Abstract
Background: Invasive fungal infections represent a concerning healthcare issue, with Candida spp. reported as the main aetiological agent. Candida spp. bloodstream infections show high mortality rates, indicating increasing antifungal-resistance episodes as a contributing feature. Despite the global prevalence of C. albicans, non-albicans species emerged as significant in the last decades. Methods: The present manuscript reports a five-year evaluation on Candida spp. bloodstream isolates and their antifungal susceptibility profiles, aiming to enrich the literature and epidemiological data. Results: According to the gathered data, antifungal-resistance cases remained uncommon. However, the study revealed rare resistance phenotypes such as a single case of pan-echinocandin resistance C. albicans. Conclusions: Finally, a comprehensive review of Candida spp. antifungal resistance integrates the data, emphasizing the extreme species-specific variability and the consequent importance of always providing species identification.
1. Introduction
Invasive fungal infections represent a significant healthcare challenge, especially regarding immunocompromised and intensive care patients. Fungal bloodstream infections mainly report Candida spp. as the aetiological agent, documenting a significant prevalence in the United States and Europe [1]. Candida spp. bloodstream infections often reach elevated mortality rates due to fungal virulence and/or patients’ underlying conditions. For instance, critical patients suffer from risk factors such as prolonged hospitalization, neutropenia, and invasive surgical procedures [1,2]. Furthermore, Candida spp. infections may meet therapeutic failure depending on the eventual biofilm formation and antifungal-resistance mechanisms [1,3]. Candida albicans is the most isolated fungal species during candidaemia episodes and is known for its relevant capability to form biofilm. Despite this species’ global prevalence, recent epidemiological data document a continuous evolution to non-albicans Candida species. Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei are responsible for numerous candidaemia cases. Additionally, uncommon species such as Candida guilliermondii, Candida lusitaniae, and Candida kefyr rarely cause systemic infections [1,4]. As regarding antifungal-resistance rates, all the above-mentioned Candida species may variably show resistance episodes through drug target alteration or reduced cellular drug concentration [4]. Antifungal-resistance mechanisms pose a significant therapeutical issue due to systemic infections’ severity and critical patients’ conditions [5]. Diagnostic workflows currently face low sensitivity rates and prolonged incubation intervals in the case of a bloodstream-infection microbiological diagnosis. Consequently, clinicians could proceed with empiric antifungal treatment before the specific options are established. These therapeutical protocols can lead to unnecessary antifungal usage and emerging resistance promotion [6].
The present manuscript reports a five-year evaluation on Candida spp. bloodstream isolates and their antifungal susceptibility profiles, aiming to enrich the literature and epidemiological data. A comprehensive review of Candida spp. resistance profiles completes the study, highlighting the importance of always integrating microbiological reports with species identification.
1.1. Antifungal Resistance in Candida albicans
Triazoles represent the major antifungal drug class in clinical usage. The main azole-resistance mechanism is efflux pumps overexpression, which leads to insufficient drug concentration within the fungal cell. Furthermore, ERG11 gene mutations contribute to altering the corresponding enzyme (lanosterol 14-α-Demethylase), avoiding the drug binding to its main target (ERG11) in the fungal cell structure [7,8]. C. albicans azole resistance demonstrated low worldwide rates, accounting for rare and limited outbreaks in North America and South America [7,8].
5-fluocytosin-resistance episodes are related to the uracil phosphoribosyltransferase enzyme, whose mutations definitively alter the 5-fluocytosin antifungal action. This resistance mechanism easily appears after drug exposition; thus, monotherapy regimens are not recommended, leading to several past changes in antifungal therapeutical plans [7,8].
Finally, mutations in the ERG3 gene lead to a significant reduction in the ergosterol concentration within the fungal cellular membrane. As a consequence, amphotericin B fails in its antifungal activity, facing difficulties in finding the main target. An increased catalase activity, along with a reduced susceptibility to the oxidative damage, may also contribute to the same resistance. However, amphotericin B-resistance mechanisms are uncommon, and this is an interesting advantage due to the increasing clinical importance of this molecule within antifungal therapeutical regimens [8].
Echinocandins (caspofungin, micafungin, and anidulafungin) are the first-line therapeutical choice in the case of Candida spp. systemic infections, due to their fungicidal activity, effectiveness, and safety. Echinocandins inhibit the β-(1,3)-glucan-synthase enzyme, causing direct damage to the fungal cell wall. Echinocandins-resistance episodes have been attributed to FKS1 or FKS2 genes mutations. Specifically, substitutions alter the already cited genes, producing the target enzyme alteration and the ineffectiveness of the echinocandins [9,10]. Echinocandin-resistance isolates have been rarely reported across European countries. However, a pan-echinocandin-resistant C. albicans case report was recently documented from a bloodstream infection in Southern Italy [11,12].
C. albicans has been extensively investigated to clarify its drug tolerance mechanisms. First, we can define as tolerance the fungal ability to surviving in drug concentrations above the minimum inhibitory concentration (MIC). The antifungal tolerance may lead to resistance episodes, explaining discrepancies between in vitro susceptibility data and in vivo therapeutical outcomes. Recent published data document heat shock proteins and calcineurin stress-response molecular pathways as possible contributions to the antifungal tolerance. The overexpression or the activation of similar pathways lead to fungal cells’ survival in presence of the antifungal molecules [7].
1.2. Antifungal Resistance in Candida glabrata
C. glabrata (whose taxonomical current name is Nakaseomyces glabrata) expresses an intrinsic low susceptibility to azoles, especially regarding fluconazole, which is the main therapeutical choice in the case of prophylaxis need. The resistance is related to ERG11 gene mutations. Fluconazole-resistant C. glabrata emerged across South America, Europe, and Africa. Itraconazole resistance often accompanies these episodes. Some American countries documented increased MIC values with an azole dose-dependent susceptibility for the same species. A similar attitude was reported for voriconazole within Europe and North America. South America revealed fluconazole and miconazole-resistant isolates, while resistance episodes also include miconazole, clotrimazole, itraconazole, and ketoconazole across the Asian South-East region. Finally, Australian regions showed an increasing percentage of fluconazole-resistant isolates [12,13].
Published surveys reveal a significant amphotericin B-resistance incidence in the Asian regions, reporting the same mechanism as C. albicans, while elevated epidemiological cut-off values emerged in South America. However, these defined outbreaks did not expand to other world countries, confirming the rare Candida spp. tendency to amphotericin B resistance [12,13]. Echinocandin resistance due to FKS genes mutations rarely appeared in C. glabrata. Remarkably, Switzerland, Italy, and the United Kingdom reported single C. glabrata isolation along with an episode of echinocandin resistance [12,13,14,15].
1.3. Antifungal Resistance in Candida parapsilosis
C. parapsilosis shows relevant biofilm production, especially in patients carrying central venous catheters or receiving parenteral nutrition. C. parapsilosis biofilm reveals high variability, including high carbohydrates and low protein rates within the biofilm extracellular matrix. The biofilm production is one of the most diffused antifungal resistance causes in C. parapsilosis isolates, which exhibit decreased antimicrobial susceptibility [16]. Similarly to other Candida species, C. parapsilosis exhibits different mechanisms related to azole resistance. These mechanisms include the upregulation of the MDR1 efflux pump and alterations in the ergosterol biosynthesis genes such as ERG11. The literature data document fluconazole-resistant C. parapsilosis isolation within Central America, Brazil, Southern Europe (especially regarding Italy, Spain, and France), and India [16,17]. Interestingly, C. parapsilosis strains recently reported a 1.3% global rate for amphotericin B resistance due to sterol composition variations, ergosterol target replacement (mutations on the ERG1, ERG4, ERG6, and ERG11 genes), and reinforced defences against amphotericin B-related oxidative damage [16]. Finally, echinocandins’ extensive usage facilitated resistance episodes due to FKS genes in C. parapsilosis isolates, which frequently show elevated MIC values for these antifungal drugs [16].
1.4. Antifungal Resistance in Candida tropicalis
The overexpression of ERG11 is frequently related to azole resistance in C. tropicalis strains, allowing a significant increase in 14-lanosterol-demethylases and the survival of the fungal cells. The resistance mechanisms may involve the overexpression of the UPC2 gene, which codifies for a transcription factor related to ergosterol biosynthesis [18]. North America, Latin America, and several European countries documented a moderate resistance rate (<5%) according to recent global surveillance programmes [19]. C. tropicalis amphotericin B resistance is very uncommon and related to the already mentioned mechanisms. Experimental data reported amphotericin B-resistant episodes only within North America [20]. Finally, the echinocandin-resistance rate remains <1% in C. tropicalis, especially regarding immunocompromised patients within healthcare settings in the USA, India, and Taiwan [21].
1.5. Antifungal Resistance in Candida krusei
C. krusei (whose taxonomical current name is Pichia kudriavzevii) harbours an intrinsic fluconazole resistance, accounting for more than 70% of resistant strains across Europe, North America, Latin America, Asia, and Africa. Voriconazole resistance is less common for this species, reporting approximately 4–14% of resistant isolates within the same geographical areas. Novel triazoles such as posaconazole and isavuconazole still express a relevant antifungal activity against C. krusei [22]. Reduced caspofungin susceptibility cases have been reported in North America [23]. Remarkably, there have been records of amphotericin B-resistant C. krusei isolates in Asian countries [22,23,24]. Azole, echinocandins, and amphotericin B resistance depends on the already cited mechanisms [22,23,24].
1.6. Antifungal Resistance in Uncommon Candida species
According to epidemiological data, several atypical yeast species emerged during the last decade, reporting severe fungal infections among critically ill patients. Clinical practice has to confront insufficient evidence about uncommon species’ antimicrobial susceptibility. Candida kefyr, Candida lusitaniae, Candida guilliermondii, Candida nivariensis, and Candida famata are the most isolated Candida spp. rare species [25].
C. kefyr (current taxonomical name Kluyveromyces marxianus) has recently been reported in systemic infection episodes, accounting for some multi-drug-resistant strains. Specifically, the literature data reported rare high fluconazole, voriconazole, posaconazole, amphotericin B, and echinocandins MIC values. These uncommon resistant isolates mainly appeared in Kuwait and Turkey [26,27]. The identified resistance mechanisms were the same as for other Candida species, along with a relevant biofilm formation tendency.
C. lusitaniae (current taxonomical name Clavispora lusitaniae) expressed a moderate rate of amphotericin B resistance due to the above-mentioned mechanisms. These episodes were reported in experimental data from Central Europe (France) [25,28]. C. famata (whose current taxonomical name is Debaryomyces hansenii) rarely causes systemic infections, occasionally demonstrating a reduced susceptibility to azoles and echinocandins in North America [25,29]. C. guilliermondii (current taxonomical name Meyerozyma guilliermondii) occasionally revealed high antifungal resistance to fluconazole and echinocandins [25,30]. C. nivariensis (current taxonomical name Nakaseomyces nivariensis) integrates the C. glabrata complex as a cryptic fungal species, reporting high virulence and resistance rates. For instance, ERG11 mutations demonstrate a higher incidence than C. glabrata [25]. Furthermore, previously published articles documented a C. nivariensis therapeutical failure after fluconazole regimens in a Spanish healthcare setting [31].
1.7. Antifungal Resistance in Candida auris
Candida auris represents a global healthcare challenge due to its extensive antifungal drug resistance. The most intricate feature of this species is the common coexistence of azoles-, echinocandins-, and amphotericin B-resistance mechanisms [32]. Although the specific resistance mechanisms have not been completely clarified, C. auris combats the antifungal drugs through the already cited alterations [32]. C. auris revealed a significant capability to survive under hard environmental conditions, demonstrating persistence on surfaces and in healthcare settings. These characteristics emphasize the increasing concern about the potential for C. auris invasive infections among critical patients [33]. As regards geographical distribution, North America, Brazil, most of the European countries, some Asian regions, and Australia reported pan-drug C. auris infection episodes [5,32].
Figure 1, Figure 2 and Figure 3 illustrates the epidemiological incidence of antifungal-resistant Candida isolates across the world, the European countries, and Italy. Furthermore, Figure 4 shows the most important antifungal-resistance mechanisms reported in Candida spp.
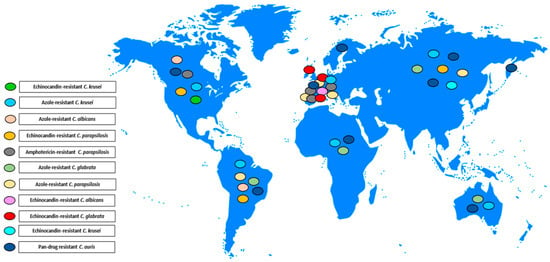
Figure 1.
Antifungal-resistant Candida spp. isolates distribution across the world [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,29,32]. Coloured marks signal these isolates’ presence within the countries according to the reported colours legend.
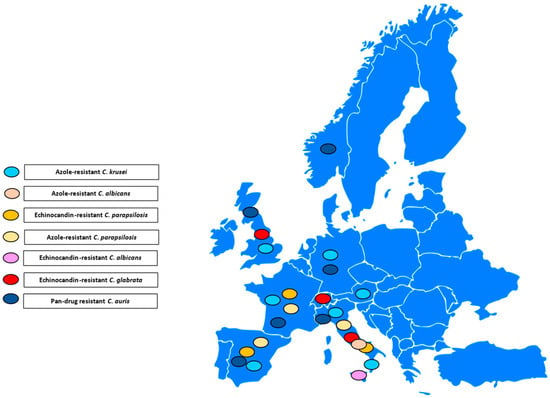
Figure 2.
Antifungal-resistant Candida spp. isolates distribution across the European countries [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,29,32]. Coloured marks signal these isolates’ presence within the countries according to the reported colours legend.
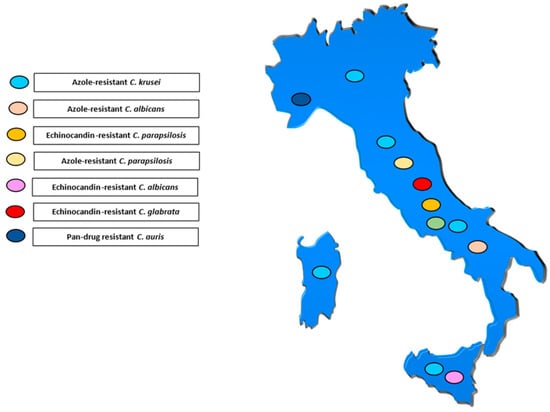
Figure 3.
Antifungal-resistant Candida spp. isolates distribution across Italy [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,29,32]. Coloured marks signal these isolates’ presence within the country according to the reported colours legend.
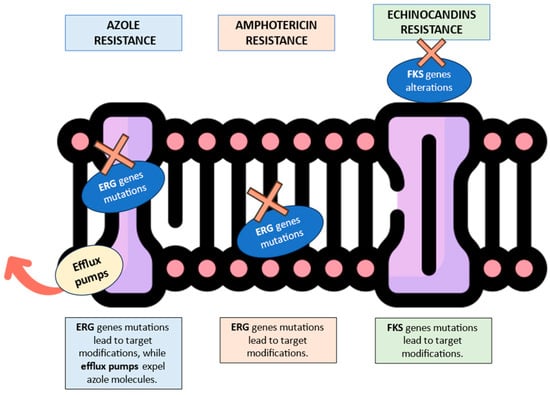
Figure 4.
Graphical summary of the most important antifungal-resistance mechanisms reported in Candida spp. The red “X” symbols indicate the resistance mechanism in its specific cell location. The red arrow illustrates the efflux pump’s capability to expel antifungal molecules from the fungal cell (https://www.flaticon.com/search?wordcell%20membrane, accessed on 27 August 2024).
2. Results
The evaluation reported a total number of 172 clinical isolates. Globally, the study identified C. albicans (82), C. parapsilosis (44), C. glabrata (18), C. tropicalis (16), C. krusei (7), C. lusitaniae (2), C. guilliermondii (1), C. famata (1), and Candida nivariensis (1). The Candida species incidence varied slightly, depending on the analysed period. Specifically, C. albicans reached the same incidence rate as non-albicans species in 2020, showing its supremacy in 2021. The non-albicans isolation rate fluctuated during the following years, reporting percentages higher than the C. albicans numbers during 2022 and 2024. Otherwise, 2023 demonstrated a relevant (more than 50%) C. albicans percentage. Table 1 summarizes all these general data about C. albicans and non-albicans species.

Table 1.
Distribution of Candida species by year.
As regards the non-albicans species, C. parapsilosis was the most reported species during the analysed period (from 19.5% in 2020 to 36.1% in 2022). On the one hand, 2020 reported a similar C. glabrata incidence; on the other hand, C. parapsilosis significantly overcame other species during the following years. Notably, C. famata appeared in 2021, while C. guilliermondii only emerged in 2022.
In addition, C. lusitaniae emerged between 2023 and 2024, whereas C. nivariensis emerged in 2024. However, these uncommon species were rarely isolated.
Table 2 summarizes Candida spp. distribution within the different hospital units during the study period. Remarkably, internal medicine had the most candidaemia episodes. The intensive care (ICU), haematology, and surgery units documented medium candidaemia rates. Finally, the pneumology ward and neonatal intensive care unit (NICU) reported the lowest candidaemia episodes percentages. The uncommon Candida species (C. lusitaniae, C. guilliermondii, C. famata, and C. nivariensis) reported statistical significance in the hospital units. These species mainly emerged within the pneumology and the internal medicine wards.

Table 2.
Distribution of Candida species according to different hospital units.
Table 3 illustrates details on the Candida spp. isolates and their resistance percentages for the analysed antifungal drugs. The authors included amphotericin B, fluconazole, voriconazole, and echinocandins, which represent the most relevant therapeutical choices in the case of invasive candidiasis. The antifungal susceptibility patterns documented several strains with a multi-drug-resistance profile. As regards the C. albicans isolates, several strains revealed resistance MIC values for different antifungal drugs.

Table 3.
Antifungal susceptibilities to antifungal agents for various Candida species.
One strain (1.2%) reported pan-echinocandins resistance, and one strain (1.2%) showed micafungin and anidulafungin resistance.
Additionally, two strains revealed voriconazole and fluconazole resistance. Among C. parapsilosis isolates, one strain showed both fluconazole and voriconazole resistance. One C. glabrata isolate showed caspofungin and fluconazole resistance, whereas a single C. tropicalis strain revealed fluconazole and voriconazole resistance. One C. krusei isolate documented both caspofungin and fluconazole resistance. As regards the uncommon species, C. nivariensis and C. guilliermondii revealed high fluconazole MIC values. Amphotericin B resistance or non-wild-type isolation did not appear during the evaluation of all the isolated Candida species.
3. Discussion
Invasive candidiasis is one of the most severe fungal diseases in worldwide hospital settings. Despite several diagnostic improvements, Candida spp. bloodstream isolation remains difficult in most cases due to a low sensitivity rate and prolonged turn-around time. Moreover, the lack of global epidemiological data results in significant underestimation of candidaemia cases [34]. Particularly, intensive care patients have an enormous variability in their immunological status, complicating risk-factor identifications. This variability often leads to clinical and diagnostic delays for systemic fungal infections. Published data have demonstrated that infection and mortality rates are frequently underestimated [35].
Based on the relevant candidaemia rate, previously published data analysed the Candida spp. distribution within different hospital settings. Xiao et al. reported C. albicans as the most isolated Candida species in candidaemia cases. C. parapsilosis, C. glabrata, C. krusei, and C. tropicalis followed this rate in the same retrospective study, which included different hospital settings in China [36]. Interestingly, our evaluation revealed the same species distribution, which was already documented within larger European evaluations [5]. Our study rarely showed uncommon species such as C. guilliermondii, C. famata, C. lusitaniae, and C. nivariensis. These rare isolations complement the literature data [37,38,39,40] and suggest a future focus on uncommon species’ characteristics. Although it has a presence within several European countries, C. auris did not emerge across our geographical area. Current data do not exclude possible future appearances due to the extraordinary environmental persistence and diffusion of this species.
Antifungal resistance complicates candidaemia management. Our results confirm the overall presence of a moderate antifungal-resistance percentage. However, some isolates documented challenging resistance mechanisms, such as echinocandin resistance in C. albicans and fluconazole resistance in C. parapsilosis. Furthermore, azole-resistant C. albicans emerged. Previous data rarely reported isolates with the same characteristics, confirming the interest in the collected resistant strains [9,10,11,12]. Despite the typical resistance MIC values, these episodes underline the difficulties in detecting the specific molecular resistance mechanisms. Rare gene mutations may be recognized through advanced generation methodologies, suggesting sequencing analysis’ integration as a final step in the microbiological workflow.
Noticeably, several species revealed multi-drug-resistance episodes. For instance, we analysed C. glabrata and C. krusei simultaneously reporting caspofungin and fluconazole resistance. These isolates significantly complicate patients’ therapeutical plans, excluding fundamental alternatives in the case of Candida spp. dissemination. The literature data emphasized the extreme rarity of these resistance mechanisms’ co-presence and the consequent difficulties in clinical management [22,41]. Similar episodes highlight the significant clinical impact of multi-drug antifungal resistance due to the limited antifungal molecules availability.
The presented data confirmed high fluconazole MIC values for C. nivariensis and C. guilliermondii, highlighting previously published data [42,43]. The gathered results never described amphotericin B resistance. Previous literature data documented 2–3% of amphotericin B resistance in bloodstream C. parapsilosis and C. krusei isolates [44]. Additionally, Ahmady et al. stated that C. lusitaniae and C. albicans may acquire amphotericin B resistance [44]. Despite these rare worldwide isolations, our surveillance did not highlight any similar case. The reported antifungal-resistance cases emphasize the importance of always furnishing precise species identification, especially in the case of severe-infection isolation. Unfortunately, fungal species identification may become challenging in some diagnostic settings. A manuscript from A. Lau documents how essential is to apply advanced identification technologies to avoid misidentification phenomena [45]. Similar data indicates the importance of updating the diagnostic workflow for severe fungal infections.
This manuscript aims to illustrate antifungal susceptibility data to enrich the epidemiological and literature database about Candida spp. resistance patterns. This aim was satisfied through the application of coherent antifungal susceptibility testing in all the systemic infection Candida spp. isolations. Unfortunately, Italy is one of the few European countries able to provide antifungal susceptibility data, along with Spain, the United Kingdom, and Norway.
A recent survey reported that most European countries are not currently enabled to execute similar investigations, disallowing the transfer of resistance data to surveillance programmes [46]. This information may suggest the importance of diffuse monitoring of antifungal susceptibility testing methods and practice among European laboratory settings. Surveillance collections often gather resistance data on the first Candida spp. blood isolate, avoiding eventual repetitions and bias. However, some isolates develop resistance mechanisms after antifungal drug exposure; thus, secondary collection of the same candidaemia case may be useful to document changes in the susceptibility profile [47,48].
Our study showed possible variability in Candida spp. distribution and antifungal susceptibility patterns. The results illustrated how rare antifungal-resistance mechanisms may appear in common Candida species, as well as how uncommon Candida species may emerge in systemic infection episodes. Complicatedly, the data confirmed the fundamental role of a complete diagnostic workflow, integrating species identification through advanced technologies and MIC values definition through precise methods. Additionally, the possibility of confirming MIC discrepancies through standardized broth microdilution appeared essential due to some discrepancies in commercial methodologies documented by previous published experiences [49,50]. In conclusion, a periodical enrichment of the local epidemiological data may be crucial to prepare different hospital settings for infection control strategies and multi-drug-resistance diffusion in severe fungal infection episodes.
4. Materials and Methods
The present manuscript describes a five-year evaluation (2020–2024) on bloodstream infections by Candida spp. isolates. The study documents the antifungal susceptibility profiles of Candida spp. strains of recovered patients from the University Hospital Policlinico of Catania. Specifically, emergency room, intensive care, internal medicine, infectious diseases, cardiology, transplants, urology, pneumology, and surgery units reported systemic Candida spp. isolation. All the isolates were identified using a MALDI Biotyper® Sirius System (Bruker, Billerica, MA, USA). According to current guidelines [51], antifungal susceptibility testing was performed for each bloodstream isolate through the Sensititre™ YeastOne™ YO9 AST Plate (Thermo Fisher Scientific, Waltham, MA, USA). This panel includes echinocandins (micafungin, caspofungin, anidulafungin), azoles (fluconazole, voriconazole, itraconazole, posaconazole), 5-fluocytosine, and amphotericin. The susceptibility profiles were evaluated following the manufacturer’s instructions [52]. Furthermore, the MIC values interpretation was based on the current Clinical and Laboratory Standards Institute guidelines (M27M44S-Ed3 and M57S-Ed4 documents) [51,52]. All the resistance MIC values were confirmed through a CLSI-standardized broth microdilution method [53]. All the above-mentioned procedures did not involve direct interventions on human beings and only regarded clinical isolates.
The authors reported a statistical evaluation of Candida spp. distribution depending on the analysed hospital units. They applied the MedCalc Statistical Software version 17.9.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2017, accessed on 30 June 2024), reporting the corresponding p values. The χ2 and Fisher’s exact test established the categorical variables as percentages. Furthermore, the study included an analysis of the most isolated Candida spp. for each study year. Finally, details on the antifungal susceptibility profiles were summarized in tables. The categories “susceptible” (S), “intermediate” (I), “susceptible dose-dependent” (SDD), and “resistant” (R) included the isolates according to the CLSI guidelines. Epidemiological cut-off (E-COFF) allowed Candida spp. classification as wild-type (WT, with a MIC value equal or lower than the E-COFF) or non-wild-type (non-WT, with a MIC value higher than the E-COFF) in the absence of a clinical breakpoint. The wild-type isolates were considered presumptively susceptible, while the non-wild-type strains were related to a hypothetical resistance.
Author Contributions
Conceptualization, M.C. and L.T.; methodology, M.C. and L.T.; investigation, M.C. and L.T.; data curation, M.C. and L.T.; writing—original draft preparation, M.C.; writing—review and editing, M.C. and L.T.; supervision, L.T. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the gathered data were included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dawoud, A.M.; Saied, S.A.; Torayah, M.M.; Ramadan, A.E.; Elaskary, S.A. Antifungal susceptibility and virulence determinants profile of candida species isolated from patients with candidemia. Sci. Rep. 2024, 14, 11597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Posteraro, B.; De Carolis, E.; Criscuolo, M.; Ballanti, S.; De Angelis, G.; Del Principe, M.I.; Delia, M.; Fracchiolla, N.; Marchesi, F.; Nadali, G.; et al. Candidaemia in haematological malignancy patients from a SEIFEM study: Epidemiological patterns according to antifungal prophylaxis. Mycoses 2020, 63, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Prigitano, A.; Morroni, G.; Brescini, L.; Barchiesi, F. Candidemia: Evolution of Drug Resistance and Novel Therapeutic Approaches. Infect. Drug Resist. 2021, 14, 5543–5553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoenigl, M.; Salmanton-García, J.; Egger, M.; Gangneux, J.P.; Bicanic, T.; Arikan-Akdagli, S.; Alastruey-Izquierdo, A.; Klimko, N.; Barac, A.; Özenci, V.; et al. Guideline adherence and survival of patients with candidaemia in Europe: Results from the ECMM Candida III multinational European observational cohort study. Lancet Infect. Dis. 2023, 23, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61, S612–S617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trovato, L.; Bongiorno, D.; Calvo, M.; Migliorisi, G.; Boraccino, A.; Musso, N.; Oliveri, S.; Stefani, S.; Scalia, G. Resistance to Echinocandins Complicates a Case of Candida albicans Bloodstream Infection: A Case Report. J. Fungi 2021, 7, 405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martins, M.D.; Lozano-Chiu, M.; Rex, J.H. Declining rates of oropharyngeal candidiasis and carriage of Candida albicans associated with trends toward reduced rates of carriage of fluconazole-resistant C. albicans in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 1998, 27, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Garzon, A.; Peñuela, A.; Valderrama-Beltrán, S.; Vargas-Casanova, Y.; Ariza, B.; Parra-Giraldo, C.M. Emergence and circulation of azole-resistant C. albicans, C. auris and C. parapsilosis bloodstream isolates carrying Y132F, K143R or T220L Erg11p substitutions in Colombia. Front. Cell. Infect. Microbiol. 2023, 13, 1136217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frías-De-León, M.G.; Hernández-Castro, R.; Conde-Cuevas, E.; García-Coronel, I.H.; Vázquez-Aceituno, V.A.; Soriano-Ursúa, M.A.; Farfán-García, E.D.; Ocharán-Hernández, E.; Rodríguez-Cerdeira, C.; Arenas, R.; et al. Candida glabrata Antifungal Resistance and Virulence Factors, a Perfect Pathogenic Combination. Pharmaceutics 2021, 13, 1529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hassan, Y.; Chew, S.Y.; Than, L.T.L. Candida glabrata: Pathogenicity and Resistance Mechanisms for Adaptation and Survival. J. Fungi 2021, 7, 667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Posteraro, B.; Torelli, R.; Vella, A.; Leone, P.M.; De Angelis, G.; De Carolis, E.; Ventura, G.; Sanguinetti, M.; Fantoni, M. Pan-Echinocandin-Resistant Candida glabrata Bloodstream Infection Complicating COVID-19: A Fatal Case Report. J. Fungi 2020, 6, 163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Branco, J.; Miranda, I.M.; Rodrigues, A.G. Candida parapsilosis Virulence and Antifungal Resistance Mechanisms: A Comprehensive Review of Key Determinants. J. Fungi 2023, 9, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escribano, P.; Guinea, J. Fluconazole-Resistant Candida parapsilosis: A new emerging threat in the fungi arena. Front. Fungal Biol. 2022, 3, 1010782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, D.; An, N.; Yang, Y.; Yang, X.; Fan, Y.; Feng, J. Candida tropicalis distribution and drug resistance is correlated with ERG11 and UPC2 expression. Antimicrob. Resist. Infect. Control 2021, 10, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Fan, X.; Wang, H.; Kudinha, T.; Mei, Y.N.; Ni, F.; Pan, Y.H.; Gao, L.M.; Xu, H.; Kong, H.S.; et al. Continual Decline in Azole Susceptibility Rates in Candida tropicalis Over a 9-Year Period in China. Front. Microbiol. 2021, 12, 702839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rex, J.H.; Cooper, C.R., Jr.; Merz, W.G.; Galgiani, J.N.; Anaissie, E.J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob. Agents Chemother. 1995, 39, 906–909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lima, R.; Ribeiro, F.C.; Colombo, A.L.; de Almeida, J.N., Jr. The emerging threat antifungal-resistant Candida tropicalis in humans, animals, and environment. Front. Fungal Biol. 2022, 3, 957021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Hakki, M.; Staab, J.F.; Marr, K.A. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 2006, 50, 2522–2524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Xiao, J.; Du, M.; Lei, W.; Yang, W.; Xue, X. Post-Translational modifications confer amphotericin B resistance in Candida krusei isolated from a neutropenic patient. Front. Immunol. 2023, 14, 1148681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, S.; Kumar, A.; Roudbary, M.; Mohammadi, R.; Černáková, L.; Rodrigues, C.F. Overview on the Infections Related to Rare Candida Species. Pathogens 2022, 11, 963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmad, S.; Khan, Z.; Al-Sweih, N.; Alfouzan, W.; Joseph, L.; Asadzadeh, M. Candida kefyr in Kuwait: Prevalence, antifungal drug susceptibility and genotypic heterogeneity. PLoS ONE 2020, 15, e0240426. [Google Scholar] [CrossRef]
- Dagi, H.T.; Findik, D.; Senkeles, C.; Arslan, U. Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 36. [Google Scholar] [CrossRef]
- Peyron, F.; Favel, A.; Michel-Nguyen, A.; Gilly, M.; Regli, P.; Bolmström, A. Improved Detection of Amphotericin B-Resistant Isolates of Candida lusitaniae by Etest. J. Clin. Microbiol. 2001, 39, 339–342. [Google Scholar] [CrossRef]
- Beyda, N.D.; Lewis, R.E.; Garey, K.W. Echinocandin Resistance in Candida Species: Mechanisms of Reduced Susceptibility and Therapeutic Approaches. Ann. Pharmacother. 2012, 46, 1086–1096. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Puig-Asensio, M.; Pérez-García, F.; Escribano, P.; Sánchez-Carrillo, C.; Zaragoza, O.; Padilla, B.; Cuenca-Estrella, M.; Almirante, B.; Martín-Gómez, M.T.; et al. Candida guilliermondii Complex Is Characterized by High Antifungal Resistance but Low Mortality in 22 Cases of Candidemia. Antimicrob. Agents Chemother. 2017, 61, e00099-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Soria, L.M.; Bereciartua, E.; Santamaría, M.; Soria, L.M.; Hernández-Almaraz, J.L.; Mularoni, A.; Nieto, J.; Montejo, M. Primer caso de fungemia asociada a catéter por Candida nivariensis en la Península Ibérica [First case report of catheter-related fungemia by Candida nivariensis in the Iberian Peninsula]. Rev. Iberoam. Micol. 2013, 30, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Abbasi, A.F.; Prakash, S.; Mangat, J.; Hosein, Z.; Haider, N.; Chan, J. Candida auris: An Overview of the Emerging Drug-Resistant Fungal Infection. Infect. Chemother. 2022, 54, 236–246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio 2019, 10, e01397-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive candidiasis: Current clinical challenges and unmet needs in adult populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azim, A.; Ahmed, A. Diagnosis and management of invasive fungal diseases in non-neutropenic ICU patients, with focus on candidiasis and aspergillosis: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 1256158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, Z.; Wang, Q.; Zhu, F.; An, Y. Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: A retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob. Resist. Infect. Control 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Savini, V.; Catavitello, C.; Onofrillo, D.; Masciarelli, G.; Astolfi, D.; Balbinot, A.; Febbo, F.; D’Amario, C.; D’Antonio, D. What do we know about Candida guilliermondii? A voyage throughout past and current literature about this emerging yeast. Mycoses 2011, 54, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Randazza, O.; Erickson, K.; Denmeade, T.; Luther, V.; Palavecino, E.; Beardsley, J. Treatment of Candida nivariensis Blood Stream Infection with Oral Isavuconazole. Cureus 2022, 14, e32137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haseeb, U.L.; Rasool, M.; Swaminathan, G.; Hosna, A.U.; Ishfaq, S.; Trandafirescu, T. Candida lusitaniae, an Emerging Opportunistic Pathogen in Immunocompetent Populations: A Case Report. Cureus 2023, 15, e43211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beyda, N.D.; Chuang, S.H.; Alam, M.J.; Shah, D.N.; Ng, T.M.; McCaskey, L.; Garey, K.W. Treatment of Candida famata bloodstream infections: Case series and review of the literature. J. Antimicrob. Chemother. 2013, 68, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Javorova Rihova, Z.; Slobodova, L.; Hrabovska, A. Micafungin Is an Efficient Treatment of Multi Drug-Resistant Candida glabrata Urosepsis: A Case Report. J. Fungi 2021, 7, 800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. Fluconazole Resistance in Isolates of Uncommon Pathogenic Yeast Species from the United Kingdom. Antimicrob. Agents Chemother. 2019, 63, e00211-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esposto, M.C.; Prigitano, A.; Romeo, O.; Criseo, G.; Trovato, L.; Tullio, V.; Fadda, M.E.; Tortorano, A.M.; FIMUA Working Group. Looking for Candida nivariensis and C. bracarensis among a large Italian collection of C. glabrata isolates: Results of the FIMUA working group. Mycoses 2013, 56, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, L.; Gothwal, M.; Mukkoli, M.M.; Bari, V.K. Antifungal drug resistance in Candida: A special emphasis on amphotericin B. APMIS 2024, 132, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.F. Matrix-Assisted Laser Desorption Ionization Time-of-Flight for Fungal Identification. Clin. Lab. Med. 2021, 41, 267–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galia, L.; Pezzani, M.D.; Compri, M.; Callegari, A.; Rajendran, N.B.; Carrara, E.; Tacconelli, E. The Combacte Magnet Epi-Net Network. Surveillance of Antifungal Resistance in Candidemia Fails to Inform Antifungal Stewardship in European Countries. J. Fungi 2022, 8, 249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arendrup, M.C.; Dzajic, E.; Jensen, R.H.; Johansen, H.K.; Kjaeldgaard, P.; Knudsen, J.D.; Kristensen, L.; Leitz, C.; Lemming, L.E.; Nielsen, L.; et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: Data from a nationwide fungaemia surveillance programme. Clin. Microbiol. Infect. 2013, 19, e343–e353. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.amcli.it/wp-content/uploads/2015/10/DocAgg2015Candida-Aspergillus.pdf (accessed on 23 July 2024).
- Trovato, L.; Calvo, M.; Scalia, G.; Oliveri, S. A Comparative Prospective Study in Evaluating Candida spp. In Vitro Susceptibility through Micronaut-AM and Sensititre Yeast-One. Microbiol. Res. 2023, 14, 1077–1088. [Google Scholar] [CrossRef]
- Calvo, M.; Scalia, G.; Palermo, C.I.; Oliveri, S.; Trovato, L. Comparison between EUCAST Broth Microdilution and MIC Strip Test in Defining Isavuconazole In Vitro Susceptibility against Candida and Rare Yeast Clinical Isolates. Antibiotics 2023, 12, 251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.thermofisher.com/order/catalog/product/YO9 (accessed on 2 January 2020).
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI Supplement M27M44S; Clinical and Laboratory Standards Institute, 2022; Available online: https://clsi.org/media/osthhxax/m27m44sed3e_sample.pdf (accessed on 20 August 2022).
- CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 4th ed.; CLSI Supplement M57S; Clinical and Laboratory Standards Institute, 2022; Available online: https://clsi.org/media/tbvf5qr2/m57sed4e_sample.pdf (accessed on 20 August 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).