; Iczkowski, K.; Rapoport, A.; Troitsky, N. Bacteriophages show promise as antimicrobial agents. J. Infect. 1998, 36, 5–15. [Google Scholar] [CrossRef]
- Diallo, K.; Dublanchet, A. Benefits of combined phage–antibiotic therapy for the control of antibiotic-resistant bacteria: A literature review. Antibiotics 2022, 11, 839. [Google Scholar] [CrossRef]
- Rosner, D.; Clark, J. Formulations for bacteriophage therapy and the potential uses of immobilization. Pharmaceuticals 2021, 14, 359. [Google Scholar] [CrossRef]
- Moraes de Souza, C.; Tanir, T.; Orellana, M.; Escalante, A.; Koeris, M.S. Manufacturing bacteriophages (part 2 of 2): Formulation, analytics and quality control considerations. Pharmaceuticals 2021, 14, 895. [Google Scholar] [CrossRef]
- Merabishvili, M.; Pirnay, J.-P.; Vogele, K.; Malik, D.J. Production of phage therapeutics and formulations: Innovative approaches. In Phage Therapy: A Practical Approach; Springer: Cham, Switzerland, 2019; pp. 3–41. [Google Scholar]
- Azam, A.H.; Tanji, Y. Bacteriophage-host arm race: An update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl. Microbiol. Biotechnol. 2019, 103, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Le, S.; Yao, X.; Lu, S.; Tan, Y.; Rao, X.; Li, M.; Jin, X.; Wang, J.; Zhao, Y.; Wu, N.C. Chromosomal DNA deletion confers phage resistance to Pseudomonas aeruginosa. Sci. Rep. 2014, 4, 4738. [Google Scholar] [CrossRef] [PubMed]
- Holguín, A.V.; Cárdenas, P.; Prada-Peñaranda, C.; Rabelo Leite, L.; Buitrago, C.; Clavijo, V.; Oliveira, G.; Leekitcharoenphon, P.; Møller Aarestrup, F.; Vives, M.J. Host resistance, genomics and population dynamics in a Salmonella Enteritidis and phage system. Viruses 2019, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Chadha, P.; Katare, O.P.; Chhibber, S. In vivo efficacy of single phage versus phage cocktail in resolving burn wound infection in BALB/c mice. Microb. Pathog. 2016, 99, 68–77. [Google Scholar] [CrossRef]
- Azam, A.H.; Kadoi, K.; Miyanaga, K.; Usui, M.; Tamura, Y.; Cui, L.; Tanji, Y. Analysis host-recognition mechanism of staphylococcal kayvirus ɸSA039 reveals a novel strategy that protects Staphylococcus aureus against infection by Staphylococcus pseudintermedius Siphoviridae phages. Appl. Microbiol. Biotechnol. 2019, 103, 6809–6823. [Google Scholar] [CrossRef]
- Fallico, V.; Ross, R.P.; Fitzgerald, G.F.; McAuliffe, O. Genetic response to bacteriophage infection in Lactococcus lactis reveals a four-strand approach involving induction of membrane stress proteins, D-alanylation of the cell wall, maintenance of proton motive force, and energy conservation. J. Virol. 2011, 85, 12032–12042. [Google Scholar] [CrossRef]
- Bera, A.; Biswas, R.; Herbert, S.; Götz, F. The presence of peptidoglycan O-acetyltransferase in various staphylococcal species correlates with lysozyme resistance and pathogenicity. Infect. Immun. 2006, 74, 4598–4604. [Google Scholar] [CrossRef]
- Sijtsma, L.; Wouters, J.; Hellingwerf, K. Isolation and characterization of lipoteichoic acid, a cell envelope component involved in preventing phage adsorption, from Lactococcus lactis subsp. cremoris SK110. J. Bacteriol. 1990, 172, 7126–7130. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Lavigne, R.; Brüssow, H. Bacteriophage therapy: Advances in formulation strategies and human clinical trials. Annu. Rev. Virol. 2015, 2, 599–618. [Google Scholar] [CrossRef]
- Nilsson, A.S. Pharmacological limitations of phage therapy. Upsala J. Med. Sci. 2019, 124, 218–227. [Google Scholar] [CrossRef]
- Caflisch, K.M.; Suh, G.A.; Patel, R. Biological challenges of phage therapy and proposed solutions: A literature review. Expert Rev. Anti-Infect. Ther. 2019, 17, 1011–1041. [Google Scholar] [CrossRef] [PubMed]
- Regulski, K.; Champion-Arnaud, P.; Gabard, J.; Harper, D.; Abedon, S.; Burrowes, B.; McConville, M. Bacteriophages: Biology, Technology, Therapy; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Mutti, M.; Corsini, L. Robust approaches for the production of active ingredient and drug product for human phage therapy. Front. Microbiol. 2019, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.; Garton, N.J.; Stapley, A.G.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Piątek, B. Phage therapy—Challenges, opportunities and future prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Littman, D.R.; Pamer, E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 2011, 10, 311–323. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. Bmj 2018, 361, k2179. [Google Scholar] [CrossRef]
- Friedrich, M. Benefits of gut microflora under study. JAMA 2008, 299, 162. [Google Scholar] [CrossRef]
- Marco, M.L. Defining how microorganisms benefit human health. Microb. Biotechnol. 2021, 14, 35–40. [Google Scholar] [CrossRef]
- Sandhu, B.K.; McBride, S.M. Clostridioides difficile. Trends Microbiol. 2018, 26, 1049–1050. [Google Scholar] [CrossRef] [PubMed]
- Juneau, C.; Mendias, E.N.P.; Wagal, N.; Loeffelholz, M.; Savidge, T.; Croisant, S.; Dann, S.M. Community-acquired Clostridium difficile infection: Awareness and clinical implications. J. Nurse Pract. 2013, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Grambow, S.C.; Woods, C.W.; Fowler, V.G.; Moehring, R.W.; Anderson, D.J.; Lewis, S.S. Epidemiologic trends in Clostridioides difficile infections in a regional community hospital network. JAMA Netw. Open 2019, 2, e1914149. [Google Scholar] [CrossRef] [PubMed]
- Hota, S.S.; Achonu, C.; Crowcroft, N.S.; Harvey, B.J.; Lauwers, A.; Gardam, M.A. Determining mortality rates attributable to Clostridium difficile infection. Emerg. Infect. Dis. 2012, 18, 305. [Google Scholar] [CrossRef]
- Shen, E.P.; Surawicz, C.M. Current treatment options for severe Clostridium difficile–associated disease. Gastroenterol. Hepatol. 2008, 4, 134. [Google Scholar]
- Al-Jashaami, L.S.; DuPont, H.L. Management of Clostridium difficile infection. Gastroenterol. Hepatol. 2016, 12, 609. [Google Scholar]
- Rohlke, F.; Stollman, N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Ther. Adv. Gastroenterol. 2012, 5, 403–420. [Google Scholar] [CrossRef]
- Ayobami, O.I.; Sunbare-Funto, O.J.; Mbah, C.E.; Ajibade, O.A.; Oyawoye, O.M.; Aborode, A.T.; Ogunleye, S.C.; Jamiu, A.; Bolarinwa, B.; Abanikannda, M.F. Faecal microbial transplant. Adv. Biomark. Sci. Technol. 2024, 6, 20–34. [Google Scholar]
- Greenberg, S.A.; Youngster, I.; Cohen, N.A.; Livovsky, D.M.; Strahilevitz, J.; Israeli, E.; Melzer, E.; Paz, K.; Fliss-Isakov, N.; Maharshak, N. Five years of fecal microbiota transplantation-an update of the Israeli experience. World J. Gastroenterol. 2018, 24, 5403. [Google Scholar] [CrossRef]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- McFarland, L.V. From yaks to yogurt: The history, development, and current use of probiotics. Clin. Infect. Dis. 2015, 60, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, N.; Yamazaki, S. Probiotics and safety. Am. J. Clin. Nutr. 2001, 73, 465s–470s. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.F.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 2017, CD006095. [Google Scholar] [CrossRef] [PubMed]
- Al Sharaby, A.; Abugoukh, T.M.; Ahmed, W.; Ahmed, S.; Elshaikh, A.O. Do probiotics prevent Clostridium difficile-associated diarrhea? Cureus 2022, 14, e27624. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, B.; Chen, F.; Xia, R.; Zhu, D.; Chen, B.; Lin, A.; Zheng, C.; Hou, D.; Li, X. Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: A randomized, controlled, prospective study. Front. Cell. Infect. Microbiol. 2023, 12, 1089991. [Google Scholar] [CrossRef]
- Su, L.; Hong, Z.; Zhou, T.; Jian, Y.; Xu, M.; Zhang, X.; Zhu, X.; Wang, J. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci. Rep. 2022, 12, 1152. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, J.; Li, S.; Liu, M.; Han, R.; Wang, Y.; Wang, Z.; Wang, D. Efficacy of fecal microbiota transplantation in type 2 diabetes mellitus: A systematic review and meta-analysis. Endocrine 2024, 84, 48–62. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Jing, X.-B.; Chen, X.; Chen, L.-Z.; Zhang, S.-H.; Cai, X.-B. Fecal microbiota transplantation treatment for type 1 diabetes mellitus with malnutrition: A case report. Ther. Adv. Chronic Dis. 2022, 13, 20406223221117449. [Google Scholar] [CrossRef]
- Chernikova, D.A.; Zhao, M.Y.; Jacobs, J.P. Microbiome therapeutics for food allergy. Nutrients 2022, 14, 5155. [Google Scholar] [CrossRef]
- Mocanu, V.; Zhang, Z.; Deehan, E.C.; Kao, D.H.; Hotte, N.; Karmali, S.; Birch, D.W.; Samarasinghe, K.K.; Walter, J.; Madsen, K.L. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: A randomized double-blind, placebo-controlled phase 2 trial. Nat. Med. 2021, 27, 1272–1279. [Google Scholar] [CrossRef]
- Qiu, B.; Liang, J.; Li, C. Effects of fecal microbiota transplantation in metabolic syndrome: A meta-analysis of randomized controlled trials. PLoS ONE 2023, 18, e0288718. [Google Scholar] [CrossRef] [PubMed]
- Proença, I.M.; Allegretti, J.R.; Bernardo, W.M.; de Moura, D.T.; Neto, A.M.P.; Matsubayashi, C.O.; Flor, M.M.; Kotinda, A.P.; de Moura, E.G. Fecal microbiota transplantation improves metabolic syndrome parameters: Systematic review with meta-analysis based on randomized clinical trials. Nutr. Res. 2020, 83, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Keyser, P.; Elofsson, M.; Rosell, S.; Wolf-Watz, H. Virulence blockers as alternatives to antibiotics: Type III secretion inhibitors against Gram-negative bacteria. J. Intern. Med. 2008, 264, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Pais, S.V.; Kim, E.; Wagner, S. Virulence-associated type III secretion systems in Gram-negative bacteria. Microbiology 2023, 169, 001328. [Google Scholar] [CrossRef]
- Fasciano, A.C.; Shaban, L.; Mecsas, J. Promises and challenges of the type three secretion system injectisome as an antivirulence target. Protein Secret. Bact. 2019, 8, 261–276. [Google Scholar]
- Harmon, D.E.; Davis, A.J.; Castillo, C.; Mecsas, J. Identification and characterization of small-molecule inhibitors of Yop translocation in Yersinia pseudotuberculosis. Antimicrob. Agents Chemother. 2010, 54, 3241–3254. [Google Scholar] [CrossRef]
- Garrity-Ryan, L.K.; Kim, O.K.; Balada-Llasat, J.-M.; Bartlett, V.J.; Verma, A.K.; Fisher, M.L.; Castillo, C.; Songsungthong, W.; Tanaka, S.K.; Levy, S.B. Small molecule inhibitors of LcrF, a Yersinia pseudotuberculosis transcription factor, attenuate virulence and limit infection in a murine pneumonia model. Infect. Immun. 2010, 78, 4683–4690. [Google Scholar] [CrossRef]
- François, B.; Luyt, C.-E.; Dugard, A.; Wolff, M.; Diehl, J.-L.; Jaber, S.; Forel, J.-M.; Garot, D.; Kipnis, E.; Mebazaa, A. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: A randomized, double-blind, placebo-controlled trial. Crit. Care Med. 2012, 40, 2320–2326. [Google Scholar] [CrossRef]
- Milla, C.E.; Chmiel, J.F.; Accurso, F.J.; VanDevanter, D.R.; Konstan, M.W.; Yarranton, G.; Geller, D.E.; KB001 Study Group. Anti-PcrV antibody in cystic fibrosis: A novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr. Pulmonol. 2014, 49, 650–658. [Google Scholar] [CrossRef]
- Jain, R.; Beckett, V.; Konstan, M.; Accurso, F.; Burns, J.; Mayer-Hamblett, N.; Milla, C.; VanDevanter, D.; Chmiel, J.; KB001-A Study Group. KB001-A, a novel anti-inflammatory, found to be safe and well-tolerated in cystic fibrosis patients infected with Pseudomonas aeruginosa. J. Cyst. Fibros. 2018, 17, 484–491. [Google Scholar] [CrossRef]
- Eickhoff, M.J.; Bassler, B.L. SnapShot: Bacterial quorum sensing. Cell 2018, 174, 1328.e1321. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Joseph Sexton, D.; Diggle, S.P.; Peter Greenberg, E. Acyl-homoserine lactone quorum sensing: From evolution to application. Annu. Rev. Microbiol. 2013, 67, 43–63. [Google Scholar] [CrossRef]
- Sturme, M.H.; Kleerebezem, M.; Nakayama, J.; Akkermans, A.D.; Vaughan, E.E.; De Vos, W.M. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek 2002, 81, 233–243. [Google Scholar] [CrossRef]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum sensing: A prospective therapeutic target for bacterial diseases. BioMed Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef]
- Sosto, F.; Benvenuti, C.; CANVA Study Group. Controlled study on thymol+ eugenol vaginal douche versus econazole in vaginal candidiasis and metronidazole in bacterial vaginosis. Arzneimittelforschung 2011, 61, 126–131. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Wang, C.; Yue, Y.; Wei, B.; Zhang, H.; Wang, H.; Chen, J. Research Progress on the Combination of Quorum-Sensing Inhibitors and Antibiotics against Bacterial Resistance. Molecules 2024, 29, 1674. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Salinas-Delgado, G.A.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Battle royale: Immune response on biofilms–host-pathogen interactions. Curr. Res. Immunol. 2023, 4, 100057. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Velliyagounder, K.; Ragunath, C.; Rohde, H.; Mack, D.; Knobloch, J.K.-M.; Ramasubbu, N. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J. Bacteriol. 2004, 186, 8213–8220. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Ragunath, C.; Velliyagounder, K.; Fine, D.H.; Ramasubbu, N. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2004, 48, 2633–2636. [Google Scholar] [CrossRef]
- Le Norcy, T.; Niemann, H.; Proksch, P.; Linossier, I.; Vallée-Réhel, K.; Hellio, C.; Faÿ, F. Anti-biofilm effect of biodegradable coatings based on hemibastadin derivative in marine environment. Int. J. Mol. Sci. 2017, 18, 1520. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Kim, Y.S.; Han, D.K.; Kim, Y.H.; Lee, E.H.B.; Suh, H.; Choi, K.S. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials 1998, 19, 851–859. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Lisa Brancatisano, F.; Esin, S.; Campa, M. Use of antimicrobial peptides against microbial biofilms: Advantages and limits. Curr. Med. Chem. 2011, 18, 256–279. [Google Scholar] [CrossRef] [PubMed]
- Jorge, P.; Lourenco, A.; Pereira, M.O. New trends in peptide-based anti-biofilm strategies: A review of recent achievements and bioinformatic approaches. Biofouling 2012, 28, 1033–1061. [Google Scholar] [CrossRef]
- Pulido, D.; Prats-Ejarque, G.; Villalba, C.; Albacar, M.; González-López, J.J.; Torrent, M.; Moussaoui, M.; Boix, E. A novel RNase 3/ECP peptide for Pseudomonas aeruginosa biofilm eradication that combines antimicrobial, lipopolysaccharide binding, and cell-agglutinating activities. Antimicrob. Agents Chemother. 2016, 60, 6313–6325. [Google Scholar] [CrossRef]
- Segev-Zarko, L.-a.; Saar-Dover, R.; Brumfeld, V.; Mangoni, M.L.; Shai, Y. Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem. J. 2015, 468, 259–270. [Google Scholar] [CrossRef]
- Lopes, L.Q.S.; de Almeida Vaucher, R.; Giongo, J.L.; Gündel, A.; Santos, R.C.V. Characterisation and anti-biofilm activity of glycerol monolaurate nanocapsules against Pseudomonas aeruginosa. Microb. Pathog. 2019, 130, 178–185. [Google Scholar] [CrossRef]
- Torelli, R.; Cacaci, M.; Papi, M.; Sterbini, F.P.; Martini, C.; Posteraro, B.; Palmieri, V.; De Spirito, M.; Sanguinetti, M.; Bugli, F. Different effects of matrix degrading enzymes towards biofilms formed by E. faecalis and E. faecium clinical isolates. Colloids Surf. B Biointerfaces 2017, 158, 349–355. [Google Scholar] [CrossRef]
- Mukherji, R.; Patil, A.; Prabhune, A. Role of extracellular proteases in biofilm disruption of gram positive bacteria with special emphasis on Staphylococcus aureus biofilms. Enzym. Eng. 2015, 4, 126. [Google Scholar]
- Darmani, H.; Tawalbeh, K.H.; Al-Hiyasat, A.S.; Al-Akhras, M.-A. Comparison of the photosensitivity of biofilms of different genera of cariogenic bacteria in tooth slices. Pol. J. Microbiol. 2018, 67, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Gholibegloo, E.; Karbasi, A.; Pourhajibagher, M.; Chiniforush, N.; Ramazani, A.; Akbari, T.; Bahador, A.; Khoobi, M. Carnosine-graphene oxide conjugates decorated with hydroxyapatite as promising nanocarrier for ICG loading with enhanced antibacterial effects in photodynamic therapy against Streptococcus mutans. J. Photochem. Photobiol. B Biol. 2018, 181, 14–22. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.B.; Ferrisse, T.M.; Marques, R.S.; de Annunzio, S.R.; Brighenti, F.L.; Fontana, C.R. Effect of photodynamic therapy on microorganisms responsible for dental caries: A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 3585. [Google Scholar] [CrossRef]
- Blenkinsopp, S.A.; Khoury, A.E.; Costerton, J.W. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 1992, 58, 3770–3773. [Google Scholar] [CrossRef]
- Van der Borden, A.; Van der Mei, H.; Busscher, H. Electric-current-induced detachment of Staphylococcus epidermidis strains from surgical stainless steel. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 68, 160–164. [Google Scholar] [CrossRef]
- Del Pozo, J.; Rouse, M.; Patel, R. Bioelectric effect and bacterial biofilms. A systematic review. Int. J. Artif. Organs 2008, 31, 786–795. [Google Scholar] [CrossRef]
- Sastalla, I.; Monack, D.M.; Kubatzky, K.F. Bacterial exotoxins: How bacteria fight the immune system. Front. Immunol. 2016, 7, 300. [Google Scholar] [CrossRef]
- Sharma, S.; Pellett, S.; Morse, S.A. Gram-Positive Bacterial Toxins. Microorganisms 2023, 11, 2054. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.W.; Seu, M.Y.; Dorafshar, A.H.; Shafikhani, S.H. Pseudomonas aeruginosa cytotoxins: Mechanisms of cytotoxicity and impact on inflammatory responses. Cells 2023, 12, 195. [Google Scholar] [CrossRef]
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, M.; Lu, R.; Uzzau, S.; Wang, W.; Margaretten, K.; Pazzani, C.; Maimone, F.; Fasano, A. Zonula occludens toxin structure-function analysis: Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001, 276, 19160–19165. [Google Scholar] [CrossRef] [PubMed]
- Geny, B.; Popoff, M.R. Bacterial protein toxins and lipids: Pore formation or toxin entry into cells. Biol. Cell 2006, 98, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Gentschev, I.; Dietrich, G.; Goebel, W. The E. coli α-hemolysin secretion system and its use in vaccine development. Trends Microbiol. 2002, 10, 39–45. [Google Scholar] [CrossRef]
- Cohen, T.S.; Parker, D.; Prince, A. Pseudomonas aeruginosa host immune evasion. In Pseudomonas: Volume 7: New Aspects of Pseudomonas Biology; Springer: Dordrecht, The Netherlands, 2015; pp. 3–23. [Google Scholar]
- Gupta, S.; Pellett, S. Recent developments in vaccine design: From live vaccines to recombinant toxin vaccines. Toxins 2023, 15, 563. [Google Scholar] [CrossRef]
- Hirai, K.; Arimitsu, H.; Umeda, K.; Yokota, K.; Shen, L.; Ayada, K.; Kodama, Y.; Tsuji, T.; Hirai, Y.; Oguma, K. Passive oral immunization by egg yolk immunoglobulin (IgY) to Vibrio cholerae effectively prevents cholera. Acta Medica Okayama 2010, 64, 163–170. [Google Scholar]
- Hill, D.R.; Ford, L.; Lalloo, D.G. Oral cholera vaccines: Use in clinical practice. Lancet Infect. Dis. 2006, 6, 361–373. [Google Scholar] [CrossRef]
- Pierce, N.F.; Reynolds, H.Y. Immunity to experimental cholera: I. Protective effect of humoral IgG antitoxin demonstrated by passive immunization. J. Immunol. 1974, 113, 1017–1023. [Google Scholar] [CrossRef]
- Hobbs, Z.; Abedon, S.T. Diversity of phage infection types and associated terminology: The problem with ‘Lytic or lysogenic’. FEMS Microbiol. Lett. 2016, 363, fnw047. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B. Bacteriophages and phage-derived proteins–application approaches. Curr. Med. Chem. 2015, 22, 1757–1773. [Google Scholar] [CrossRef]
- Loponte, R.; Pagnini, U.; Iovane, G.; Pisanelli, G. Phage therapy in veterinary medicine. Antibiotics 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of phage-based inhibition of Listeria monocytogenes in food products and food processing environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef] [PubMed]
- Endersen, L.; Coffey, A. The use of bacteriophages for food safety. Curr. Opin. Food Sci. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Khan, M.A.S.; Rahman, S.R. Use of phages to treat antimicrobial-resistant Salmonella infections in poultry. Vet. Sci. 2022, 9, 438. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E. A combination therapy of Phages and Antibiotics: Two is better than one. Int. J. Biol. Sci. 2021, 17, 3573. [Google Scholar] [CrossRef]
- Jensen, C.; Antonsen, M.F.; Lied, G.A. Gut microbiota and fecal microbiota transplantation in patients with food allergies: A systematic review. Microorganisms 2022, 10, 1904. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Liu, Y.; Lin, L.; Lin, X.; Gong, W.; Xia, R.; He, J.; Sheng, J.; Cai, H. Efficacy and safety of fecal microbiota transplantation in the treatment of ulcerative colitis: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 14494. [Google Scholar] [CrossRef]
- Seida, I.; Al Shawaf, M.; Mahroum, N. Fecal microbiota transplantation in autoimmune diseases–An extensive paper on a pathogenetic therapy. Autoimmun. Rev. 2024, 23, 103541. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Cai, J. Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J. Autoimmun. 2023, 141, 103038. [Google Scholar] [CrossRef]
- Marrs, T.; Walter, J. Pros and cons: Is faecal microbiota transplantation a safe and efficient treatment option for gut dysbiosis? Allergy 2021, 76, 2312–2317. [Google Scholar] [CrossRef]
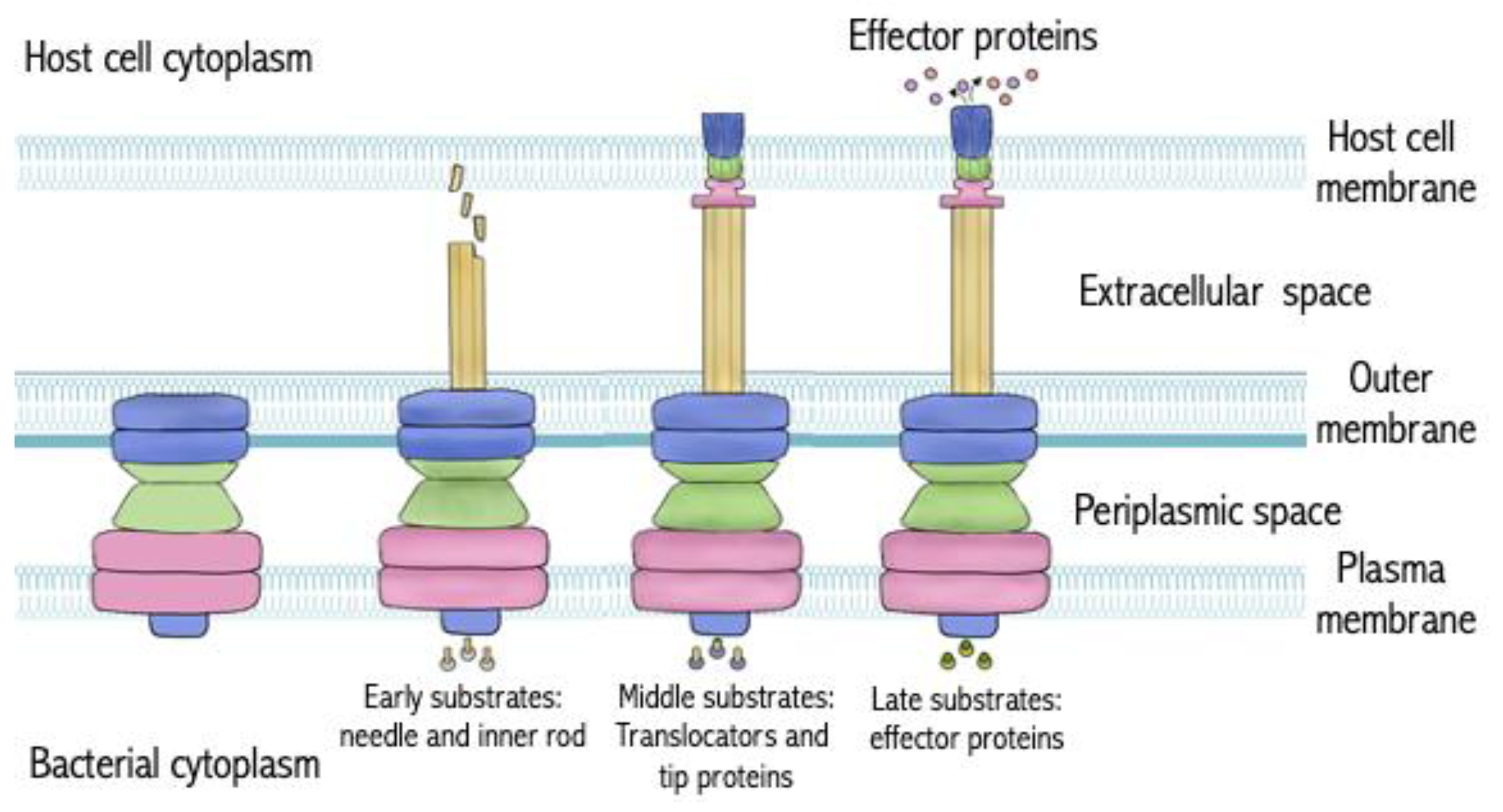
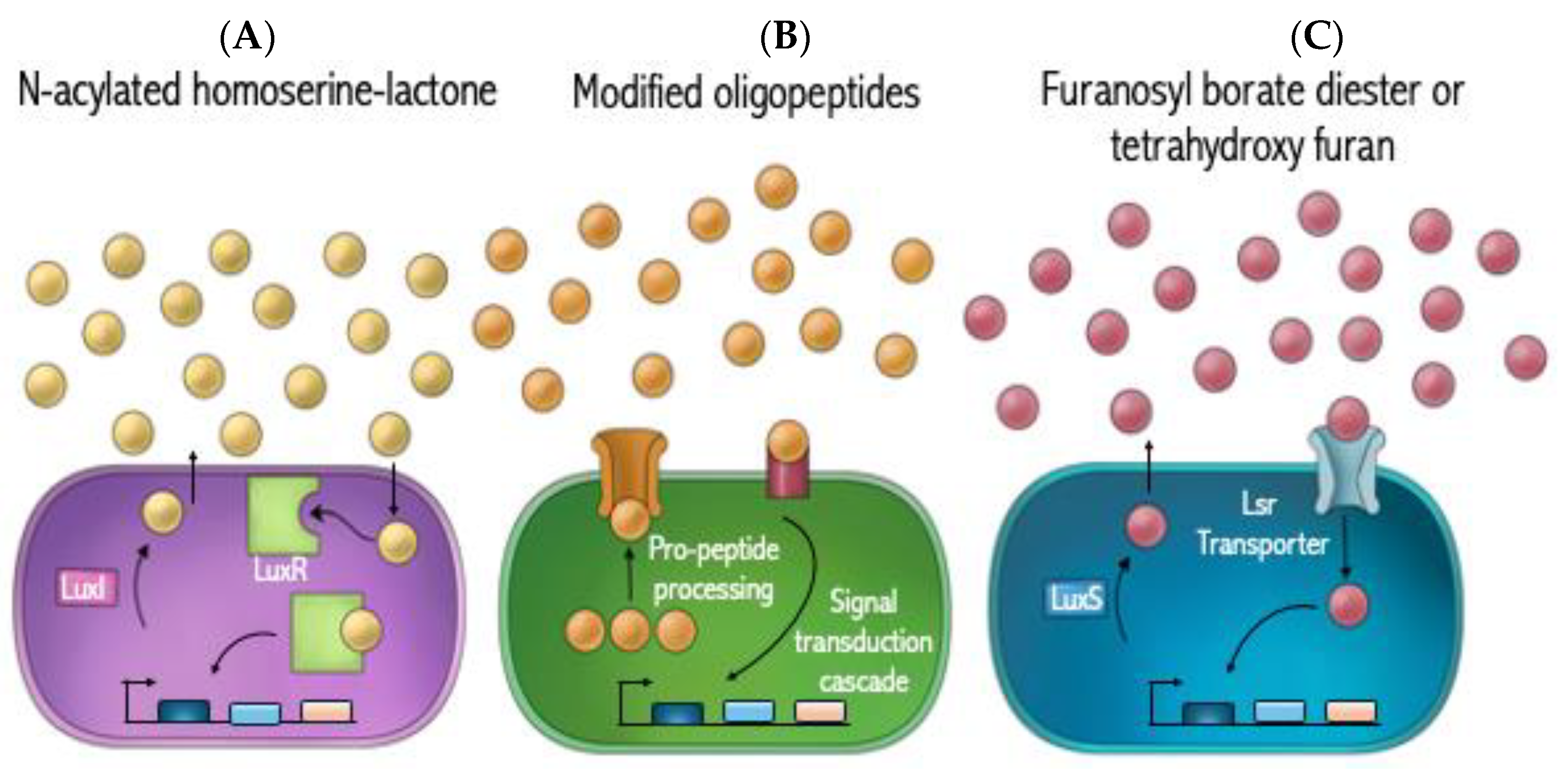
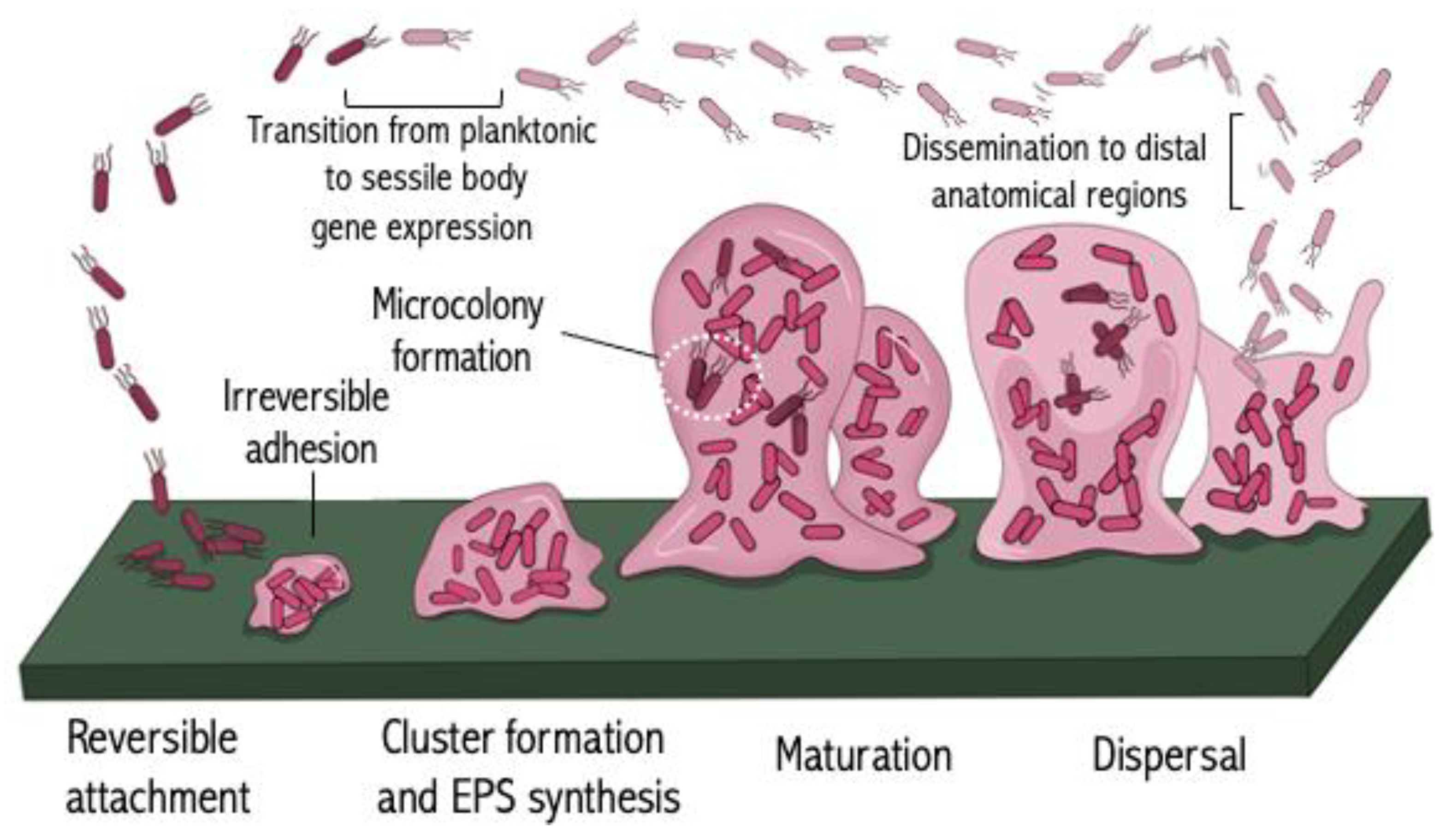
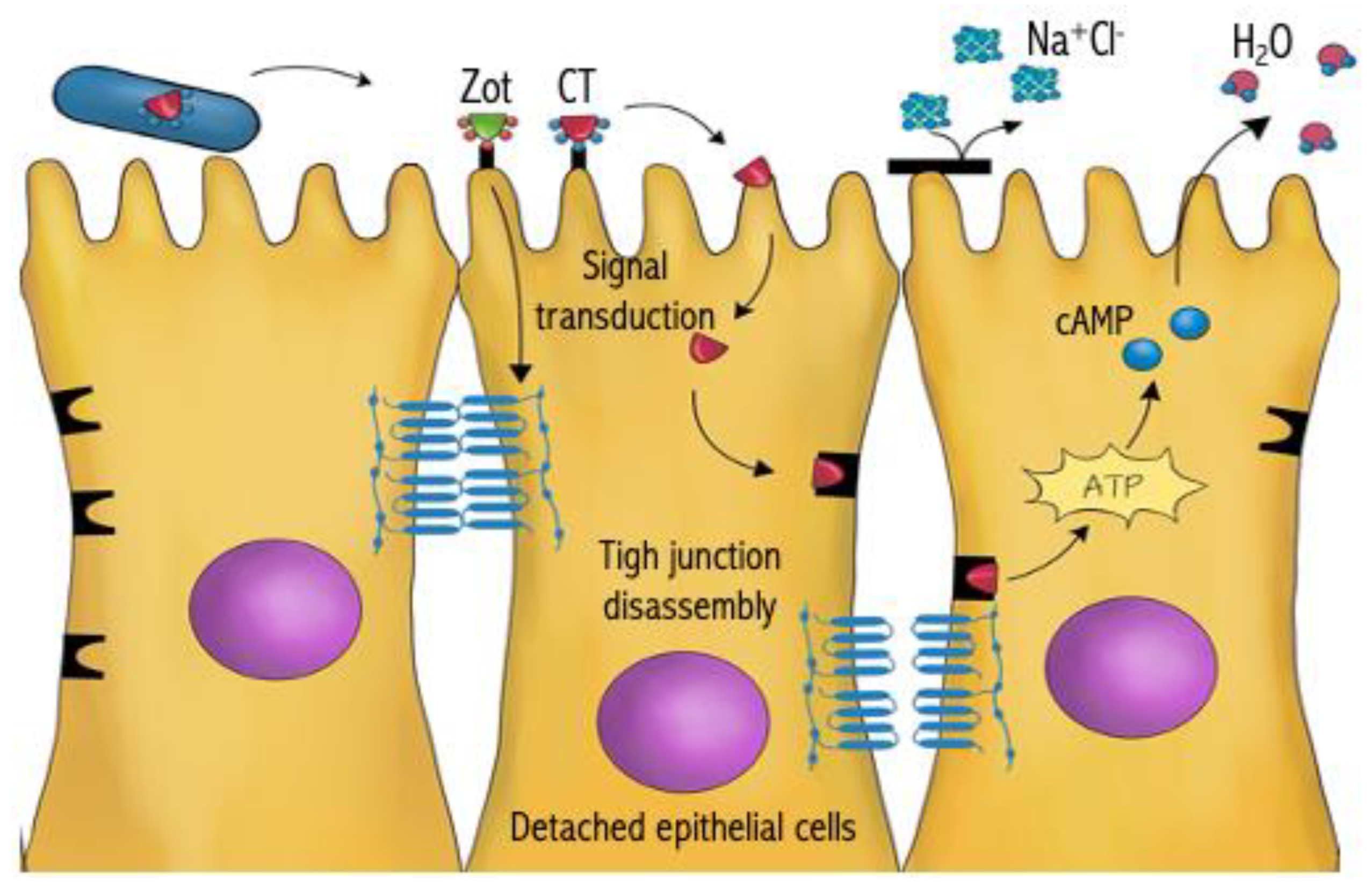

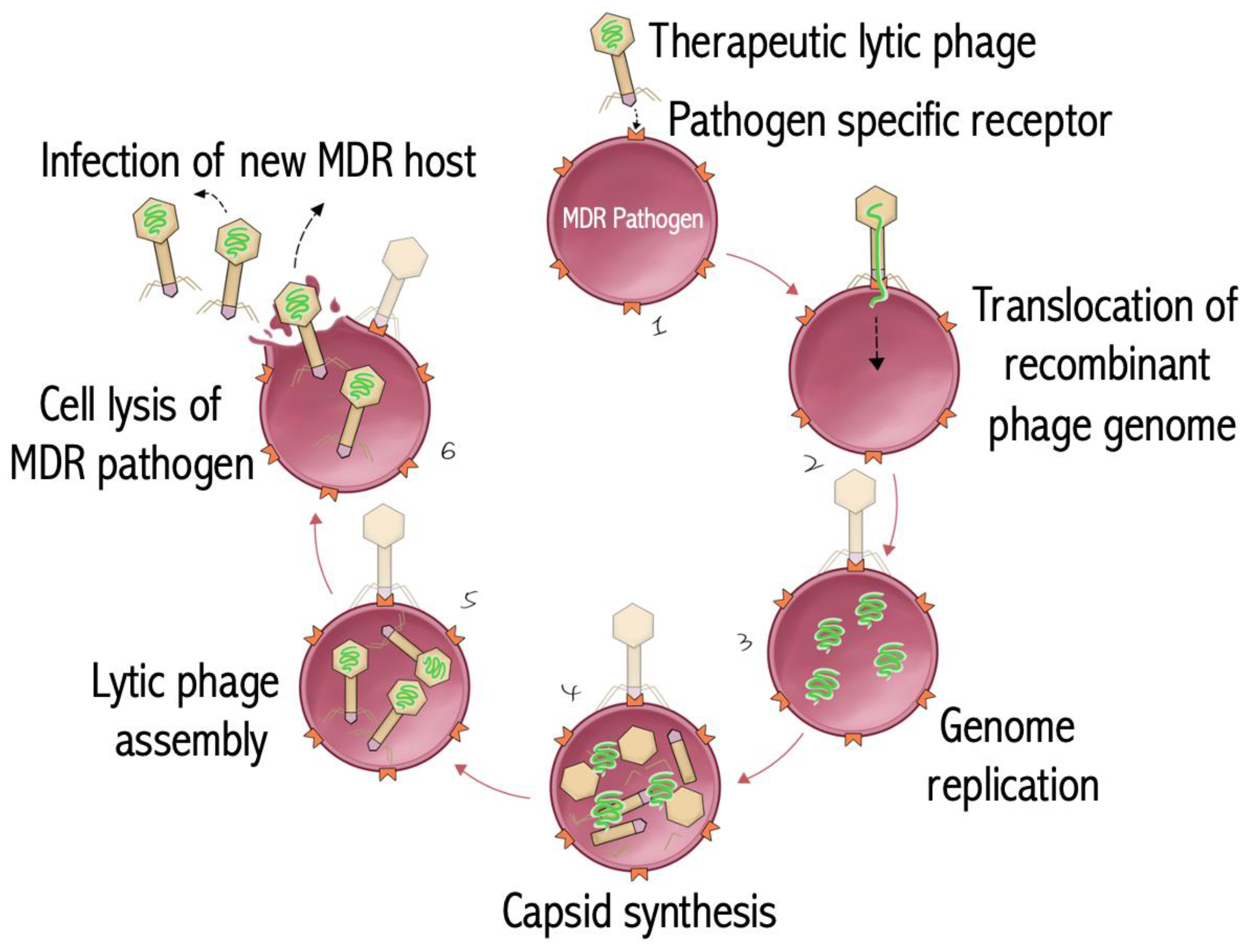

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benyamini, P. Beyond Antibiotics: What the Future Holds. Antibiotics 2024, 13, 919. https://doi.org/10.3390/antibiotics13100919
Benyamini P. Beyond Antibiotics: What the Future Holds. Antibiotics. 2024; 13(10):919. https://doi.org/10.3390/antibiotics13100919
Chicago/Turabian StyleBenyamini, Payam. 2024. "Beyond Antibiotics: What the Future Holds" Antibiotics 13, no. 10: 919. https://doi.org/10.3390/antibiotics13100919
APA StyleBenyamini, P. (2024). Beyond Antibiotics: What the Future Holds. Antibiotics, 13(10), 919. https://doi.org/10.3390/antibiotics13100919




