Abstract
Plants of the genus Prangos are intensively investigated as potential new sources of bioactive isolated products. In this work, the chemical composition of volatile constituents (essential oils and headspace volatiles) and dichloromethane extracts, as well as antimicrobial and antibiofilm activities of essential oils and MFDEs (methanol fractions of dichloromethane extracts) of Prangos trifida from Serbia, were investigated. Volatiles of roots, leaves, stems and fruits, and fatty acids and phytosterols in dichloromethane extracts of roots and fruits were analyzed by GC-FID-MS, whereas coumarins in MFDEs by LC–MS and some isolated coumarins by 1H-NMR. Minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations/minimum fungicidal concentrations (MBCs/MFCs) of essential oils and MFDEs were determined against 13 microorganisms. Antibiofilm activity was assessed against four microorganisms. Additionally, congo red and ergosterol binding assays were conducted to elucidate selected mechanisms of antibiofilm action in the case of Candida albicans. Total of 52 volatile constituents, 16 fatty acids, eight phytosterols and 10 coumarins were identified. Essential oils demonstrated significant activity, surpassing that of commercial food preservatives, against six tested molds from the Aspergillus, Penicillium and Trichoderma genera, as well as against bacteria Staphylococcus aureus and Bacillus cereus. Most of the oils strongly inhibited the formation of biofilms by S. aureus, Listeria monocytogenes and Escherichia coli. MFDEs exhibited noteworthy effects against B. cereus and the tested Aspergillus species, particularly A. niger, and significantly inhibited C. albicans biofilm formation. This inhibition was linked to a marked reduction in exopolysaccharide production, while antifungal mechanisms associated with ergosterol remained unaffected.
1. Introduction
In an era of growing antimicrobial resistance, it is crucial to discover new antibacterial and antifungal agents [1,2]. Plants are well-known to be rich sources of compounds, which have a wide range of biological activities, including effects on broad spectrum of microorganisms [3], the most researched plant preparations are essential oils and extracts [4]. Their extensive chemical characterization is a fundamental requirement in the initial phases of antimicrobial activity research. This approach not only makes it easier to identify preparations with possible antimicrobial abilities, but it also enables understanding of the active components and their potential interactions.
The genus Prangos Lindl. (Apiaceae) comprises about 50 species distributed in Eurasia (from Portugal to Tibet), with the center of diversity in the Irano-Turanian region [5,6]. Both underground and aerial parts of various plants of this genus are used in folk medicine. For example, different organs of P. ferulacea (L.) Lindl. are used orally for digestive disorders, and as antidiabetic and antihypertensive agents, and externally against parasites and for the treatment of wounds [6,7].
Past phytochemical studies on these plants were dealing primarily with essential oils and coumarins, and to a smaller extent with flavonoids, phenolic acids, γ-pyrones, phytosterols, carotenoids and polyacetylenes [6]. For example, recently, the composition of essential oils of leaves and flowers of P. ferulacea [8], flowering aerial parts of P. platychlaena Boiss. [9], aerial parts and roots of P. heyniae H. Duman & M. F. Watson [10,11], and aerial parts of P. meliocarpoides Boiss. and P. uechtritzii Boiss. & Hausskn. [10], as well as of coumarin-containing extracts of roots of P. pabularia Lindl. and P. hulusii Şenol, Yıldırım & Seçmen [12,13], and aerial parts of P. ferulacea, P. peucedanifolia Fenzl [14], P. heyniae, P. meliocarpoides and P. uechtritzii [15], were investigated.
Also, for extracts of different polarity and/or essential oils from various plant parts of Prangos species, interesting bioactivities were previously demonstrated. These include in vitro antimicrobial, cytotoxic, antioxidant, enzyme (α-amylase, α-glucosidase, lipase, tyrosinase, acetyl and butyrylcholinesterase, and angiotensin-converting enzyme) inhibitory and spasmolytic, and in vivo hypoglycemic and analgesic effects [6,7]. It should be noted that the antimicrobial activity is among the most commonly investigated bioactivities of Prangos species. For example, it was evaluated for abovementioned P. ferulacea and P. heyniae essential oils, and for P. hulusii, P. ferulacea and P. peucedanifolia extracts [8,11,13,14]. However, despite this fact, the data on the antibiofilm activities of isolated products of these species are very scarce [6,7]. Namely, the first studies on this topic were published only recently and were focused on the antibiofilm activity of P. ferulacea and P. acaulis (DC.) Bornm. extracts [16,17].
Prangos trifida (Mill.) Herrnst. & Heyn (Figure S1) is sporadically distributed on rocky slopes in European Sub-Mediterranean, from Portugal in the West to Crimea in the East [18,19]. It is a branched glabrous perennial up to 1 m height, with pinnatisect leaves and linear lobes. Fruit umbels are 10–25 rayed, with few small bracts and bracteoles and ovate wingless fruits with thick mesocarp and numerous secretory channels with essential oils [18,20]. More recent molecular studies [21,22] showed separation of two well supported groups: western an eastern clade (including Balkans and Crimea), but with insufficient morphological support. According to some authors [5,20], eastern populations belong to a separate species (under the name Cachrys alpina Bieb.).
So far, the composition of essential oils of P. trifida fruits from Turkey (plant investigated under the name C. alpina) [23], fruits, flowers, stems with leaves and roots from Spain (plant investigated under the name C. trifida L.) [24], fruits from Crimea [25], and aerial parts (collected before flowering) from Italy [26,27] were investigated. Essential oil from Italy was also tested for antioxidant [26,27] and antimicrobial activities [26]. Regarding antimicrobial activity, this essential oil was active against Bacillus cereus and B. subtilis, but not against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Salmonella Typhimurium (it should be noted that concentrations of the oil of only up to 0.2 mg/mL were tested) [26]. Also, in a search for novel anti-inflammatory agents, for three coumarins, i.e., imperatorin, isoimperatorin and prantschimgin, isolated from P. trifida (investigated under the name C. trifida), effects on some macrophage functions were demonstrated. Most notably, imperatorin caused strong reduction in nitric oxide generation, imperatorin and isoimperatorin inhibited both cyclooxygenase and lipoxygenase pathways of arachidonate metabolism, whereas prantschimgin showed effect only on the lipoxygenase pathway [28].
In summary, despite there are several studies on the composition of P. trifida essential oils, the knowledge on this topic is still insufficient because these essential oils were obtained from the plants collected at only a few localities. Moreover, other P. trifida metabolites, as well as bioactivity of isolates of this plant, were very scarcely investigated. Therefore, the aim of the current study was to perform a comprehensive analysis of isolated products obtained from P. trifida collected in Serbia. This included chemical analysis, investigation of antimicrobial and antibiofilm activities, and exploration of potential mechanisms of action. We focused on analyzing the compositions of volatile constituents in different plant parts, i.e., roots, leaves, stems and fruits, as well as of dichloromethane (CH2Cl2) extracts from roots and fruits. Subsequently, we assessed the antimicrobial and antibiofilm activities of the isolates, for which we found relevant metabolites during phytochemical investigations, i.e., the activities of essential oils and coumarin-rich methanol fractions of CH2Cl2 extracts (MFDEs). Additionally, we screened MFDEs for selected mechanisms of action against Candida albicans.
2. Results
2.1. Chemical Composition of P. trifida
2.1.1. Composition of Essential Oils
The yields of essential oils obtained by hydrodistillation were the lowest in stems (0.10 ± 0.05% w/w) and roots (0.05 ± 0.01% w/w), and were not significantly different (p = 0.24). Leaves and fruits were richer in essential oils (yields 0.50 ± 0.04% w/w and 0.37 ± 0.04% w/w, respectively), and the content of essential oils differed significantly both between these two plant organs and also in relation to the contents of essential oils in stems and roots (p < 0.05). The composition of the isolated essential oils was analyzed using GC-FID-MS (Table 1). In this way, 32 components were identified in both the root and leaf essential oils, 39 in the stem oil and 43 in the fruit oil, comprising 95.6, 95.1, 94.0 and 95.2% of the total essential oils, respectively. Dominant constituents of these essential oils were monoterpenes (66.2–87.2%). Monoterpene hydrocarbons, i.e., non-oxygenated monoterpenes, were more abundant in the root (56.2%), leaf (67.2%) and fruit essential oils (66.5%), whereas the stem oil differed due to the prevalence of oxygenated monoterpenes (45.6%). Accordingly, non-oxygenated monoterpenes terpinolene (36.2%) and p-cymene (11.5%) were dominant in the root essential oil, (E)-β-ocimen (23.2%), terpinolene (18.1%) and α-pinene (11.3%) in the leaf oil, and p-cymene (25.4%), limonene (14.4%) and γ-terpinene (11.4%) in the fruit oil. In the stem essential oil, oxygenated monoterpene p-cymen-8-ol (21.8%) was the most abundant and it was followed by non-oxygenated compound p-cymene (14.1%). Additionally, the stem oil contained a considerable amount of the oxygenated sesquiterpene caryophyllene oxide (13.1%).

Table 1.
Chemical composition of P. trifida volatile constituents.
2.1.2. Composition of Headspace Volatiles
Headspace volatiles, i.e., the most volatile compounds of investigated P. trifida roots, leaves, stems and fruits, were isolated using the headspace sampler and analyzed using GC-FID-MS (Table 1). In total, 15 constituents isolated in this way were identified in the roots, 16 in the leaves, 15 in the stems and 14 in the fruits, representing 100.0, 96.7, 100.0 and 99.7% of the total headspace volatiles, respectively. In all instances, the dominant were monoterpene hydrocarbons (92.3, 93.0, 93.3 and 99.4%, respectively), which was in accordance with their higher volatility compared to oxygenated monoterpenes. α-Pinene (17.3, 21.7 and 19.5% in the leaves, stems and fruits, respectively), sabinene (12.5% in the fruits), p-cymene (11.1, 40.3 and 23.3% in the roots, stems and fruits, respectively), limonene (11.6, 21.6 and 23.9% in the roots, stems and fruits, respectively), (E)-β-ocimene (40.9% in the leaves) and terpinolene (58.5 and 18.6% in the roots and leaves, respectively) were the most abundant headspace volatiles.
2.1.3. Fatty Acid, Phytosterol and Triterpene Composition of CH2Cl2 Extracts
Prangos trifida roots and fruits were extracted with CH2Cl2. After evaporation of the solvent, semi-solid (oily) extracts were obtained. The yields of the extracts of roots and fruits were significantly different (p = 0.03), and amounted to 4.4 ± 0.1% w/w and 7.6 ± 0.9% w/w, respectively.
Fatty acids of roots and fruits CH2Cl2 extracts were analyzed using GC-FID-MS as volatile fatty acid methyl esters (FAME), i.e., after saponification and subsequent methylation; FAME yields: 39.1% w/w (fruits extract) and 35.5% w/w (roots extract). Sixteen fatty acids were identified in each sample, comprising 92.6 and 98.7% of all detected fatty acids, respectively (Table 2). Dominant ones in the roots extract were polyunsaturated fatty acids (55.4%), mainly linoleic (51.8%). Notable amounts of saturated palmitic (14.9%) and monounsaturated oleic acid (14.2%) were also revealed. The most abundant in the fruits extract were monounsaturated fatty acids (61.3%), mostly petroselinic (49.9%) and oleic (10.2%). Appreciable amount of linoleic acid (28.3%) was also present.

Table 2.
Fatty acid composition of P. trifida roots and fruits CH2Cl2 extracts.
Yields of residual unsaponifiable fractions were 5.1 and 4.8% w/w of the roots and fruits extracts, respectively. In these fractions, phytosterols and triterpenes were analyzed after silanization, i.e., as volatile trimethylsilyl (TMS) derivatives, using GC-FID-MS (Table 3). In the case of unsaponifiable fraction of the roots extract (UFRE), phytosterols and triterpenes accounted for 93.7%, and the unsaponifiable fraction of the fruits extract (UFFE) accounted for 69.6% of the total unsaponifiable fractions. Among them, the dominant was β-sitosterol (60.0%) in UFRE, and a mixture of β-sitosterol and α-spinasterol (52.7%) in UFFE, followed by stigmasterol (18.5 and 26.3%) in both cases. A small quantity (4.1%) of one triterpene, β-amyrin, was detected in UFFE.

Table 3.
Phytosterol and triterpene composition of unsaponifiable fractions of P. trifida roots and fruits CH2Cl2 extracts (UFRE and UFFE).
2.1.4. Coumarin Composition of CH2Cl2 Extracts and Their MeOH Fractions (MFDEs)
Two coumarins, oxypeucedanin (6) and prantschimgin (10), were obtained as crystalline precipitates from the semi-solid (oily) CH2Cl2 extracts (6 from the fruits extract and 10 from the roots extract) after treating the extracts with diethyl ether (precipitates accounted for 2.5 and 3.2% w/w of the fruits and roots extracts, respectively). The structures of these coumarins were elucidated by comparing 1H-NMR data with the literature [30,31,32]. The purity of the compounds was determined using LC-MS: 6 (78.20%) and 10 (93.55%).
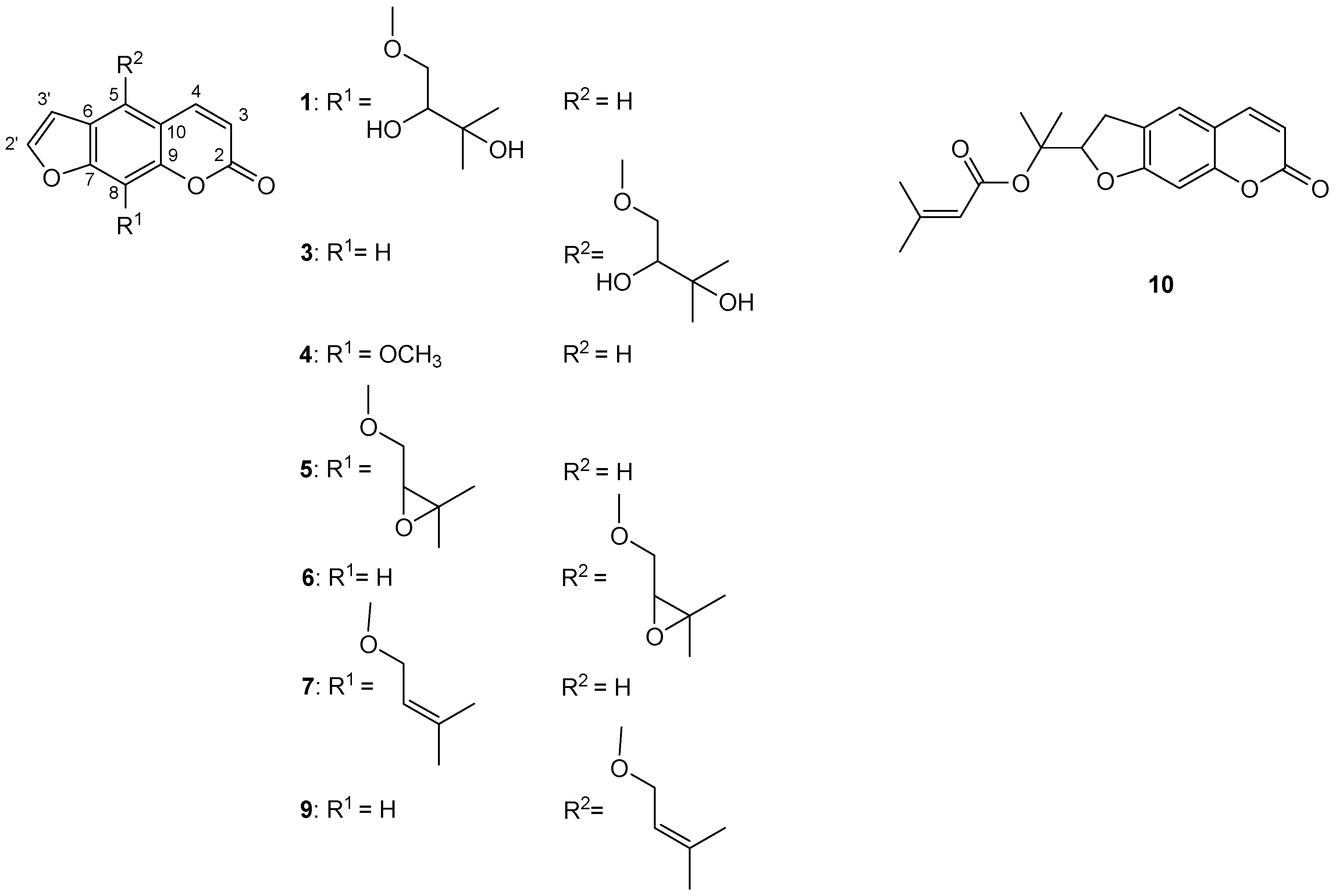
To further investigate coumarins of P. trifida roots and fruits CH2Cl2 extracts, the MeOH fractions of CH2Cl2 extracts (MFDEs) were prepared; yields of MFDEs: 90.9% w/w (roots extract) and 69.5% w/w (fruits extract). The results are presented in Table 4 and Table 5 and Figure 1. LC–MS analysis of MFDEs enabled elucidation of the structures of ten coumarins [seven (2–4, 6, 7, 9, 10) in roots extract and nine (1–3, 5–10) in fruits extract] (Table 4, Figure 1). The furanocoumarins xanthotoxin (4) and imperatorin (7) were identified using commercial standard compounds, and heraclenol (1) and heraclenin (5) using a previously chemically characterized CH2Cl2 extract of Heracleum ternatum Velen. fruits [33]. Based on comparison of their mass and UV spectra to those of imperatorin (7), heraclenol (1) and heraclenin (5), the next three furanocoumarins were identified as isoimperatorin (9), oxypeucedanin hydrate (3) and oxypeucedanin (6), respectively. Namely, isoimperatorin (9) had similar mass spectrum to imperatorin (7), oxypeucedanin hydrate (3) to heraclenol (1), and oxypeucedanin (6) to heraclenin (5). On the other hand, isoimperatorin (9), oxypeucedanin hydrate (3) and oxypeucedanin (6) had characteristic UV spectra of furanocoumarins oxygenated at C(5), in contrast with imperatorin (7), heraclenol (1) and heraclenin (5), whose UV spectra were typical for furanocoumarins oxygenated at C(8) [34,35]. This led to the conclusion that these three coumarin pairs [i.e., isoimperatorin (9) and imperatorin (7), oxypeucedanin hydrate (3) and heraclenol (1), and oxypeucedanin (6) and heraclenin (5)] are C(5)-C(8) positional isomers. The identity of oxypeucedanin (6) was confirmed using the external standard of this compound (obtained from P. trifida fruits crude CH2Cl2 extract). Compared to the identified furanocoumarins, compounds 2, 8 and 10 had different UV spectra, similar to those of the simple coumarins. The coumarin 10 was identified as prantschimgin (senecioyl ester of marmesin) using the external standard of this 2′,3′-dihydrofuranocoumarin (obtained from P. trifida roots crude CH2Cl2 extract). The compound 8 had the same UV and mass spectra as 10, suggesting that this was also a 2′,3′-dihydrofuranocoumarin derivative. The compound 2 had the same UV, but a different mass spectrum, compared to 10, which indicated that this is a 2′,3′-dihydrofuranocoumarin without senecioyl group, most likely marmesin, a precursor in the biosynthesis of all abovementioned coumarins [36].

Table 4.
UV and MS data of coumarins identified in P. trifida roots and fruits MFDEs obtained by LC–MS in positive ESI mode at fragmentor voltage of 100 V and 250 V.

Table 5.
Coumarin composition of P. trifida roots and fruits MFDEs.
Figure 1.
Structures of identified coumarins in MFDEs: 1—heraclenol, 3—oxypeucedanin hydrate, 4—xanthotoxin, 5—heraclenin, 6—oxypeucedanin, 7—imperatorin, 9—isoimperatorin, 10—prantschimgin.
Quantitative LC–MS analysis of the MFDEs was performed using the external standard method (Table 5). Besides in the analyzed MFDEs, the contents of coumarins in the crude (whole) CH2Cl2 extracts were calculated. The dominant coumarin in the root isolates was prantschimgin (10; 118.7 mg/g MFDE and 107.9 mg/g extract), followed by imperatorin (7; 36.6 mg/g MFDE and 33.2 mg/g extract). In the fruit isolates, oxypeucedanin (6; 346.0 mg/g MFDE and 240.4 mg/g extract), prantschimgin (10; 96.3 mg/g MFDE and 66.9 mg/g extract) and oxypeucedanin hydrate (3; 87.5 mg/g MFDE and 60.8 mg/g extract) prevailed.
2.2. Antimicrobial and Antibiofilm Activities of P. trifida
2.2.1. Antimicrobial Activity of Essential Oils
Antimicrobial activity of the essential oils of P. trifida roots, leaves, stems and fruits was tested against 13 microorganisms (Table 6). It was revealed that all investigated essential oils showed considerable antifungal activity against all six tested strains of molds from Aspergillus, Penicillium and Trichoderma genera (MIC = 0.10–0.78 mg/mL; MFC = 0.20–1.56 mg/mL), whereas the effect on Candida albicans was somewhat lower (MIC = 1.56–3.13 mg/mL; MFC = 3.13–6.25 mg/mL). The highest antifungal activity was demonstrated for all investigated essential oils against both tested Penicillium strains, the root and leaf oils against T. harzianum, and the root oil against A. fumigatus (MIC = 0.10 mg/mL; MFC = 0.20 mg/mL), followed by the stem and fruit oils against A. fumigatus, root and stem oils against A. niger, and stem oil against T. harzianum (MIC = 0.20 mg/mL; MFC = 0.39 mg/mL). Every investigated essential oil also exhibited activity on all six tested bacteria (MIC = 0.20–6.25 mg/mL; MBC = 0.39–12.5 mg/mL). The highest antibacterial effect was observed for the root and stem oils against Staphylococcus aureus and Bacillus cereus (MIC = 0.20 mg/mL; MBC = 0.39 mg/mL).

Table 6.
Antimicrobial activity of P. trifida essential oils and positive controls (mg/mL).
2.2.2. Antibiofilm Activity of Essential Oils
Antibiofilm activity of the essential oils of P. trifida roots, leaves, stems and fruits was assessed against bacterial Staphylococcus aureus, Listeria monocytogenes and Escherichia coli, and fungal Candida albicans biofilms (Table 7). All tested essential oils exhibited a noteworthy inhibition of the formation of all investigated bacterial biofilms. The most intriguing observation within our study pertained to the significant inhibition of L. monocytogenes biofilm formation, with root and leaf essential oils demonstrating reductions of 80.2% and 82.6%, respectively. These effects were achieved when the oils were applied at sub-inhibitory concentrations, specifically at half of their minimum inhibitory concentrations (½MIC). Moreover, the leaf essential oil achieved high inhibition (50.3%) of the biofilm formation by this pathogen even in a lower concentration (¼MIC). Another noteworthy result pointed to the inhibition of E. coli biofilm formation by the fruit and root essential oils (46.3% and 27.3%); in both instances, the oils were applied in sub-inhibitory concentrations (½MIC). Finally, the root and stem essential oils that were applied at concentrations equal to half of their minimum inhibitory concentrations (½MIC), which is particularly noteworthy given their low MIC value of 0.2 mg/mL, exhibited effectiveness in inhibiting the formation of S. aureus biofilm, resulting in reductions of 37.7% and 35.5%, respectively. In contrast to tested bacterial biofilms, C. albicans biofilm was not significantly affected by any tested essential oil.

Table 7.
Antibiofilm activity of P. trifida essential oils (%).
2.2.3. Antimicrobial Activity of MFDEs
The experiments performed on P. trifida roots and fruits MFDEs, using the same 13 microorganisms (like in investigations of essential oils), provided intriguing results, both in terms of antibacterial (MIC = 0.125–4 mg/mL; MBC = 0.25–8 mg/mL) and antifungal activities (MIC = 0.0625–1 mg/mL; MFC = 0.125–2 mg/mL) (Table 8).

Table 8.
Antimicrobial activity of P. trifida MFDEs and positive controls (mg/mL).
When it comes to antibacterial effects, Bacillus cereus emerged as the most sensitive strain in relation to the action of these MFDEs. This pathogen was particularly susceptible to the impacts of the MFDEs, exhibiting an MIC value of 0.125 mg/mL and an MBC value of 0.25 mg/mL. This indicates that B. cereus was affected by the smallest concentration of the MFDEs among all the tested bacterial strains, demonstrating its high sensitivity to the MFDEs.
As for antifungal activity, it was observed that the MFDEs displayed identical effects on each tested fungal species. The fungal species Aspergillus niger was found to be the most susceptible to the antifungal properties of the MFDEs, with an MIC value of 0.0625 mg/mL and an MFC value of 0.125 mg/mL. Notable activities of both MFDEs were also shown against A. fumigatus (MIC = 0.25 mg/mL; MFC = 0.5 mg/mL), whereas effects against A. versicolor and Penicillium funiculosum were somewhat lower (MIC = 0.5 mg/mL; MFC = 1 mg/mL).
2.2.4. Antibiofilm Activity of MFDEs
Our study also explored the P. trifida roots and fruits MFDEs’ effectiveness in impeding the formation of Staphylococcus aureus, Listeria monocytogenes, Escherichia coli and Candida albicans biofilms (Table 9).

Table 9.
Antibiofilm activity of P. trifida MFDEs (%).
The most considerable inhibition of bacterial biofilm formation was noted in the case of S. aureus, when the fruits MFDE was applied at a sub-inhibitory concentration, which is half of the minimum inhibitory concentration (½MIC). Under this condition, the fruits MFDE was able to inhibit S. aureus biofilm formation by 28.3%. In a similar vein, the roots MFDE demonstrated appreciable L. monocytogenes biofilm inhibition when applied at one-fourth of the minimum inhibitory concentration (¼MIC). Under this condition, it was capable of inhibiting biofilm formation of this pathogen by 27.6%. On the other hand, neither MFDE inhibited formation of E. coli biofilm to a notable extent.
Our results also suggest that both MFDEs hold substantial promise in inhibiting C. albicans biofilm formation. Namely, antibiofilm activity was noteworthy in the case of almost all applied concentrations of the roots and fruits MFDEs, reaching 53.5 and 48.6% (at MICs), respectively.
2.2.5. Testing MFDEs in Congo Red and Ergosterol Binding Assays in Candida albicans
Tested P. trifida roots and fruits MFDEs caused marked reduction in exopolysaccharide (EPS) production in C. albicans in congo red binding assay (Table 10). Specifically, when C. albicans was exposed to the fruits MFDE at its MIC, we noted a 31.5% reduction in EPS production. This reduction was even more pronounced (33.7%) when the roots MFDE was applied. Moreover, this effect was observed at ¼MIC.

Table 10.
Effects of P. trifida MFDEs on C. albicans EPS production inhibition (%).
The results of ergosterol binding assay were negative in the case of both MFDEs, i.e., no changes in the MIC values were observed after the addition of exogenous ergosterol.
3. Discussion
3.1. Chemical Composition of P. trifida
3.1.1. Composition of Volatile Constituents
In this work, the chemical composition of P. trifida root, leaf, stem and fruit essential oils isolated by hydrodistillation was investigated. Obtained results were additionally confirmed by analyzing the composition of the headspace volatiles of these plant organs. Namely, static headspace extraction is a nondestructive, solvent-free and rapid method for the isolation of the most volatile components from plants [37]. Thus, using this procedure and subsequent GC-FID-MS analysis, the presence of the most volatile constituents of P. trifida essential oils was also revealed in the intact dried plant material. It should be noted that headspace volatiles are obtained in a gaseous state and cannot be used in investigations of antimicrobial and antibiofilm activities.
Previously, the essential oils of P. trifida originating from some other regions were analyzed. In comparison with previously investigated fruit essential oils, the sample from Serbia, investigated in the current work, was similar to those from Spain and Crimea and different from the one from Turkey [23,24,25]. Namely, the fruit oils from two localities in Spain and one in Crimea were also dominated by p-cimene (14.1, 24.5 and 9.1%), limonene (14.2, 34.5 and 31.3%) and γ-terpinene (28.3, 37.3 and 12.5%) [24,25]. On the other hand, in the fruit oil from Turkey the sesquiterpene α-humulene (33.1%) was the most abundant and it was followed by p-cymene (9.3%) [23]. α-Humulene was present in very low amounts in the samples from Serbia (2.1%) and Spain (traces), and it was absent in the sample from Crimea [24,25]. Regarding previously investigated root essential oils from two localities in Spain, they had similar compositions to the Serbian root essential oil sample: terpinolene (61.8 and 70.7%), γ-terpinene (8.2 and 13.6%) and p-cymene (8.0 and 10.9%) prevailed [24]. In the case of Spanish plants, essential oils of leaves with stems from four localities were also investigated. In the oils from two localities, (E)-β-ocimene was dominant (61.0 and 70.7%), as was also in the case in leaf essential oil of the plant from Serbia, while in the oils from the other two localities, the isomer (Z)-β-ocimene prevailed (20.5 and 51.5%) [24]. This isomer was also the dominant in the aerial parts (collected before flowering) essential oil of Italian P. trifida (18.1%) [26,27], while it was present in low quantities in the leaf and stem essential oils of the plant from Serbia (≤1.3%).
Regarding essential oils of other Prangos species, monoterpene hydrocarbons, such as α-pinene, β-pinene, γ-terpinene, β-phellandrene and p-cymene, were usually the most abundant [6]. As expected, some of them were also present as major and/or minor constituents in the P. trifida essential oils investigated in our work. However, one of specificities of investigated P. trifida essential oils was the dominance of p-cymen-8-ol in the stem essential oil (21.8%) and its presence in notable amounts in the root and leaf essential oils (6.2 and 13.0%). In the essential oils of other Prangos species, this oxygenated monoterpene was usually present in lower amounts or it was absent [6].
Our study is the first to reveal the chemical composition of essential oils isolated from P. trifida roots, leaves, stems and fruits collected in Serbia. Moreover, through comparison with previously published data [23,24,25,26,27], we provided initial insights about variation in the composition of P. trifida essential oils depending on the geographical origin of this species, and established a good basis for further research on this topic.
3.1.2. Chemical Composition of CH2Cl2 Extracts
In P. trifida roots and fruits CH2Cl2 extracts, fatty acids, phytosterols, triterpenes and coumarins were investigated. Fatty acids, phytosterols and triterpenes were analyzed by GC-FID-MS. In that aim, more volatile derivatives of these compounds were prepared: methyl esters of fatty acids (FAME) and trimethylsilyl (TMS) derivatives of phytosterols and triterpenes. Regarding coumarins, two of them were obtained from CH2Cl2 extracts and identified using 1H-NMR. In order to further investigate these secondary metabolites, i.e., to perform their LC–MS analysis, MeOH fractions of CH2Cl2 extracts (MFDEs) were prepared. Namely, semi-solid (oily) CH2Cl2 extracts cannot be directly injected into a standard reversed-phase LC–MS system and coumarins have very similar solubility in CH2Cl2 and MeOH, i.e., those extracted using CH2Cl2 from the plant material will be also dissolved by MeOH [38].
In comparison with the current study, a very similar fruits fatty acid pattern, i.e., the prevalence of petroselinic acid (47.1–56.9%), which was followed by linoleic (21.8–30.4%) and oleic acids (9.5–14.0%), was observed for five Turkish Prangos species (P. meliocarpoides, P. pabularia, P. platychlaena, P. uechtritzii and P. uloptera DC.) [39,40]. On the other hand, the fatty acids in roots of Prangos species were investigated for the first time in our study. The abundance of petroselinic acid in P. trifida fruits extract and its absence from the roots extract, in which widespread linoleic acid was present in markedly higher amounts compared to other compounds, is in accordance with literature data for other Apiaceae species [39].
The two ubiquitous phytosterols β-sitosterol and stigmasterol, which were dominant in unsaponifiable fractions of both roots and fruits extracts (UFRE and UFFE) investigated in our work, were also previously isolated from the roots of P. hulusii [13]. Up to date, there is no literature data on phytosterols and triterpenes from the fruits of Prangos species. For example, α-spinasterol, which was also present in notable amounts in UFFE, was previously identified in some spices originating from the Apiaceae family, such as fruits of anise (Pimpinella anisum L.) and ajwain (Trachyspermum ammi L.) [41].
Regarding coumarins, it should be noted that prantschimgin (10), imperatorin (7) and isoimperatorin (9), identified in both roots and fruits (i.e., in their MFDEs) in the current work, were also previously identified in P. trifida, but the plant part from which these coumarins were isolated was not reported in the available literature [28]. These and all other coumarins identified in our study were also previously identified in different plant parts of various Prangos species [6]. For example, heraclenol (1), oxypeucedanin hydrate (3), heraclenin (5), oxypeucedanin (6), imperatorin (7), isoimperatorin (9) and prantschimgin (10) were isolated from the chloroform extract of the roots of P. pabularia [12]. Also, heraclenin (5) was identified in P. heyniae aerial parts MeOH and water extracts, and imperatorin (7) in P. meliocarpoides aerial parts hexane, ethyl acetate, MeOH and water extracts. Quantitative analysis of these extracts revealed that amounts of coumarins in the aerial parts of these species (phenophase was not specified) were much lower (up to 14.72 mg/g in the case of 7 in P. meliocarpoides water extract) [15] compared to P. trifida roots and fruits investigated in the current work. Similarly, in MeOH fractions of CH2Cl2 extracts (MFDEs) of P. trifida leaves and stems, prepared in the same way as MFDEs of roots and fruits in this work, we established notably lower amounts of coumarins (only up to 5 mg/g) using LC–MS. Therefore, as well as because fatty oils are present only in roots and fruits of this plant, CH2Cl2 extracts and MFDEs of leaves and stems were not the focus of this work.
Prantschimgin (10), which was firstly isolated from the ethanol extract of the roots of P. tschimganica O. Fedtsch. [42], is certainly the most specific coumarin identified in our work. Besides in the Prangos species, this coumarin was reported only for representatives of a few other related Apiaceae genera, e.g., Ferulago W.D.J.Koch [14].
In summary, the present study is, to the best of our knowledge, the first investigation of fatty acid, phytosterol and triterpene profiles of P. trifida. It also notably complements the data on coumarin composition of this plant, as it led to the identification of five new coumarins for this species.
3.2. Antimicrobial and Antibiofilm Activities, and Potential Mechanisms of Selected P. trifida Isolated Products
In our study, we adopted a multifaceted approach to evaluate the antimicrobial and antibiofilm properties of selected isolates derived from P. trifida. Specifically, we chose to investigate essential oils and coumarin-rich MeOH fractions of CH2Cl2 extracts (MFDEs). Our decision to focus on these particular isolates was guided by the presence and amounts of metabolites that we identified during our phytochemical investigations, as described in Section 2.1 (MFDEs contained notably higher shares of coumarins than crude CH2Cl2 extracts; Table 5). By focusing on these key antimicrobial constituents, we gained a deeper understanding of the potential antimicrobial properties of P. trifida isolates. In doing so, we contributed to a more thorough understanding of the biological properties of P. trifida isolates, shedding light on their potential applications in antimicrobial or antibiofilm contexts.
For this research, we selected 13 microorganisms, most of which are generally known to be food contaminants. They can cause food spoilage, produce various harmful toxins and/or cause foodborne infections. However, it should be noted that a number of tested microorganisms can also be spread in various other ways. Among these 13 microorganisms, 4 were selected for antibiofilm activity study. Due to biofilm production, they are significant from the aspect of food safety and/or hospital-acquired infections [43,44,45,46,47,48].
3.2.1. Antimicrobial and Antibiofilm Activities of Essential Oils
There is an ongoing trend in the search for essential oils that can be used as new natural food preservatives. Moreover, essential oils are also being investigated as potential new natural products for the treatment of infections. Thus, we compared antimicrobial activity revealed for investigated essential oils of P. trifida roots, leaves, stems and fruits to the activity of the two commercial food preservatives of synthetic origin (sodium benzoate—E211 and potassium metabisulphite—E224), as well as to the activity of the standard antibiotic streptomycin and antimycotic ketoconazole. The most intriguing were the effects of all investigated essential oils against all six tested strains of molds, as well as of the root and stem oils against Staphylococcus aureus and Bacillus cereus, since they were all better compared to the effects of both E211 and E224 against these microorganisms. Despite essential oils generally showing weaker activity compared to standard drugs, it should be noted that obtained MIC values were in many cases ca. 0.1 mg/mL, which is regarded, according to some authors [49], as promising and justifies deeper investigations. Such MIC values were exhibited by all investigated essential oils against both tested Penicillium strains, the root, leaf and stem oils against Trichoderma harzianum, the root, stem and fruit oils against Aspergillus fumigatus, and the root and stem oils against A. niger, S. aureus and B. cereus. Generally, tested essential oils showed good potential for control of tested Aspergillus and Penicillium species, which are known food contaminants and producers of a number of potentially carcinogenic mycotoxins. For example, A. niger and P. verrucosum produce nephrotoxic, hepatotoxic, teratogenic and immunosuppressive ochratoxin A [43,44].
Furthermore, all investigated P. trifida essential oils (i.e., those of roots, leaves, stems and fruits) strongly inhibited biofilm formation by all tested bacteria (Listeria monocytogenes, Escherichia coli and S. aureus), but not of the tested yeast (Candida albicans). The significance of these results is reflected in the fact that L. monocytogenes, E. coli and S. aureus biofilms are emerging as a major safety concern in food processing plants due to their persistence on various surfaces, causing food spoilage and even foodborne diseases. Furthermore, E. coli and S. aureus biofilms are, among others, also found on medical devices in healthcare facilities and can cause hospital-acquired infections [45,46,47,48].
In previous studies [50,51,52,53,54], both the antimicrobial and antibiofilm activities against some microorganisms tested in our work, such as S. aureus, L. monocytogenes or E. coli, were demonstrated for certain essential oil constituents. For example, limonene, α-pinene, p-cymene, γ-terpinene and (E)-caryophyllene, which were present as either major or minor components in investigated P. trifida essential oils, exhibited these activities [50,51,52,53,54]. This fact can at least partly explain the activities observed in the current study.
Our study significantly widens the knowledge of antibacterial activity of P. trifida essential oils, which has been hinted at in previous research in which the authors investigated the potential of essential oil of aerial parts of this plant from Italy (collected before flowering) against six bacteria. The activity of the Italian essential oil was the same as the activity of the root and stem oils, investigated in the current work, against B. cereus (MIC = 0.2 mg/mL). The sample from Italy was also effective towards B. subtilis (MIC = 0.12 mg/mL). Activity was not found in the case of S. aureus, E. coli, Pseudomonas aeruginosa and Salmonella Typhimurium. However, it should be noted that concentrations of the oil of only up to 0.2 mg/mL were tested [26]. Regarding other Prangos species, antibacterial and/or antifungal activities were for example previously demonstrated for essential oils of P. ferulacea roots, leaves and flowers, P. peucedanifolia leaves and fruits, and P. pabularia, P. asperula, P. platychlaena and P. uechtritzii fruits [6,8,55,56,57,58,59]. However, antibiofilm activity of Prangos essential oils was not previously studied.
3.2.2. Antimicrobial and Antibiofilm Activities of MFDEs
Coumarin-rich P. trifida roots and fruits MFDEs were investigated in a search for sources of compounds that could potentially represent new therapeutic options against the 13 aforementioned tested microorganisms. Thus, obtained MIC and MBC/MFC values were compared to those of standard antibiotic streptomycin and antimycotic ketoconazole. Despite the fact that MFDEs were active against all 13 microorganisms, the antimicrobial effects of the standard drugs were generally better. However, several notable results were revealed.
Overall, it is noteworthy that the antifungal impact of the tested MFDEs appeared to be more pronounced, or at least more evident than the antibacterial effect. Namely, in the case of Penicillium funiculosum and three tested Aspergillus species, MICs were below 1 mg/mL, and in the case of A. niger, even below 0.1 mg/mL. In addition to being producers of mycotoxins, some of these molds can cause serious infections in immunocompromised people, such as invasive aspergillosis caused by A. fumigatus [60]. Regarding tested bacteria, foodborne pathogen Bacillus cereus was by far the most susceptible microorganism in the case of both MFDEs with MICs close to 0.1 mg/mL.
Amongst coumarins identified in investigated MFDEs, antimicrobial activity was previously shown for prantschimgin (10) against Staphylococcus aureus, Escherichia coli and Candida albicans [61], imperatorin (7) against E. coli and C. albicans [62], as well as for oxypeucedanin (6), oxypeucedanin hydrate (3), and isoimperatorin (9) against all bacteria tested in the present study (including B. cereus) [63], at least partly clarifying our results. Regarding coumarin-rich isolated products of other Prangos species, for the CH2Cl2 extract of the roots of P. hulusii, as well as for its ten isolated coumarins (including 6 and 9), the activity against 15 bacterial strains, including S. aureus and E. coli strains, was shown [13]. Very interesting effects of MFDEs tested in our work against molds, particularly A. niger, demand further research in order to reveal their active constituents.
The best biofilm inhibiting abilities of both the roots and fruits MFDEs were revealed in the case of C. albicans. Biofilms are an important aspect of this yeast’s life cycle and contribute to its ability to resist antimicrobial treatments and cause infections (e.g., oral thrush and vaginitis) [64]. Therefore, the potential of these MFDEs to reduce biofilm formation by about 50% at their MICs indicates that they may be useful tools in the fight against C. albicans infections. By inhibiting the biofilm formation of C. albicans, these MFDEs could hinder the yeast’s ability to establish resilient communities, thereby potentially facilitating its control and elimination. This is particularly important because biofilms are often linked to the development of chronic and recurrent infections that are difficult to treat with traditional antifungal agents. Besides on formation of C. albicans biofilm, the fruits MFDE also had notable effect on formation of S. aureus biofilm and the roots MFDE on formation of Listeria monocytogenes biofilm. Previously, reduction in the transcription of genes involved in the biofilm formation ability of L. monocytogenes was shown for P. ferulacea water extract [17].
However, despite the aforementioned biofilm inhibition capabilities, it is crucial to note that the efficiency of these MFDEs was not universal across all tested microorganisms. For example, these MFDEs exhibited a markedly reduced effect on the biofilm formation of the bacterium E. coli. In the case of this bacterium, the biofilm inhibitory effect of both MFDEs was minimal, showing that E. coli was relatively resilient to the biofilm inhibition properties of the MFDEs. This outcome suggests that the biofilm formation of E. coli might be governed by more complex mechanisms that may not be easily disrupted by these MFDEs, and warrants further investigation to understand the underlying processes. Our findings highlight the significant biofilm inhibition capability of the MFDEs against C. albicans when applied at their MICs. This provides a promising foundation for future research, possibly leading to the development of novel treatment strategies to combat infections caused by C. albicans.
In addition, the demonstrated antimicrobial activity of these extracts’ fractions represent a good basis for future investigation of the possibility of their application for the synthesis of bioactive multicomponent nanoparticles, in accordance with the growing trend in this field [65,66].
It should be noted that despite there are previous studies about the antimicrobial activity of coumarins identified in investigated MFDEs, data on their antibiofilm activity are lacking, which justifies further research on this matter.
3.2.3. Potential Mechanisms of Action against Candida albicans of MFDEs
Our study explored potential mechanisms of action through which the MFDEs might inhibit C. albicans, a common fungal pathogen known for its ability to form biofilms that contribute to its resistance against antifungal treatments. The MFDEs were selected for testing their possible mechanisms because their MIC values were lower compared to the MIC values of the studied essential oils.
One significant observation from our results was the marked reduction in exopolysaccharide (EPS) production in C. albicans. EPS, a crucial component of the biofilm matrix that provides a protective barrier for biofilm-forming microorganisms, was noticeably reduced in the presence of both roots and fruits MFDEs. This substantial decrease in EPS production implies that these extracts may interfere with the EPS synthesis or export process, disrupting the formation of the protective biofilm matrix and leaving the fungal cells more vulnerable.
However, when we explored whether the antimicrobial action of the MFDEs could be attributed to binding to ergosterol—a major component of the fungal cell membrane—we found no changes in the MICs using the ergosterol binding assay. Ergosterol plays a vital role in maintaining the integrity, fluidity, and functionality of the fungal cell membrane, and many antifungal drugs exert their effects by interacting with ergosterol or inhibiting its synthesis [67]. However, the lack of change in MICs in our experiment indicates that the antimicrobial action of these MFDEs is not due to an interaction with ergosterol. Therefore, binding to ergosterol does not seem to be a mechanism of antimicrobial action for these MFDEs.
While the exact mechanisms of action remain to be fully elucidated, our study has revealed valuable insights into how these MFDEs might impede biofilm formation and disrupt the growth of C. albicans. The substantial reduction in EPS production is a promising lead for further investigation into the potential applications of these MFDEs in the fight against C. albicans and other biofilm-forming pathogens.
4. Materials and Methods
4.1. Plant Material
The plant material (roots, leaves, stems and fruits of P. trifida) was collected in Sićevo Gorge (Kusača), Serbia in 2020 (22.0810369° E, 43.315107° N). The plant was identified by Dr. Marjan Niketić, curator/botanist of the Natural History Museum, Belgrade (Serbia), and the voucher specimen is deposited in the Herbarium of the Natural History Museum, Belgrade (BEO); voucher number: 20200603. The plant material was air-dried prior to analyses.
4.2. Isolation of the Essential Oils
The powdered roots, leaves, stems and fruits were hydrodistilled for 2.5 h using a Clevenger-type apparatus, according to the procedure of European Pharmacopoeia 11.0 [68]. n-Hexane was used as the collecting solvent. Essential oils were dried over anhydrous sodium sulfate, n-hexane was evaporated, and essential oils were stored at 4 °C until analysis. The determination of essential oil content was done in triplicate.
4.3. Chemical Analysis of the Essential Oils
GC-FID-MS analysis was performed on an Agilent 6890N Gas Chromatograph (Agilent Technologies, Palo Alto, CA, USA), equipped with split/splitless injector, capillary column (Agilent HP-5MS 30 m × 0.25 mm, 0.25 μm film thickness) and flame ionization detector (FID), and coupled to an Agilent 5975C MS detector [33]. Briefly, injector and FID temperatures: 200 and 300 °C, respectively. Carrier gas: helium; flow: 1.0 mL/min. The oven temperature: 60 to 280 °C at 3 °C/min (final temperature held for 10 min). Split ratio: 1:10. Injected volume: 1 μL of 1.5% (v/v) solutions of essential oils in n-hexane. MSD was operating in EI mode at 70 eV. MSD transfer line, ion source and analyzer (single quadrupole) temperatures: 250, 230 and 150 °C, respectively. Range m/z: 35–550. Scan speed: 2.83 scans/s. The analysis was done using the MSD ChemStation E.01.00.237 software. Linear retention indices (RIs) of the constituents were determined in relation to the homologue series of n-alkanes (C8-C40) (Fluka, Buchs, Switzerland) ran under the same operating conditions. The identification of the compounds was based on the comparison of their retention indices (RI) and mass spectra to those from the NIST/NBS 05 and Wiley 8th edition mass spectra libraries, as well as the literature [29]. Relative percentages of the compounds were calculated using peak areas from the FID data.
4.4. Static Headspace (HS) Extraction and Chemical Analysis of HS Volatiles
HS extractions were performed using an Agilent G1888 automatic HS sampler coupled with Agilent 6890N Gas Chromatograph. Roots, leaves, stems and fruits (0.5 g) were ground and hermetically sealed in HS vials. Experimental conditions of HS extractions were as follows [69]: oven temperature 90 °C, loop temperature 100 °C, transfer line 110 °C, equilibration time 30 min, shaking low; pressurization time 0.08, carrier gas helium, in vial pressure 15 psi, loop fill 0.5, loop equilibration 0.05, inject time 1.00. GC-FID-MS experimental conditions, as well as methods of identification and quantification of compounds, were the same as in the case of the essential oils analysis.
4.5. Obtaining of CH2Cl2 Extracts
Roots and fruits were powdered and extracted with CH2Cl2 at room temperature. Two successive extractions with CH2Cl2 were performed. The first extraction lasted for 3 days, and then after filtration, the residual material was subjected to the second extraction, which lasted for 2 days (herbal drug:solvent ratio in both steps of extraction 1:10 w/v). After filtration, two resulting extracts were combined. CH2Cl2 was removed under reduced pressure to obtain semi-solid (oily) extracts.
4.6. Saponification and Transesterification of CH2Cl2 Extracts
The saponification of the fatty oils in 1 g of roots and fruits CH2Cl2 extracts was done using 50% potassium hydroxide (5 mL)/ethanol (30 mL) at 90 °C for 60 min. Unsaponifiable fractions were separated using petroleum ether, and the soap-rich polar fractions were treated with hydrochloric acid to obtain free fatty acids, which were then collected using diethyl ether. Afterwards, the fatty acids were esterified using 98% sulphuric acid (1 mL)/methanol (150 mL, purity ≥ 99.9%) at 80 °C for 60 min to obtain volatile fatty acid methyl esters (FAME), which were then collected using petroleum ether. To analyze phytosterols and triterpenes, the unsaponifiable fractions (300 μL of 5 mg/mL solution in CH2Cl2) were treated with bis-(trimethylsilyl)-trifluoroacetamide (BSTFA; Sigma-Aldrich, St. Louis, MO, USA) (200 μL) and held at 60 °C for 60 min to obtain volatile trimethylsilyl (TMS) derivatives [70].
4.7. Analysis of the FAME, Phytosterols and Triterpenes in CH2Cl2 Extracts
The same GC-FID-MS system as in the case of the analysis of the essential oils was used, except that for analysis of FAME, different column (Agilent J&W HP-88 100 m × 0.25 mm, 0.20 μm film thickness) was installed. In the case of FAME, injector and FID temperatures were both 260 °C. Carrier gas: helium; flow: 1.2 mL/min. Oven temperature was initially held at 140 °C for 5 min, then increased linearly from 140 to 240 °C at 4 °C/min, and finally held at 240 °C for 10 min. Split ratio: 1:25. Injected volume: 1 μL of 1.0% (v/v) solution of FAME in CH2Cl2. MSD parameters were the same as for the analysis of essential oils. The identification of FAME was based on the comparison of their retention times (Rt) and mass spectra to those of commercial standards ran under the same chromatographic conditions (Supelco 37 Component FAME Mix, petroselinic acid methyl ester and cis-11-vaccenic acid methyl ester; all Sigma-Aldrich) [70].
The experimental conditions for the phytosterol and triterpene analysis were the same as in the case of the essential oils, except that for phytosterols and triterpenes, the final oven temperature (280 °C) was held for 20 min. The phytosterols and triterpenes were identified based on comparison of their mass spectra to those from the NIST/NBS 05 and Wiley 8th edition libraries, as well as the literature [41,71]. Identity of stigmasterol, β-sitosterol (both Val-de-Reuil, France) and β-amyrin (Sigma-Aldrich) was confirmed using commercial standard compounds.
The relative percentages of the FAME, phytosterols, and triterpenes were calculated based on the peak areas from the FID data.
4.8. Obtaining of Oxypeucedanin (6) and Prantschimgin (10) from CH2Cl2 Extracts
To semi-solid roots and fruits CH2Cl2 extracts (470.0 and 1000.0 mg, respectively), diethyl ether (5 mL in both cases) was added. Obtained solid (crystalline) precipitates were separated by filtering through filter papers and additionally washed with diethyl ether until oily parts were completely removed.
1H-NMR spectra of the crystalline precipitates were recorded on Avance III (Bruker, Billerica, MA, USA), operating at 400 MHz. CDCl3 (Sigma-Aldrich) was used as solvent and TMS as internal standard. The precipitate obtained from the fruits extract (25.0 mg) contained compound 6 and the one obtained from the roots extract (15.1 mg) contained compound 10.
Oxypeucedanin (6) 1H-NMR (400 MHz, CDCl3, J in Hz): 8.21 (1H, d, J = 9.8, H-4), 7.62 (1H, d, J = 2.2, H-2′), 7.21 (1H, s, H-8), 6.95 (1H, d, J = 2.2, H-3′), 6.32 (1H, d, J = 9.8, H-3), 4.60 (1H, dd, J = 10.8, 4.3, H-1″), 4.44 (1H, dd, J = 10.8, 6.5, H-1″) 3.23 (1H, dd, J = 4.6, 6.2, H-2″), 1.41 (3H, s, Me-3″), 1.33 (3H, s, Me-3″).
Prantschimgin (10) 1H-NMR (400 MHz, CDCl3, J in Hz): 7.59 (1H, d, J = 9.5, H-4), 7.21 (1H, s, H-5), 6.74 (1H, s, H-8), 6.21 (1H, d, J = 9.5, H-3), 5.55 (1H, s, H-2 (OSen)), 5.14 (1H, t, J = 8.6, H-2′), 3.23 (2H, m, H-3′), 2.10 (3H, s, H-4 (OSen)), 1.85 (3H, s, H-5 (OSen)), 1.60 (3H, s, H-2″), 1.53 (3H, s, H-3″).
The purity of compounds was determined based on peak areas recorded on diode array detector (DAD) at 250 nm (6) and 350 nm (10), using the LC–MS method described in the Section 4.10.
4.9. Obtaining of Methanol Fractions of CH2Cl2 Extracts (MFDEs)
The semi-solid roots and fruits CH2Cl2 extracts were suspended in MeOH (5 mg/mL) and filtered through membrane filters (0.45 μm). MeOH was removed from filtrate under reduced pressure.
4.10. LC–MS Analysis of Coumarins in MFDEs
The coumarins were analyzed on an Agilent Infinity 1200 Liquid Chromatograph (Agilent Technologies), equipped with autosampler, quaternary pump, Agilent Zorbax SB-Aq column (150 × 3.0 mm; 3.5 μm particle size), diode array detector (DAD) and Single quad MS detector with an electrospray ionization (ESI) ion source (LC–MS). MFDEs were dissolved in MeOH (5 mg/mL). Injection volume: 5 μL (roots MFDE) or 2.5 μL (fruits MFDE). Binary mobile phase, consisting of 0.1% formic acid and 10% isopropanol (A), and MeOH (B) (all Sigma-Aldrich; LC–MS purity), was applied at 0.3 mL/min; gradient: 25–100% B (30 min). DAD chromatograms were recorded at 210, 250, 270, 320 and 350 nm. ESI mass spectra were recorded in positive ion mode, with nebulization with nitrogen at 10 L/min and pressure of 40 psi, at temperature of 350 °C and capillary voltage at 3500 V. Signals were registered by fragmentor voltage of 100 V or 250 V. Analysis was done in triplicate.
Identification of coumarins was performed using commercial standards of xanthotoxin (4) and imperatorin (7) (obtained from Sigma-Aldrich), oxypeucedanin (6) and prantschimgin (10) obtained in this work, and previously chemically characterized CH2Cl2 extract of Heracleum ternatum fruits [33] (1 and 5), or their structures were elucidated to the highest possible extent based on analysis of their UV and mass spectra (2, 3, 8 and 9). Quantification of compounds was done using imperatorin (7), oxypeucedanin (6) and prantschimgin (10) by the external standard method using peak areas obtained by DAD at 250 nm (7 and 6) or 350 nm (10). Based on similarity of their UV spectra, imperatorin (7) was used for quantification of furanocoumarins oxygenated at C(8), oxypeucedanin (6) for furanocoumarins oxygenated at C(5), and prantschimgin (10) for 2′,3′-dihydrofuranocoumarins. For the purpose of quantification of isoimperatorin (9; which co-eluted with prantschimgin, 10), the calibration curve of imperatorin (7) using peak area obtained by Single Ion Monitoring (SIM) of m/z 271.1 was used. Limits of detection (LODs) and quantification (LOQs) for the coumarins were determined using the standard deviations of the intercepts (SDb) and the slopes (a) as follows: LOD = 3.3 × SDb/a and LOQ = 10 × SDb/a. Regression equations, r2, LODs and LOQs are given in Table S1.
4.11. Investigation of Antibacterial and Antifungal Activities of Essential Oils and MFDEs
The following Gram-positive bacteria: Staphylococcus aureus (ATCC 11632), Bacillus cereus (food isolate) and Listeria monocytogenes (NCTC 7973), as well as Gram-negative bacteria Escherichia coli (ATCC 25922), Enterobacter cloacae (ATCC 35030) and Salmonella Typhimurium (ATCC 13311) were tested. Also, the following micromycetes were used: Aspergillus fumigatus (ATCC 9197), Aspergillus niger (ATCC 6275), Aspergillus versicolor (ATCC 11730), Penicillium funiculosum (ATCC 36839), Penicillium verrucosum var. cyclopium (food isolate), Trichoderma harzianum (TH-IS005-12) and Candida albicans (ATCC 10231). The microorganisms are deposited at Mycological laboratory, Department of Plant Physiology, Institute for Biological research “Sinisa Stanković”, National Institute of Republic of Serbia, University of Belgrade, Serbia. By using the microdilution method [72], minimal inhibitory concentrations (MICs) and minimal bactericidal/fungicidal concentrations (MBCs/MFCs) were calculated. The 96-well microtiter plates were incubated at 37 °C for 24 h with serially diluted essential oils/MFDEs in liquid broth. After incubation, the MICs and MFCs were identified. MIC was defined as the lowest concentrations at which no growth was seen under a microscope. After successive sub-cultivation of 10 µL of samples at 37 °C for 24 h, MBC/MFC values were observed as concentrations without discernible growth. As positive controls, artificial food preservatives E211 and E224, as well as standard antibiotic streptomycin (Sigma-Aldrich) and antimycotic ketoconazole (Sigma-Aldrich), were used.
4.12. Inhibition of Biofilm Formation of Essential Oils and MFDEs
Strains used for inhibition of biofilm formation assay were Staphylococcus aureus (ATCC 11632), Listeria monocytogenes (NCTC 7973), Escherichia coli (ATCC 25922) and Candida albicans (ATCC 10231).
The ability of essential oils/MFDEs to inhibit the formation of biofilm was examined as described previously [73]. Selected strains were cultured with MIC, ½MIC and ¼MIC concentrations of the essential oils/MFDEs in TSB/YPD medium at 37 °C for 24 h in 96-well microtiter plates with adhesive bottoms (Sarstedt, Germany). Following three sterile PBS (Phosphate Buffered Saline, pH 7.4) well washes, biofilms were fixed in methanol for 20 min. After that, the methanol was taken out and biofilms were dyed for 30 min with 0.1% crystal violet (Bio-Merieux, France). Ethanol (96%) (Zorka, Serbia) was applied to dissolve bonded crystal violet after the plate had been slowly cleaned (to remove excess dye) and dried on the air. Thermo Scientific’s Multiskan FC Microplate Photometer was used to measure the absorbance (620 nm). Percentage of inhibition of biofilm formation was calculated using absorbances of samples treated with essential oils/MFDEs (A620 sample) and untreated samples (A620 control), using the following equation:
Inhibition (%) = [(A620 control − A620 sample)/A620control)] × 100
4.13. Congo Red Binding Assay
According to a previously described approach [74], the effect of the tested MFDEs on the exopolysaccharide (EPS) synthesis by the C. albicans ATCC 10231 biofilm was assessed with some changes. Tested MFDEs were applied to preformed 24-h biofilms in microtiter plates for 24 h at 37 °C at its MIC, ½MIC and ¼MIC concentrations. The adherent cells were then rinsed with PBS after the planktonic cells were removed. Wells received congo red (1%, w/v) and were then left in the dark for 30 min. The excess dye was removed, and 200 μL DMSO was used to dissolve the bound congo red. A microtiter plate reader at 450 nm was used to measure the absorbance. Percentage of EPS inhibition was calculated using absorbances of samples treated with MFDEs (A450 sample) and untreated samples (A450 control), using the following equation:
Inhibition (%) = [(A450 control − A450 sample)/A450 control)] × 100
4.14. Ergosterol Binding Assay in C. albicans
Ergosterol binding assay was performed according to the method described by Leite et al. [64], with some modifications. Briefly, MFDEs microdilutions were prepared in a similar manner as for the testing of antimicrobial activity, with the exception that ergosterol (400 μg/mL) was added to the plate’s rows. After a 24-h incubation period at 37 °C, the MIC values of the samples without added ergosterol were contrasted with the MIC values of ergosterol-added samples. The increase of MIC value in ergosterol-added samples would indicate that the antimicrobial action is due to an interaction with ergosterol.
4.15. Statistical Analysis
The results were presented as the mean value of three replicates ± standard deviation (SD). When applicable, the data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s HSD test with α = 0.05 using the program IBM SPSS version 22.0, IBM Corp., Armonk, NY, USA. Values of p < 0.05 were regarded for statistically significant.
5. Conclusions
In this work, the data about P. trifida volatile constituents and coumarins were significantly complemented, whereas fatty acids and phytosterols of this plant were, to the best of our knowledge, investigated for the first time. Essential oils showed strong activity against a wide range of food contaminants, as well as promising inhibition of biofilm formation of some tested bacteria, justifying their further investigation as raw materials for pharmaceutical and food industries. Also, results obtained for MFDEs provide a good basis for the investigation of antimicrobial and antibiofilm activities of identified coumarins, particularly against tested molds, as well as Candida albicans. As we move forward, continued exploration of P. trifida chemical composition and its practical applications in pharmaceuticals and food safety will undoubtedly pave the way for innovative solutions to real-world challenges in these industries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13010041/s1, Figure S1: Prangos trifida aerial parts in fruit (A and B), fruits (C-F) and dried leaf (G). Sićevo Gorge (Kusača), Serbia (2020).; Table S1: Regression equations, r2, limits of detection (LOD) and quantification (LOQ) of the compounds.
Author Contributions
Conceptualization, S.P. and D.S.; methodology, L.U., S.P., D.S. and M.S.; investigation, L.U., D.S., T.C. and V.M.; resources, S.P., M.N., D.S. and M.S.; writing—original draft preparation, L.U., D.S., T.C. and V.M.; writing—review and editing, S.P., M.S. and M.N.; visualization, L.U., D.S., T.C. and V.M.; supervision, S.P., D.S. and M.S.; project administration, S.P., D.S. and M.S.; funding acquisition, S.P., D.S. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant numbers 451-03-47/2023-01/200007 and 451-03-47/2023-01/200161.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stojković, D.; Petrović, J.; Carević, T.; Soković, M.; Liaras, K. Synthetic and Semisynthetic Compounds as Antibacterials Targeting Virulence Traits in Resistant Strains: A Narrative Updated Review. Antibiotics 2023, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging Antifungal Targets and Strategies. Int. J. Mol. Sci. 2022, 23, 2756. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Ivanov, M.; Markovic, T.; Sanković Babić, S.; Barros, L.; Calhelha, R.; Sokovic, M.; Ciric, A. An in vitro study of the Origanum minutiflorum O. Schwarz & P. H. Davis and Coriandrum sativum L. essential oils as chronic tonsillitis therapeutics: Antibacterial, antibiofilm, antioxidant, and cytotoxic activities. J. Essent. Oil Res. 2022, 34, 533–543. [Google Scholar] [CrossRef]
- Plants of the World Online. Available online: https://powo.science.kew.org (accessed on 14 December 2023).
- Mottaghipisheh, J.; Kiss, T.; Tóth, B.; Csupor, D. The Prangos genus: A comprehensive review on traditional use, phytochemistry, and pharmacological activities. Phytochem. Rev. 2020, 19, 1449–1470. [Google Scholar] [CrossRef]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. The nonvolatile and volatile metabolites of Prangos ferulacea and their biological properties. Planta Med. 2019, 85, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, N.; Maresca, V.; Di Napoli, M.; Bruno, M.; Basile, A.; Zanfardino, A. Chemical composition and biological activities of Prangos ferulacea essential oils. Molecules 2022, 27, 7430. [Google Scholar] [CrossRef]
- Azarkish, P.; Moghaddam, M.; Ghasemi Pirbalouti, A.; Khakdan, F. Variability in the essential oil of different wild populations of Prangos platychlaena collected from Southwestern Iran. Plant Biosyst. 2021, 155, 1100–1110. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Yıldıztugay, E.; Jugreet, S.; Khan, S.U.; Dall’Acqua, S.; Mollica, A.; Bouyahya, A.; Montesano, D. Chemical composition, biological activities and in silico analysis of essential oils of three endemic Prangos species from Turkey. Molecules 2022, 27, 1676. [Google Scholar] [CrossRef]
- Karahisar, E.; Köse, Y.B.; İşcan, G.; Kurkcuoglu, M.; Tugay, O. Chemical Composition and Anticandidal Activity of Essential Oils Obtained from Different Parts of Prangos heyniae H. Duman & MF Watson. Rec. Nat. Prod. 2022, 16, 74–83. [Google Scholar] [CrossRef]
- Sevin, G.; Alan, E.; Demir, S.; Albayrak, G.; Demiroz, T.; Yetik-Anacak, G.; Baykan, S. Comparative evaluation of relaxant effects of three Prangos species on mouse corpus cavernosum: Chemical characterization and the relaxant mechanisms of action of P. pabularia and (+)-oxypeucedanin. J. Ethnopharmacol. 2022, 284, 114823. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sinan, K.I.; Ak, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamočlija, J.; Soković, M.; Jekő, J.; Cziáky, Z.; et al. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: A comparative study. Ind. Crops Prod. 2020, 153, 112572. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Sut, S.; Zengin, G.; Peron, G.; Elbasan, F.; Yildiztugay, E.; Bibi Sadeer, N.; Mahomoodally, M.F. Phytochemical screening, antioxidant, and enzyme inhibitory properties of three Prangos species (P. heyniae, P. meliocarpoides var. meliocarpoides, and P. uechtritzii) depicted by comprehensive LC-MS and multivariate data analysis. Antioxidants 2022, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, M.; Ranjbar, R. Investigation of the antibacterial and biofilm inhibitory activities of Prangos acaulis (DC.) Bornm in nanoparticulated formulation. Nanotechnology 2022, 33, 385103. [Google Scholar] [CrossRef] [PubMed]
- Sarghaleh, S.J.; Behbahani, B.A.; Hojjati, M.; Vasiee, A.; Noshad, M. Evaluation of the constituent compounds, antioxidant, anticancer, and antimicrobial potential of Prangos ferulacea plant extract and its effect on Listeria monocytogenes virulence gene expression. Front. Microbiol. 2023, 14, 1202228. [Google Scholar] [CrossRef]
- Herrnstadt, I.; Heyn, C.C. A monographic study of the genus Prangos (Umbelliferae). Boissiera 1977, 26, 1–91. [Google Scholar]
- Niketić, M. Cachrys alpina L. In The Red Data Book of Flora of Serbia. Extinct and Critically Endangered Taxa; Stevanović, V., Ed.; Ministry of Environment of the Republic of Serbia, Faculty of Biology, University of Belgrade, Institution for Protection of Nature of the Republic of Serbia: Beograd, Serbia, 1999; pp. 174–176+454–455, (In Serbian and English). [Google Scholar]
- Hand, R. Apiaceae. In Euro+Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity; Dipartimento di Scienzeambientali e Biodiversità ed Orto botanico, Università degli Studi di Palermo: Palermo, Italy, 2011; Available online: https://europlusmed.org/cdm_dataportal/taxon/368c1360-c3ad-41c6-8a61-e46168b44d47#footnote-T (accessed on 10 September 2023).
- Lyskov, D.F. Systematics of the Genus Prangos (Umbelliferae, Apioideae) and Related Taxa: Comparison of Morphological-Anatomic and Molecular Data. Ph.D. Thesis, Faculty of Biology, M.V. Lomonosov Moscow State University, Moscow, Russia, 2015. (In Russian). [Google Scholar]
- Lyskov, D.; Samigullin, T. European Prangos species complexes: When classic morphological features are not enough to distinguish similar species. In Abstract Book, Proceedings of the IX Apiales Symposium, The Gold Coast Marina Club, Guangzhou, China, 31 July–2 August 2017; Oskolski, A., Nuraliev, M., Tilney, P., Eds.; University of Johannesburg: Johannesburg, South Africa; Komarov Botanical Institute: St. Petersburg, Russia; Lomonosov Moscow State University: Moscow, Russia, 2017; p. 23. [Google Scholar]
- Baser, K.H.C.; Demirci, B.; Akalin, E.; Özhatay, N. Composition of the Essential Oil of Cachrys alpine Bieb. J. Essent. Oil Res. 2004, 16, 167–168. [Google Scholar] [CrossRef]
- Palá-Paül, J.; Velasco-Negueruela, A.; Pérez-Alonso, J.; Maqueda, J.; Sanz, J. Volatile oil Constituents from Different Parts of Cachrys trifida L. J. Essent. Oil Res. 2004, 16, 347–349. [Google Scholar] [CrossRef]
- Korotkov, O.I.; Shevchuk, O.M.; Shatko, V.G.; Timashova, L.A.; Feskov, S.A. Some biochemical characteristics of Prangos trifida (Mill.) Herrnst. & Heyn. Bull. State Nikit. Botan. Gard. 2018, 76–83. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Vaglica, A.; Ilardi, V.; Varcamonti, M.; Bruno, M.; Zanfardino, A. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Italian Prangos trifida (Mill.) Herrnst. & Heyn. Nat. Prod. Res. 2022, 37, 3772–3786. [Google Scholar] [CrossRef]
- Maresca, V.; Badalamenti, N.; Ilardi, V.; Bruno, M.; Basile, A. The Antioxidant Properties and Protective Capacity of Prangos trifida and Cachrys cristata Essential Oils against Cd Stress in Lunularia cruciata and Brassica napus. Antioxidants 2023, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; De Las Heras, B.; Silván, A.M.; Pascual, R.; Bermejo, P.; Rodriguez, B.; Villar, A.M. Effects of furocoumarins from Cachrys trifida on some macrophage functions. J. Pharm. Pharmacol. 2001, 53, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1 ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017. [Google Scholar]
- Khalighi-Sigaroodi, F.; Hadjiakhoondi, A.; Shafiee, A.; Mozaffarian, V.A.; Shahverdi, A.R.; Alavi, S.H. Phytochemical analysis of Ferulogo bernardii Tomk & M. Pimen. DARU J. Pharm. Sci. 2006, 14, 214–221. [Google Scholar]
- Wei, Y.; Ito, Y. Preparative isolation of imperatorin, oxypeucedanin and isoimperatorin from traditional Chinese herb “bai zhi” Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook using multidimensional high-speed counter-current chromatography. J. Chromatogr. A 2006, 1115, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, S.E.; Jamali, M.; Shokoohinia, Y.; Abdi, G.; Shahbazi, B.; Fattahi, A. Antiproliferative evaluation of terpenoids and terpenoid coumarins from Ferulago macrocarpa (Fenzl) Boiss. fruits. Pharmacogn. Res. 2015, 7, 322–328. [Google Scholar] [CrossRef]
- Ušjak, L.J.; Drobac, M.M.; Niketić, M.S.; Petrović, S.D. Chemosystematic Significance of Essential Oil Constituents and Furanocoumarins of Underground Parts and Fruits of Nine Heracleum L. Taxa from Southeastern Europe. Chem. Biodivers. 2018, 15, e1800412. [Google Scholar] [CrossRef] [PubMed]
- Kiyonga, A.N.; Hong, G.; Kim, H.S.; Suh, Y.G.; Jung, K. Facile and Rapid Isolation of Oxypeucedanin Hydrate and Byakangelicin from Angelica dahurica by Using [Bmim] Tf2N Ionic Liquid. Molecules 2021, 26, 830. [Google Scholar] [CrossRef]
- Frérot, E.; Decorzant, E. Quantification of Total Furocoumarins in Citrus Oils by HPLC Coupled with UV, Fluorescence, and Mass Detection. J. Agric. Food Chem. 2004, 52, 6879–6886. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Chichester, UK, 2009; pp. 161–165. [Google Scholar]
- Kusano, M.; Kobayashi, M.; Iizuka, Y.; Fukushima, A.; Saito, K. Unbiased profiling of volatile organic compounds in the headspace of Allium plants using an in-tube extraction device. BMC Res. Notes 2016, 9, 133. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Głowniak, K. Pressurized liquid extraction of coumarins from fruits of Heracleum leskowii with application of solvents with different polarity under increasing temperature. Molecules 2012, 17, 4133–4141. [Google Scholar] [CrossRef]
- Bagci, E. Fatty acids and tocochromanol patterns of some Turkish Apiaceae (Umbelliferae) plants; a chemotaxonomic approach. Acta Bot. Gallica 2007, 154, 143–151. [Google Scholar] [CrossRef]
- Küçükboyacι, N.; Ayaz, F.; Adιgüzel, N.; Bani, B.; Gören, A.C. Fatty Acid Methyl Ester Composition of Some Turkish Apiaceae Seed Oils: New Sources for Petroselinic Acid. Nat. Prod. Commun. 2016, 11, 1934578X1601101118. [Google Scholar] [CrossRef]
- Saini, R.K.; Song, M.H.; Yu, J.W.; Shang, X.; Keum, Y.S. Phytosterol Profiling of Apiaceae Family Seeds Spices Using GC-MS. Foods 2021, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, G.A.; Belenovskaya, L.M. Prantschimgin—A new coumarin from the roots of Prangos tschimganica. Chem. Nat. Compd. 1966, 2, 190–192. [Google Scholar] [CrossRef]
- Basilico, M.Z.; Basilico, J.C. Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Lett. Appl. Microbiol. 1999, 29, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Bayman, P.; Baker, J.L.; Doster, M.A.; Michailides, T.J.; Mahoney, N.E. Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus. Appl. Environ. Microbiol. 2002, 68, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, T.; Cervantes-Huamán, B.R.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes biofilms in the food industry: Is the current hygiene program sufficient to combat the persistence of the pathogen? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef]
- Zhao, L.; Poh, C.N.; Wu, J.; Zhao, X.; He, Y.; Yang, H. Effects of electrolysed water combined with ultrasound on inactivation kinetics and metabolite profiles of Escherichia coli biofilms on food contact surface. Innov. Food Sci. Emerg. Technol. 2022, 76, 102917. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Villa, K.; Sopha, H.; Zelenka, J.; Motola, M.; Dekanovsky, L.; Beketova, D.C.; Macak, J.M.; Ruml, T.; Pumera, M. Enzyme-photocatalyst tandem microrobot powered by urea for Escherichia coli biofilm eradication. Small 2022, 18, 2106612. [Google Scholar] [CrossRef]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J. Anti-biofilm and Virulence Factor-Reducing Activities of Essential Oils and Oil Components as a Possible Option for Bacterial Infection Control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Evaluation of antibiofilm efficacy of essential oil components β-caryophyllene, cinnamaldehyde and eugenol alone and in combination against biofilm formation and preformed biofilms of Listeria monocytogenes and Salmonella typhimurium. Lett. Appl. Microbiol. 2020, 71, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tabassum, N.; Jeong, G.J.; Jung, W.K.; Kim, Y.M. Inhibition of Mixed Biofilms of Candida albicans and Staphylococcus aureus by β-Caryophyllene-Gold Nanoparticles. Antibiotics 2023, 12, 726. [Google Scholar] [CrossRef]
- Khoury, M.; El Beyrouthy, M.; Eparvier, V.; Ouaini, N.; Stien, D. Chemical diversity and antimicrobial activity of the essential oils of four Apiaceae species growing wild in Lebanon. J. Essent. Oil Res. 2018, 30, 25–31. [Google Scholar] [CrossRef]
- Yousefi, K.; Hamedeyazdan, S.; Hodaei, D.; Lotfipour, F.; Baradaran, B.; Orangi, M.; Fathiazad, F. An in vitro ethnopharmacological study on Prangos ferulacea: A wound healing agent. Bioimpacts 2017, 7, 75–82. [Google Scholar] [CrossRef][Green Version]
- Özek, G.; Özek, T.; İşcan, G.; Başer, K.H.C.; Hamzaoglu, E.; Duran, A. Comparison of hydrodistillation and microdistillation methods for the analysis of fruit volatiles of Prangos pabularia Lindl., and evaluation of its antimicrobial activity. S. Afr. J. Bot. 2007, 73, 563–569. [Google Scholar] [CrossRef][Green Version]
- Brusotti, G.; Ibrahim, M.F.; Dentamaro, A.; Gilardoni, G.; Tosi, S.; Grisoli, P.; Dacarro, C.; Guglielminetti, M.L.; Hussain, F.H.S.; Caccialanza, G.; et al. Chemical composition and antimicrobial activity of the volatile fractions from leaves and flowers of the wild Iraqi Kurdish plant Prangos peucedanifolia Fenzl. Chem. Biodivers. 2013, 10, 274–280. [Google Scholar] [CrossRef]
- Uzel, A.; Dirmenci, T.; Çelik, A.; Arabaci, T. Composition and antimicrobial activity of Prangos platychlaena and P. uechtritzii. Chem. Nat. Compd. 2006, 42, 169–171. [Google Scholar] [CrossRef]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Şimşek, D.; Özbek, H.; Güvenalp, Z.; Altanlar, N.; Kazaz, C.; Kiliç, C.S. Antimicrobial Activities of Extracts and Isolated Coumarins from the Roots of Four Ferulago Species Growing in Turkey. Iran. J. Pharm. Res. 2019, 18, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Topcu, G.; Tan, N.; Ölçal, S.; Johansson, C.; Üçer, M.; Birman, H.; Tamer, Ş. Biological activities of a Turkish medicinal plant, Prangos platychlaena. J. Ethnopharmacol. 1995, 45, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Mileski, K.S.; Trifunović, S.S.; Ćirić, A.D.; Šakić, Z.M.; Ristić, M.S.; Todorović, N.M.; Matevski, V.S.; Marin, P.D.; Tešević, V.V.; Džamić, A.M. Research on Chemical Composition and Biological Properties Including Antiquorum Sensing Activity of Angelica pancicii Vandas Aerial Parts and Roots. J. Agric. Food Chem. 2017, 65, 10933–10949. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.A.; De Brito Bezerra, A.P.; De Sousa, J.P.; De Oliveira Lima, E. Investigating the antifungal activity and mechanism(s) of geraniol against Candida albicans strains. Med. Mycol. 2015, 53, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Sudha, K.G.; Thirumalaivasan, N.; Ahamed, M.; Pandiaraj, S.; Rajeswari, V.D.; Vinayagam, Y.; Thiruvengadam, M.; Govindasamy, R. Green Synthesis of Magnesium Oxide Nanoparticles by Using Abrus precatorius Bark Extract and Their Photocatalytic, Antioxidant, Antibacterial, and Cytotoxicity Activities. Bioengineering 2023, 10, 302. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Kumaravel, P.; Jayaprakash, J.; Ragunathan, M.G.; Sankar, S.; Pandiaraj, S.; Thirumalaivasan, N.; Thiruvengadam, M.; Govindasamy, R. Computational approaches for modeling and structural design of biological systems: A comprehensive review. Prog. Biophys. Mol. Biol. 2023, 185, 17–32. [Google Scholar] [CrossRef]
- Pemán, J.; Cantón, E.; Espinel-Ingroff, A. Antifungal drug resistance mechanisms. Expert Rev. Anti-Infect. Ther. 2009, 7, 453–460. [Google Scholar] [CrossRef]
- EDQM. European Pharmacopoeia 11.0; European Directorate for the Quality of Medicine & Health Care of the Council of Europe (EDQM): Strasbourg, France, 2023. [Google Scholar]
- Arsenijević, J.; Marković, J.; Šoštarić, I.; Ražić, S. A chemometrics as a powerful tool in the elucidation of the role of metals in the biosynthesis of volatile organic compounds in Hungarian thyme samples. Plant Physiol. Biochem. 2013, 71, 298–306. [Google Scholar] [CrossRef]
- Ušjak, L.; Sofrenić, I.; Tešević, V.; Drobac, M.; Niketić, M.; Petrović, S. Fatty acids, sterols, and triterpenes of the fruits of 8 Heracleum taxa. Nat. Prod. Commun. 2019, 14, 1934578X19856788. [Google Scholar] [CrossRef]
- Tang, J.J.; Zhao, N.; Gao, Y.Q.; Han, R.; Wang, X.Y.; Tian, J.M.; Gao, J.M. Phytosterol profiles and iridoids of the edible Eucommia ulmoides Oliver seeds and their anti-inflammatory potential. Food Biosci. 2021, 43, 101295. [Google Scholar] [CrossRef]
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Ćirić, A.; Soković, M. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom: Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid–Modes of antimicrobial and antibiofilm activities of common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Ivanov, M.; Gašić, U.; Stojković, D.; Kostić, M.; Mišić, D.; Soković, M. New Evidence for Artemisia absinthium L. Application in Gastrointestinal Ailments: Ethnopharmacology, Antimicrobial Capacity, Cytotoxicity, and Phenolic Profile. Evid. Based Complement. Alternat. Med. 2021, 2021, 9961089. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).