Infective Endocarditis by Clostridioides and Clostridium Species—A Narrative Review
Abstract
1. Introduction
2. Methods
3. Results
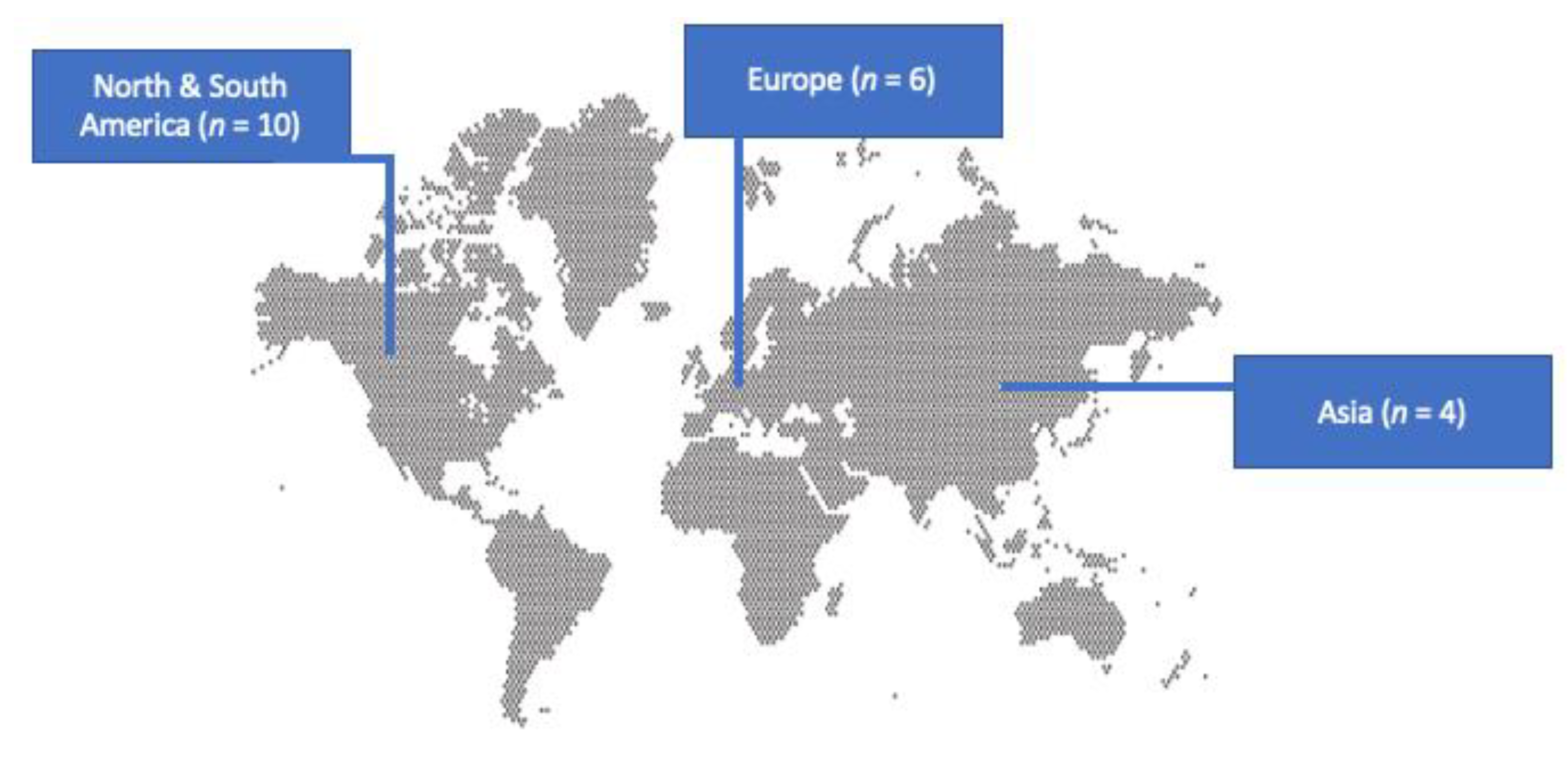
3.1. Included Studies’ Characteristics
3.2. Epidemiology of IE by Clostridioides and Clostridium Species
3.3. Microbiology and Diagnosis of IE by Clostridioides and Clostridium Species
3.4. Clinical Characteristics of IE by Clostridioides and Clostridium Species
3.5. Treatment and Outcomes of IE by Clostridioides and Clostridium Species
3.6. Comparison of Patients with IE by Clostridioides and Clostridium Species Who Survived with Those Who Died
3.7. Statistical Analysis of IE by Clostridioides and Clostridium Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Gaca, J.G.; Chu, V.H. Management Considerations in Infective Endocarditis: A Review. JAMA 2018, 320, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Cresti, A.; Chiavarelli, M.; Scalese, M.; Nencioni, C.; Valentini, S.; Guerrini, F.; D’Aiello, I.; Picchi, A.; De Sensi, F.; Habib, G. Epidemiological and Mortality Trends in Infective Endocarditis, a 17-Year Population-Based Prospective Study. Cardiovasc. Diagn. Ther. 2017, 7, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, P.E.; Samonis, G.; Andrianaki, A.M.; Christofaki, M.; Dimopoulou, D.; Papadakis, J.; Gikas, A.; Kofteridis, D.P. Epidemiology, Microbiological and Clinical Features, Treatment, and Outcomes of Infective Endocarditis in Crete, Greece. Infect. Chemother. 2018, 50, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Endocarditis Due to Anaerobic Bacteria. Cardiology 2002, 98, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Infective Endocarditis Caused by Anaerobic Bacteria. Arch. Cardiovasc. Dis. 2008, 101, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E.; Dolin, E.; Blaser, M.J. Mandell, Douglas, And Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar]
- Grenda, T.; Jarosz, A.; Sapała, M.; Grenda, A.; Patyra, E.; Kwiatek, K. Clostridium Perfringens-Opportunistic Foodborne Pathogen, Its Diversity and Epidemiological Significance. Pathogens 2023, 12, 768. [Google Scholar] [CrossRef]
- Leiblein, M.; Wagner, N.; Adam, E.H.; Frank, J.; Marzi, I.; Nau, C. Clostridial Gas Gangrene—A Rare but Deadly Infection: Case Series and Comparison to Other Necrotizing Soft Tissue Infections. Orthop. Surg. 2020, 12, 1733–1747. [Google Scholar] [CrossRef]
- Lawson, P.A.; Rainey, F.A. Proposal to Restrict the Genus Clostridium Prazmowski to Clostridium Butyricum and Related Species. Int. J. Syst. Evol. Microbiol. 2016, 66, 1009–1016. [Google Scholar] [CrossRef]
- The Lancet Infectious Diseases. C difficile—A Rose by Any Other Name… Lancet Infect. Dis. 2019, 19, 449. [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium Difficile Colitis: Pathogenesis and Host Defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Ziogou, A.; Ziogos, E.; Giannakodimos, I.; Giannakodimos, A.; Sifakis, S.; Ioannou, P.; Tsiodras, S. Bacterial Vaginosis and Post-Operative Pelvic Infections. Healthcare 2023, 11, 1218. [Google Scholar] [CrossRef]
- Parker, M.T. Postoperative Clostridial Infections in Britain. Br. Med. J. 1969, 3, 671–676. [Google Scholar] [CrossRef][Green Version]
- Brook, I. Clostridial Infections in Children: Spectrum and Management. Curr. Infect. Dis. Rep. 2015, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Kolander, S.A.; Cosgrove, E.M.; Molavi, A. Clostridial Endocarditis. Report of a Case Caused by Clostridium Bifermentans and Review of the Literature. Arch. Intern. Med. 1989, 149, 455–456. [Google Scholar] [CrossRef]
- Ridgway, E.J.; Grech, E.D. Clostridial Endocarditis: Report of a Case Caused by Clostridium Septicum and Review of the Literature. J. Infect. 1993, 26, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; DiBernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-ISCVID Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023, 77, ciad271. [Google Scholar] [CrossRef]
- More, R.H. Bacterial Endocarditis Due to Clostridium Welchii. Am. J. Pathol. 1943, 19, 413–421. [Google Scholar]
- Alvarez-Elcoro, S. Clostridium Perfringens Bacteremia in Prosthetic Valve Endocarditis: Diagnosis by Peripheral Blood Smear. Arch. Intern. Med. 1984, 144, 849. [Google Scholar] [CrossRef]
- Gordon, G.; Axelrod, J.L. Case Report: Prosthetic Valve Endocarditis Caused by Pseudallescheria Boydii and Clostridium Limosum. Mycopathologia 1985, 89, 129–134. [Google Scholar] [CrossRef]
- Barnes, P.; Leedom, J.M. Infective Endocarditis Due to Clostridium Sordellii. Am. J. Med. 1987, 83, 605. [Google Scholar] [CrossRef] [PubMed]
- Moyano, R.; Gomez-Mateos, J.M.; De Leon, F.L.; Florez, C.; Jimenez-Ocana, C.; Gamboa, F. Clostridium Bifermentans: An Exceptional Agent of Endocarditis. Clin. Infect. Dis. 1994, 18, 837. [Google Scholar] [CrossRef] [PubMed]
- Cutrona, A.F.; Watanakunakorn, C.; Schaub, C.R.; Jagetia, A. Clostridium Innocuum Endocarditis. Clin. Infect. Dis. 1995, 21, 1306–1307. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.M.; Oplustil, C.P.; Lemos Dos Santos, T.J.; Mady, C. Clostridium Perfringens as a Cause of Infectious Endocarditis in a Patient with a Vascular Prosthesis. Clin. Infect. Dis. 1996, 22, 866–867. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holland, F.; Fernandez, L.; Jacobs, J.; Bolooki, H. Clostridial Endocarditis Following Penetrating Cardiac Trauma. Clin. Infect. Dis. 1997, 24, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.A.; Almeder, L.M.; Israni, A.; Maslow, J.N. Clostridium septicum Endocarditis Complicated by Aortic-Ring Abscess and Aortitis. Clin. Infect. Dis. 1998, 26, 495–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fisker, N.; Søgaard, P. Early Clostridium Septicum Endocarditis and Myocarditis after Reconstruction of Congenital Heart Disease. Clin. Microbiol. Infect. 1998, 4, 533–535. [Google Scholar] [CrossRef]
- Koch, F.; Estrella, C. Features of Infectious Endocarditis by Clostridium sp. in the Elderly Population of a General Hospital. Anaerobe 1999, 5, 381–383. [Google Scholar] [CrossRef]
- Durmaz, B.; Agel, H.E.; Sönmez, E.; Turköz, R.; Aydin, E. Infective Endocarditis Due to Clostridium Histolyticum. Clin. Microbiol. Infect. 2000, 6, 561–563. [Google Scholar] [CrossRef]
- Chaudhry, R.; Venugopal, S.; Bahadur, T.; Verma, N.; Chaudhary, P.; Chowdhury, U.K. Prosthetic Valve Endocarditis Due to Clostridium Bifermentans: A Rare Entity. JMM Case Rep. 2014, 1, e001230. [Google Scholar] [CrossRef][Green Version]
- Yung, L.; Urban, C.; Niknam, N.; Turett, G. Prosthetic Valve Endocarditis Due to Clostridium limosum: A Case Report and Review of the Literature. Am. J. Infect. Dis. 2019, 15, 95–98. [Google Scholar] [CrossRef]
- Berkefeld, A.; Berger, F.K.; Gärtner, B.C.; Wantia, N.; Prinzing, A.; Laugwitz, K.-L.; Busch, D.H.; Rothe, K. Clostridioides (Clostridium) Difficile Pacemaker Infection. Open Forum Infect. Dis. 2020, 7, ofaa487. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Khullar, S.; Arif, N.; Sagar, T.; Meena, D.; Kumar Chowdhury, U. Clostridioides Difficile from Intracardiac Vegetation. Anaerobe 2021, 69, 102350. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Bahadur, T.; Sagar, T.; Agrawal, S.K.; Arif, N.; Choudhary, S.K.; Verma, N. Infective Endocarditis Caused by C. Sordellii: The First Case Report from India. J. Lab. Physicians 2021, 13, 074–076. [Google Scholar] [CrossRef] [PubMed]
- Dubois, D.; Dozier, A.; Schurtz, G.; Pontana, F.; Lemesle, G. Extended Emphysematous Aortitis of the Ascending Aorta: An Unusual Fatal Presentation of Aortic Valve Endocarditis Due to Clostridium Septicum. Cardiol. J. 2022, 29, 722–723. [Google Scholar] [CrossRef] [PubMed]
- Liesman, R.M.; Pritt, B.S.; Maleszewski, J.J.; Patel, R. Laboratory Diagnosis of Infective Endocarditis. J. Clin. Microbiol. 2017, 55, 2599–2608. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Giuliano, C.; Patel, C.R.; Kale-Pradhan, P.B. A Guide to Bacterial Culture Identification and Results Interpretation. Pharm. Ther. 2019, 44, 192–200. [Google Scholar]
- Strejcek, M.; Smrhova, T.; Junkova, P.; Uhlik, O. Whole-Cell MALDI-TOF MS Versus 16S rRNA Gene Analysis for Identification and Dereplication of Recurrent Bacterial Isolates. Front. Microbiol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Hong, H.-L.; Flurin, L.; Greenwood-Quaintance, K.E.; Wolf, M.J.; Pritt, B.S.; Norgan, A.P.; Patel, R. 16S rRNA Gene PCR/Sequencing of Heart Valves for Diagnosis of Infective Endocarditis in Routine Clinical Practice. J. Clin. Microbiol. 2023, 61, e0034123. [Google Scholar] [CrossRef]
- Yutin, N.; Galperin, M.Y. A Genomic Update on Clostridial Phylogeny: G Ram-negative Spore Formers and Other Misplaced Clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morales, P.; Orellana, C.A.; Moutafis, G.; Moonen, G.; Rincon, G.; Nielsen, L.K.; Marcellin, E. Revisiting the Evolution and Taxonomy of Clostridia, a Phylogenomic Update. Genome Biol. Evol. 2019, 11, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Prinzi, A.M.; Moore, N.M. Change of Plans: Overview of Bacterial Taxonomy, Recent Changes of Medical Importance, and Potential Areas of Impact. Open Forum Infect. Dis. 2023, ofad269. [Google Scholar] [CrossRef] [PubMed]
- Giannitsioti, E.; Skiadas, I.; Antoniadou, A.; Tsiodras, S.; Kanavos, K.; Triantafyllidi, H.; Giamarellou, H. Hellenic Endocarditis Study Group Nosocomial vs. Community-Acquired Infective Endocarditis in Greece: Changing Epidemiological Profile and Mortality Risk. Clin. Microbiol. Infect. 2007, 13, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, J.D.; Reller, L.B.; Wang, W.L. Susceptibility of Clostridium Perfringens Isolated from Human Infections to Twenty Antibiotics. Antimicrob. Agents Chemother. 1977, 11, 695–697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gabay, E.L.; Rolfe, R.D.; Finegold, S.M. Susceptibility of Clostridium Septicum to 23 Antimicrobial Agents. Antimicrob. Agents Chemother. 1981, 20, 852–853. [Google Scholar] [CrossRef] [PubMed]
- Slavić, D.; Boerlin, P.; Fabri, M.; Klotins, K.C.; Zoethout, J.K.; Weir, P.E.; Bateman, D. Antimicrobial Susceptibility of Clostridium Perfringens Isolates of Bovine, Chicken, Porcine, and Turkey Origin from Ontario. Can. J. Vet. Res. 2011, 75, 89–97. [Google Scholar]
- Tally, F.P.; Armfield, A.Y.; Dowell, V.R.; Kwok, Y.Y.; Sutter, V.L.; Finegold, S.M. Susceptibility of Clostridium Ramosum to Antimicrobial Agents. Antimicrob. Agents Chemother. 1974, 5, 589–593. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamakawa, K.; Nishida, S. Antibacterial Susceptibility of Clostridium Sordellii Strains. Zentralbl Bakteriol. Mikrobiol. Hyg. A 1986, 261, 345–349. [Google Scholar] [CrossRef]
- Sárvári, K.P.; Schoblocher, D. The Antibiotic Susceptibility Pattern of Gas Gangrene-Forming Clostridium Spp. Clinical Isolates from South-Eastern Hungary. Infect. Dis. 2020, 52, 196–201. [Google Scholar] [CrossRef]
- Dornbusch, K.; Nord, C.E.; Dahlbäck, A. Antibiotic Susceptibility of Clostridium Species Isolated from Human Infections. Scand. J. Infect. Dis. 1975, 7, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.I.; Yen, L.T.M.; Loan, H.T.; Diep, T.S.; Nga, T.T.T.; Van Minh Hoang, N.; Son, L.T.; van Vinh Chau, N.; Parry, C.; Farrar, J.J.; et al. Microbiologic Characterization and Antimicrobial Susceptibility of Clostridium Tetani Isolated from Wounds of Patients with Clinically Diagnosed Tetanus. Am. J. Trop. Med. Hyg. 2009, 80, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, A.N. Antimicrobial Resistance and Susceptibility Testing of Anaerobic Bacteria. Clin. Infect. Dis. 2014, 59, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Akhi, M.T.; Bidar Asl, S.; Pirzadeh, T.; Naghili, B.; Yeganeh, F.; Memar, Y.; Mohammadzadeh, Y. Antibiotic Sensitivity of Clostridium Perfringens Isolated From Faeces in Tabriz, Iran. Jundishapur J. Microbiol. 2015, 8, e20863. [Google Scholar] [CrossRef]
- Peng, Z.; Jin, D.; Kim, H.B.; Stratton, C.W.; Wu, B.; Tang, Y.-W.; Sun, X. Update on Antimicrobial Resistance in Clostridium Difficile: Resistance Mechanisms and Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 2017, 55, 1998–2008. [Google Scholar] [CrossRef]
| Characteristic | All Patients (n = 21) * | Survived (n = 14) | Died (n = 7) |
|---|---|---|---|
| Age, years, median (IQR) | 31 (21–60) | 27 (19.5–62.3) | 52 (23–59) |
| Male gender, n (%) | 14 (66.7) | 10 (71.4) | 4 (57.1) |
| Predisposing factors | |||
| Post cardiac surgery, n (%) | 5 (23.8) | 3 (21.4) | 2 (28.6) |
| Previously on antibiotics, n (%) | 5 (23.8) | 3 (21.4) | 2 (28.6) |
| Congenital heart disease, n (%) | 5 (23.8) | 4 (28.6) | 1 (14.3) |
| Prosthetic valve, n (%) | 5 (23.8) | 3 (21.4) | 2 (28.6) |
| Rheumatic fever, n (%) | 3 (14.3) | 1 (7.1) | 2 (28.6) |
| Previous IE, n (%) | 3 (14.3) | 1 (7.1) | 2 (28.6) |
| IVDU, n (%) | 2 (9.5) | 2 (14.3) | 0 (0) |
| Bad teeth hygiene or recent dental work, n (%) | 2 (9.5) | 2 (14.3) | 0 (0) |
| Method of diagnosis | |||
| Transthoracic echocardiography, n (%) | 11 (52.4) | 9 (64.3) | 2 (28.6) |
| Transesophageal echocardiography, n (%) | 5 (23.8) | 3 (21.4) | 2 (28.6) |
| Autopsy, n (%) | 3 (14.3) | 0 (0) | 3 (42.9) |
| Valve culture, n (%) | 8 (38.1) | 6 (42.9) | 2 (28.6) |
| Valve localization | |||
| Aortic valve, n (%) | 9 out of 20 (45) | 4 out of 13 (30.8) | 5 (71.4) |
| Mitral valve, n (%) | 7 out of 20 (35) | 5 out of 13 (38.5) | 2 (28.6) |
| Tricuspid valve, n (%) | 2 out of 20 (10) | 1 out of 13 (7.7) | 1 (14.3) |
| Pulmonary valve, n (%) | 2 out of 20 (10) | 1 out of 13 (7.7) | 1 (14.3) |
| Multiple valves, n (%) | 2 out of 20 (10) | 0 out of 13 (0) | 2 (28.6) |
| CIED, n (%) | 2 out of 20 (10) | 2 out of 13 (15.4) | 0 (0) |
| Other **, n (%) | 1 out of 20 (5) | 1 out of 13 (7.7) | 0 (0) |
| Clinical characteristics | |||
| Fever, n (%) | 14 (66.7) | 8 (57.1) | 6 (85.7) |
| Sepsis, n (%) | 5 (23.8) | 2 (14.3) | 3 (42.9) |
| Heart failure, n (%) | 4 (19) | 3 (21.4) | 1 (14.3) |
| Embolic phenomena, n (%) | 5 (23.8) | 2 (14.3) | 3 (42.9) |
| Shock, n (%) | 2 (9.5) | 1 (7.1) | 1 (14.3) |
| Treatment | |||
| Duration of treatment in weeks, median (IQR) | NA | 5.3 (3.3–6) | NA |
| Metronidazole, n (%) | 10 (47.6) | 7 (50) | 3 (42.9) |
| Penicillin, n (%) | 10 (47.6) | 7 (50) | 3 (42.9) |
| Aminopenicillin, n (%) | 4 (19) | 3 (21.4) | 1 (14.3) |
| Vancomycin, n (%) | 3 (14.3) | 3 (21.4) | 0 (0) |
| Cephalosporin, n (%) | 3 (14.3) | 2 (14.3) | 1 (14.3) |
| Carbapenem, n (%) | 2 (9.5) | 2 (14.3) | 0 (0) |
| Aminoglycoside, n (%) | 2 (9.5) | 1 (7.1) | 1 (14.3) |
| Linezolid, n (%) | 1 (4.8) | 1 (7.1) | 0 (0) |
| Quinolone, n (%) | 1 (4.8) | 1 (7.1) | 0 (0) |
| Clindamycin, n (%) | 1 (4.8) | 0 (0) | 1 (14.3) |
| Antipseudomonal penicillin, n (%) | 1 (4.8) | 0 (0) | 1 (14.3) |
| Surgical management, n (%) | 9 out of 20 (45) | 6 out of 13 (46.2) | 3 (42.9) |
| Outcomes | |||
| Deaths due to infection, n (%) | 7 (33.3) | NA | NA |
| Deaths overall, n (%) | 7 (33.3) | NA | NA |
| Study | Number of Patients | Age (Years) | Gender | Site of Infection n (%) | Microbiology of Infection, n (%) | Treatment Administered, n (%) | Infection Outcomes, n (%) |
|---|---|---|---|---|---|---|---|
| More et al., 1943 [19] | 1 | 34 | Female | AoV 1 (100) | C. perfrigens (welchii) 1 (100) | Sulphanilamide 1 (100) | Clinical cure a 0 (0) Deaths overall 1 (100) Deaths due to IE 1 (100) |
| Alvarez-Elcoro et al., 1984 [20] | 1 | 23 | Male | AoV 1 (100) MV 1 (100) | C. perfringens 1 (100) | Penicillin 1 (100) | Clinical cure 0 (0) Deaths overall 1 (100)Deaths due to IE 1 (100) |
| Gordon et al., 1985 [21] | 1 | 52 | Male | AoV 1 (100) | C. limosum 1 (100) | Penicillin 1 (100) Aminoglycoside 1 (100) Metronidazole 1 (100) Surgical management 1 (100) | Clinical cure 1 (100) Deaths overall 1 (100) Deaths due to IE 0 (0) |
| Barnes et al., 1987 [22] | 1 | 61 | Male | MV 1 (100) | C. sordelli 1 (100) | Penicillin 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Kolander et al., 1989 [16] | 1 | 23 | Male | MV 1 (100) | C. bifermentans 1 (100) | Penicillin 1 (100) Metronidazole 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Ridgway et al., 1993 [17] | 1 | 59 | Female | MV 1 (100) | C. septicum 1 (100) | Penicillin 1 (100) Aminopenicillin 1 (100) Metronidazole 1 (100) Surgical management 1 (100) | Clinical cure 0 (0) Deaths overall 1 (100) Deaths due to IE 1 (100) |
| Moyano et al., 1994 [23] | 1 | 26 | Male | TrV 1 (100) | C. bifermentans 1 (100) | Penicillin 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Cutrona et al., 1995 [24] | 1 | 18 | Female | PV 1 (100) TrV 1 (100) | C. innocuum 1 (100) | Clinical cure 0 (0) Deaths overall 1 (100) Deaths due to IE 1 (100) | |
| Mendes et al., 1996 [25] | 1 | 31 | Male | AoV and aortic wall at Dacron prosthesis 1 (100) | C. perfringens 1 (100) | Penicillin 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Holland et al., 1997 [26] | 1 | 20 | Male | MV 1 (100) | Clostridium spp. 1 (100) | Cephalosporin 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Cohen et al., 1998 [27] | 1 | 77 | Male | AoV 1 (100) | C. septicum 1 (100) | Cephalosporin 1 (100) Metronidazole 1 (100) | Clinical cure 0 (0) Deaths overall 1 (100) Deaths due to IE 1 (100) |
| Fisker et al., 1998 [28] | 1 | 6 | Male | PV 1 (100) | C. septicum 1 (100) | Penicillin 1 (100) Surgical management 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Koch et al., 1999 [29] | 2 | 77, 66 | 1 Female, 1 Male | CIED 1 (50) MV 1 (50) | C. perfringens 1 (50) C. symbosium 1 (50) | Penicillin 1 (50) Aminopenicillin 1 (50) Metronidazole 1 (50) | Clinical cure 2 (100) Deaths overall 0 (0) |
| Durmaz et al., 2000 [30] | 1 | 18 | Female | AoV 1 (100) | C. histolyticum 1 (100) | Penicillin 1 (100) Aminoglycoside 1 (100) Metronidazole 1 (100) Surgical management 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Chaudhry et al., 2014 [31] | 1 | 22 | Male | MV 1 (100) | C. bifermentans 1 (100) | Carbapenem 1 (100) Vancomycin 1 (100) Linezolid 1 (100) Quinolone 1 (100) Metronidazole 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Yung et al., 2019 [32] | 1 | 59 | Male | AoV 1 (100) | C. limosum 1 (100) | Aminopenicillin 1 (100) Cephalosporin 1 (100)Carbapenem 1 (100) Vancomycin 1 (100)Metronidazole 1 (100) Surgical management 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Berkefeld et al., 2020 [33] | 1 | 75 | Male | CIED 1 (100) | C. difficile 1 (100) | Aminopenicillin 1 (100) Vancomycin 1 (100) Metronidazole 1 (100) Surgical management 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Chaudhry et al., 2021 [34] | 1 | 11 | Female | NR 1 (100) | C. difficile 1 (100) | Metronidazole 1 (100) Surgical management 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Chaudhry et al., 2021 [35] | 1 | 28 | Female | AoV 1 (100) | C. sordelli 1 (100) | Metronidazole 1 (100) Surgical management 1 (100) | Clinical cure 1 (100) Deaths overall 0 (0) |
| Dubois et al., 2022 [36] | 1 | 58 | Male | AoV 1 (100) | C. septicum 1 (100) | Antipseudomonal penicillin 1 (100) Clindamycin 1 (100) Surgical management 1 (100) | Clinical cure 0 (0) Deaths overall 1 (100) Deaths due to IE 1 (100) |
| Characteristic | Univariate Analysis p-Value |
|---|---|
| Age | 0.4477 |
| Male gender | 0.5367 |
| Prosthetic cardiac valve | 0.7334 |
| Bad oral and teeth hygiene or recent dental work | 0.3171 |
| Previous episode of IE | 0.2044 |
| History of rheumatic heart disease | 0.2044 |
| IE in the aortic valve | 0.0893 |
| IE in the mitral valve | 0.6783 |
| IE in the pulmonary valve | 0.6601 |
| IE in the tricuspid valve | 0.6601 |
| IE in multiple valves | 0.0442 |
| Fever | 0.0293 |
| Sepsis | 0.1626 |
| Embolic phenomena | 0.1626 |
| Heart failure | 0.7117 |
| Surgical management | 0.8948 |
| Penicillin | 0.7715 |
| Ampicillin | 0.7117 |
| Metronidazole | 0.7715 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannou, P.; Kopidakis, I.; Makraki, E.; Baliou, S.; Samonis, G. Infective Endocarditis by Clostridioides and Clostridium Species—A Narrative Review. Antibiotics 2024, 13, 33. https://doi.org/10.3390/antibiotics13010033
Ioannou P, Kopidakis I, Makraki E, Baliou S, Samonis G. Infective Endocarditis by Clostridioides and Clostridium Species—A Narrative Review. Antibiotics. 2024; 13(1):33. https://doi.org/10.3390/antibiotics13010033
Chicago/Turabian StyleIoannou, Petros, Ioannis Kopidakis, Eirini Makraki, Stella Baliou, and George Samonis. 2024. "Infective Endocarditis by Clostridioides and Clostridium Species—A Narrative Review" Antibiotics 13, no. 1: 33. https://doi.org/10.3390/antibiotics13010033
APA StyleIoannou, P., Kopidakis, I., Makraki, E., Baliou, S., & Samonis, G. (2024). Infective Endocarditis by Clostridioides and Clostridium Species—A Narrative Review. Antibiotics, 13(1), 33. https://doi.org/10.3390/antibiotics13010033






