The Direction of the Antibacterial Effect of Rutin Hydrate and Amikacin
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
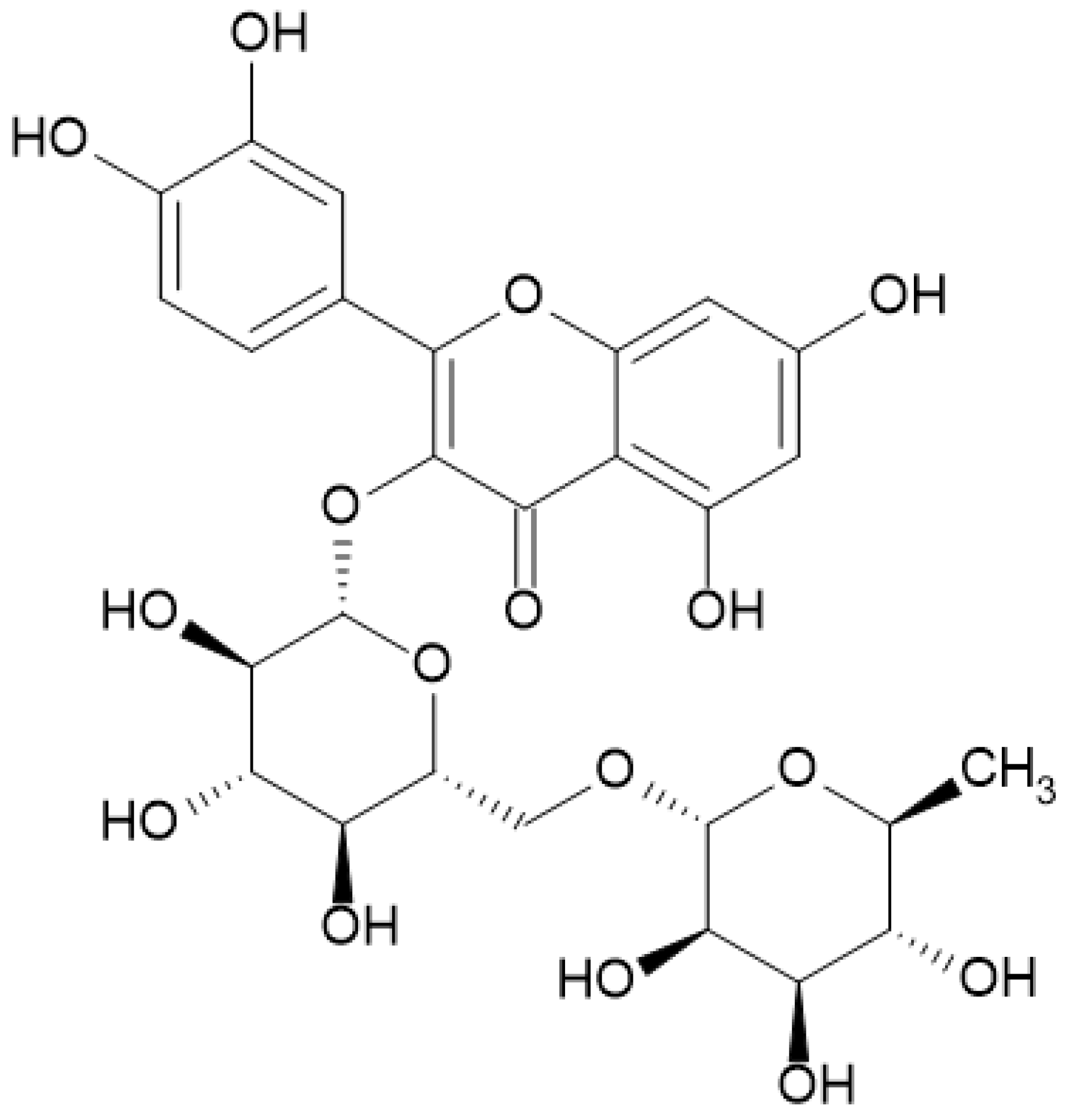
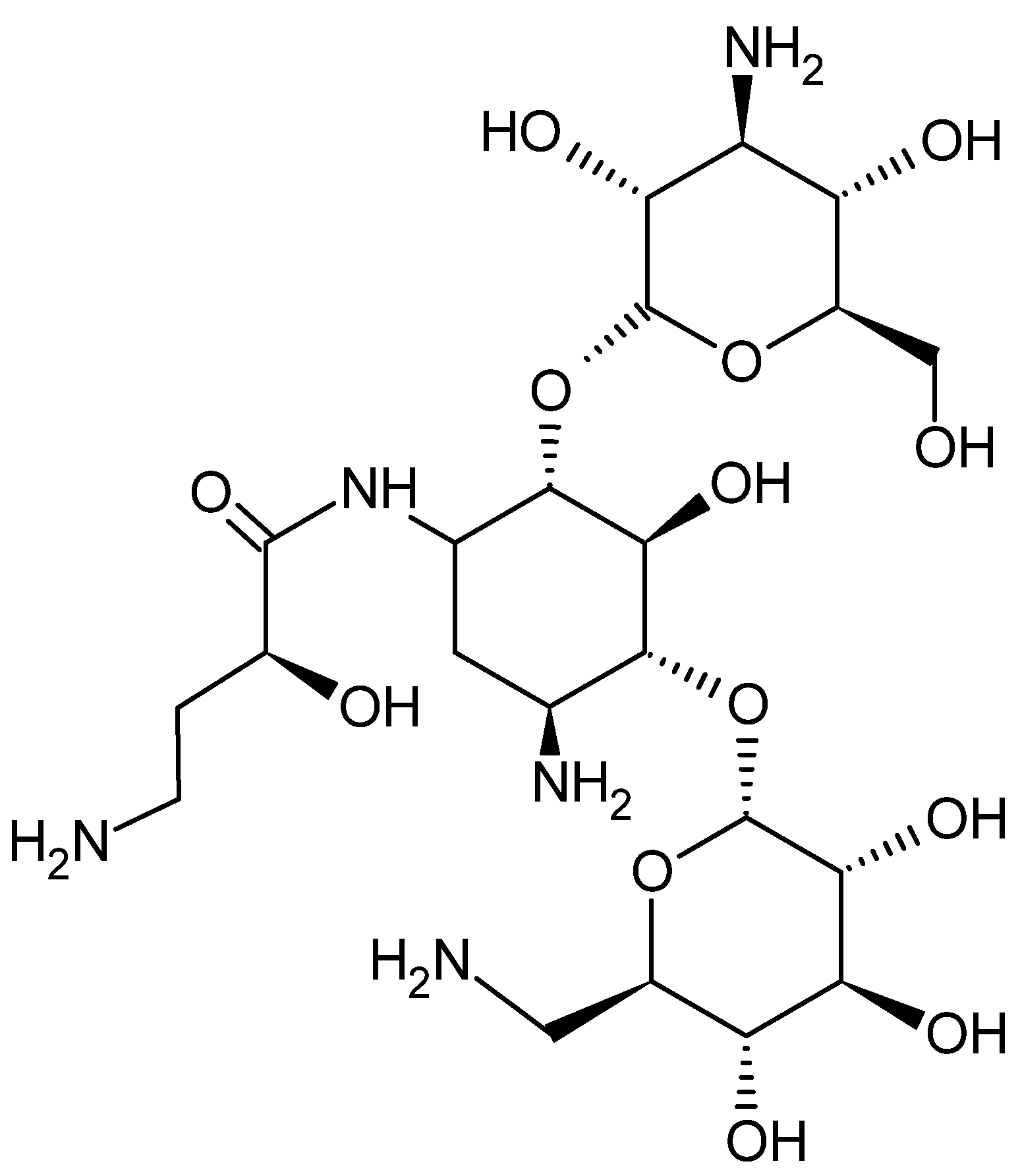
4.1. Materials
4.2. Determination of the MIC Values of the Tested Bacterial Strains
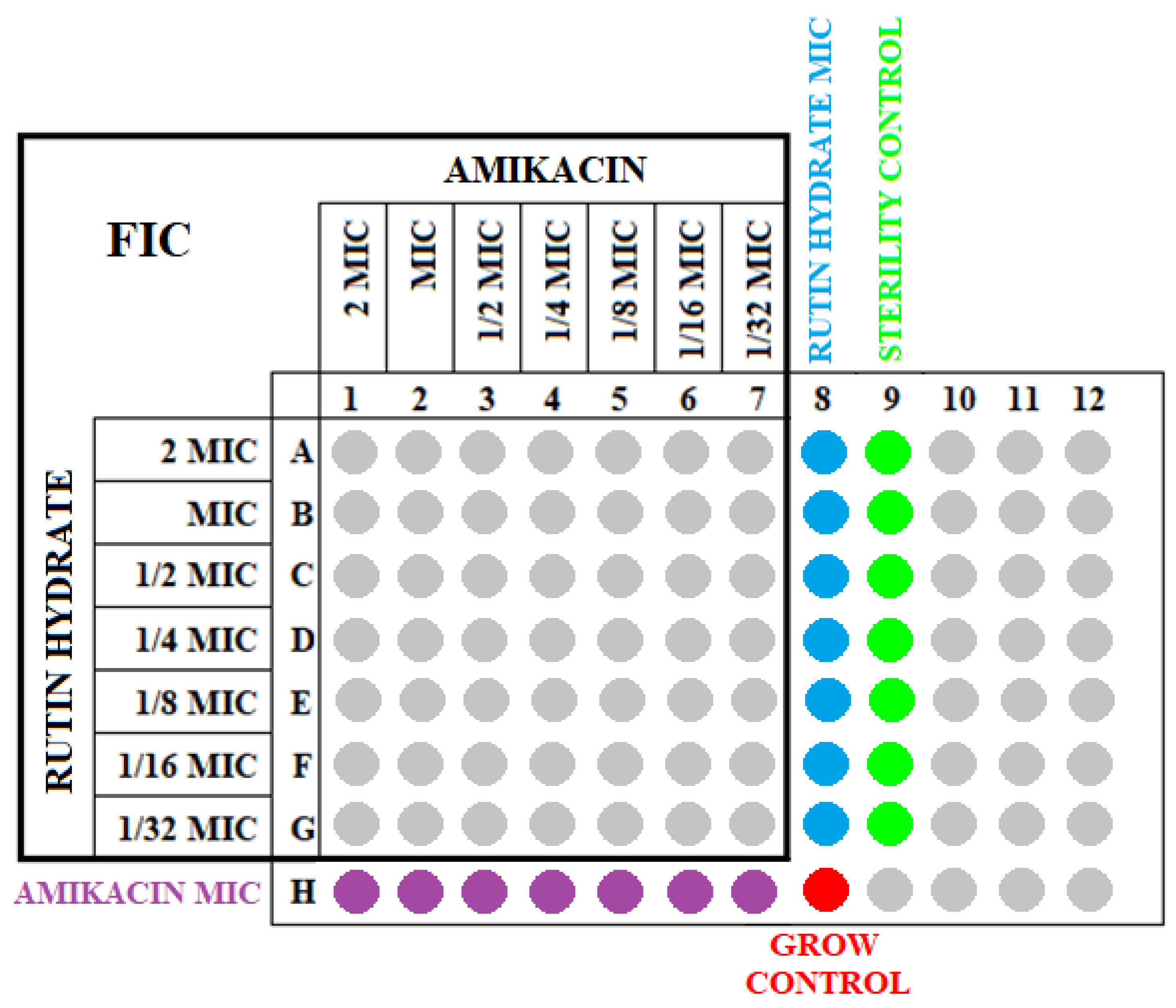
4.3. Determination of the FIC Values for the Tested Strains
- MICA + B—MIC of the antibiotic in the presence of a polyphenolic compound;
- MICB + A—MIC of a polyphenolic compound in the presence of an antibiotic;
- MICA—MIC of the antibiotic itself;
- MICB—MIC of the polyphenolic compound itself.
- FIC ≤ 0.5—synergism;
- 0.5 < FIC ≤ 1—additive action;
- 1 < FIC ≤ 4—indifference;
- FIC > 4—antagonism [35].
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.; Sharma, S. Role of Alternatives to Antibiotics in Mitigating the Antimicrobial Resistance Crisis. Indian J. Med. Res. 2022, 156, 464–477. [Google Scholar] [PubMed]
- Allen, H.K.; Trachsel, J.; Looft, T.; Casey, T.A. Finding Alternatives to Antibiotics. Ann. N. Y. Acad. Sci. 2014, 1323, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhao, L.; Hao, Z. Mechanism of Synergy Between Tetracycline and Quercetin Against Antibiotic Resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Bai, Y.; Qiu, Y.; Zhang, X.; Zeng, Z.; Chen, L.; Cheng, F.; Zhang, J. Mechanism of Synergy between Piceatannol and Ciprofloxacin against Staphylococcus Aureus. Int. J. Mol. Sci. 2022, 23, 15341. [Google Scholar] [CrossRef]
- Aonofriesei, F. Effect of Methylpyrazoles and Coumarin Association on the Growth of Gram-Negative Bacteria. Arch. Microbiol. 2022, 204, 160. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial Activity of Cinnamon Essential Oils and Their Synergistic Potential with Antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Demonstration of Bactericidal and Synergistic Activity of Quercetin with Meropenem among Pathogenic Carbapenem Resistant Escherichia Coli and Klebsiella Pneumoniae. Microb. Pathog. 2020, 143, 104120. [Google Scholar] [CrossRef]
- Samaszko-Fiertek, J.; Roguszczak, P.; Dmochowska, B.; Ślusarz, R.; Madaj, J. Rutin—Structure and Properities. Wiadomości Chem. 2016, 70, 439–441. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-433ed886-5b08-41bd-a785-41e097496418 (accessed on 23 August 2023).
- Rotimi, D.E.; Elebiyo, T.C.; Ojo, O.A. Therapeutic Potential of Rutin in Male Infertility: A Mini Review. J. Integr. Med. 2023, 21, 130–135. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Dizaj, S.M.; Saadat, Y.R.; Khezri, K.; Jafari, S.; Ahmadian, E.; Jahandizi, N.G.; Raeesi, S. Therapeutic Benefits of Rutin and Its Nanoformulations. Phyther. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Valdovinos, K.Y.; Salgado-Garciglia, R.; Vázquez-Sánchez, M.; Álvarez-Bernal, D.; Oregel-Zamudio, E.; Ceja-Torres, L.F.; Medina-Medrano, J.R. Quantitative Analysis of Rutin by HPTLC and In Vitro Antioxidant and Antibacterial Activities of Phenolic-Rich Extracts from Verbesina sphaerocephala. Plants 2021, 10, 475. [Google Scholar] [CrossRef]
- Jhanji, R.; Singh, A.; Kumar, A. Antibacterial Potential of Selected Phytomolecules: An Experimental Study. Microbiol. Immunol. 2021, 65, 325–332. [Google Scholar] [CrossRef]
- Jhanji, R.; Bhati, V.; Singh, A.; Kumar, A. Phytomolecules against Bacterial Biofilm and Efflux Pump: An In Silico and In Vitro Study. J. Biomol. Struct. Dyn. 2020, 38, 5500–5512. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Z.; Li, Z.; Ding, Y.; Jiang, F.; Liu, J. Antioxidant and Antibacterial Study of 10 Flavonoids Revealed Rutin as a Potential Antibiofilm Agent in Klebsiella Pneumoniae Strains Isolated from Hospitalized Patients. Microb. Pathog. 2021, 159, 105121. [Google Scholar] [CrossRef]
- Hryniewicz, W.; Żukowska, A. Rekomendacje Diagnostyki, Terapii i Profilaktyki Antybiotykowej Zakażeń w Szpitalu—2020: Materiał Przeznaczony dla Komitetów Terapeutycznych i Zespołów ds. Antybiotykoterapii Polskich Szpitali, Wydanie Drugie; Narodowy Instytut Leków: Warszawa, Poland, 2020. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Xia, X.; Song, X.; Li, Y.; Hou, W.; Lv, H.; Li, F.; Li, Y.; Liu, J.; Li, X. Antibacterial and Anti-Inflammatory ZIF-8@Rutin Nanocomposite as an Efficient Agent for Accelerating Infected Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 1026743. [Google Scholar] [CrossRef]
- Arima, H.; Ashida, H.; Danno, G. Rutin-Enhanced Antibacterial Activities of Flavonoids against Bacillus Cereus and Salmonella Enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Motallebi, M.; Khorsandi, K.; Sepahy, A.A.; Chamani, E.; Hosseinzadeh, R. Effect of Rutin as Flavonoid Compound on Photodynamic Inactivation against P aeruginosa and S. aureus. Photodiagnosis Photodyn. Ther. 2020, 32, 102074. [Google Scholar] [CrossRef] [PubMed]
- Obistioiu, D.; Cocan, I.; Tîrziu, E.; Herman, V.; Negrea, M.; Cucerzan, A.; Neacsu, A.-G.; Cozma, A.L.; Nichita, I.; Hulea, A.; et al. Phytochemical Profile and Microbiological Activity of Some Plants Belonging to the Fabaceae Family. Antibiotics 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic Additive and Synergistic Action of Rutin, Morin and Quercetin against Methicillin Resistant Staphylococcus Aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef]
- Xu, P.; Xu, X.B.; Khan, A.; Fotina, T.; Wang, S.H. Antibiofilm Activity against Staphylococcus Aureus and Content Analysis of Taraxacum Officinale Phenolic Extract. Pol. J. Vet. Sci. 2021, 24, 243–251. [Google Scholar] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic Compounds in Antimicrobial Therapy. J. Med. Food. 2017, 20, 1031–1038. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Amikacin: Uses, Resistance, and Prospects for Inhibition. Molecules 2017, 22, 2267. [Google Scholar] [CrossRef]
- Amsterdam, D. Susceptibility Testing of Antimicrobials in Liquid Media. In Antibiotics in Laboratory Medicine, 5th ed.; Loman, V., Ed.; Williams and Wilkins: Philadelphia, PA, USA, 2005; pp. 61–143. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Agar Dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar] [CrossRef]
- Cudic, M.; Condie, B.A.; Weiner, D.J.; Lysenko, E.S.; Xiang, Z.Q.; Insug, O.; Bulet, P.; Otvos, L. Development of Novel Antibacterial Peptides That Kill Resistant Isolates. Peptides 2002, 23, 2071–2083. [Google Scholar] [CrossRef]
- Devienne, K.F.; Raddi, M.S.G. Screening for Antimicrobial Activity of Natural Products Using a Microplate Photometer. Braz. J. Microbiol. 2002, 33, 166–168. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determinantion of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. dis 5.1. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- Chai, B.; Jiang, W.; Hu, M.; Wu, Y.; Si, H. In Vitro Synergistic Interactions of Protocatechuic Acid and Chlorogenic Acid in Combination with Antibiotics against Animal Pathogens. Synergy 2019, 9, 100055. [Google Scholar] [CrossRef]
- Mazur, P.; Skiba-Kurek, I.; Mrowiec, P.; Karczewska, E.; Drożdż, R. Synergistic ROS-Associated Antimicrobial Activity of Silver Nanoparticles and Gentamicin Against Staphylococcus Epidermidis. IJN 2020, 15, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
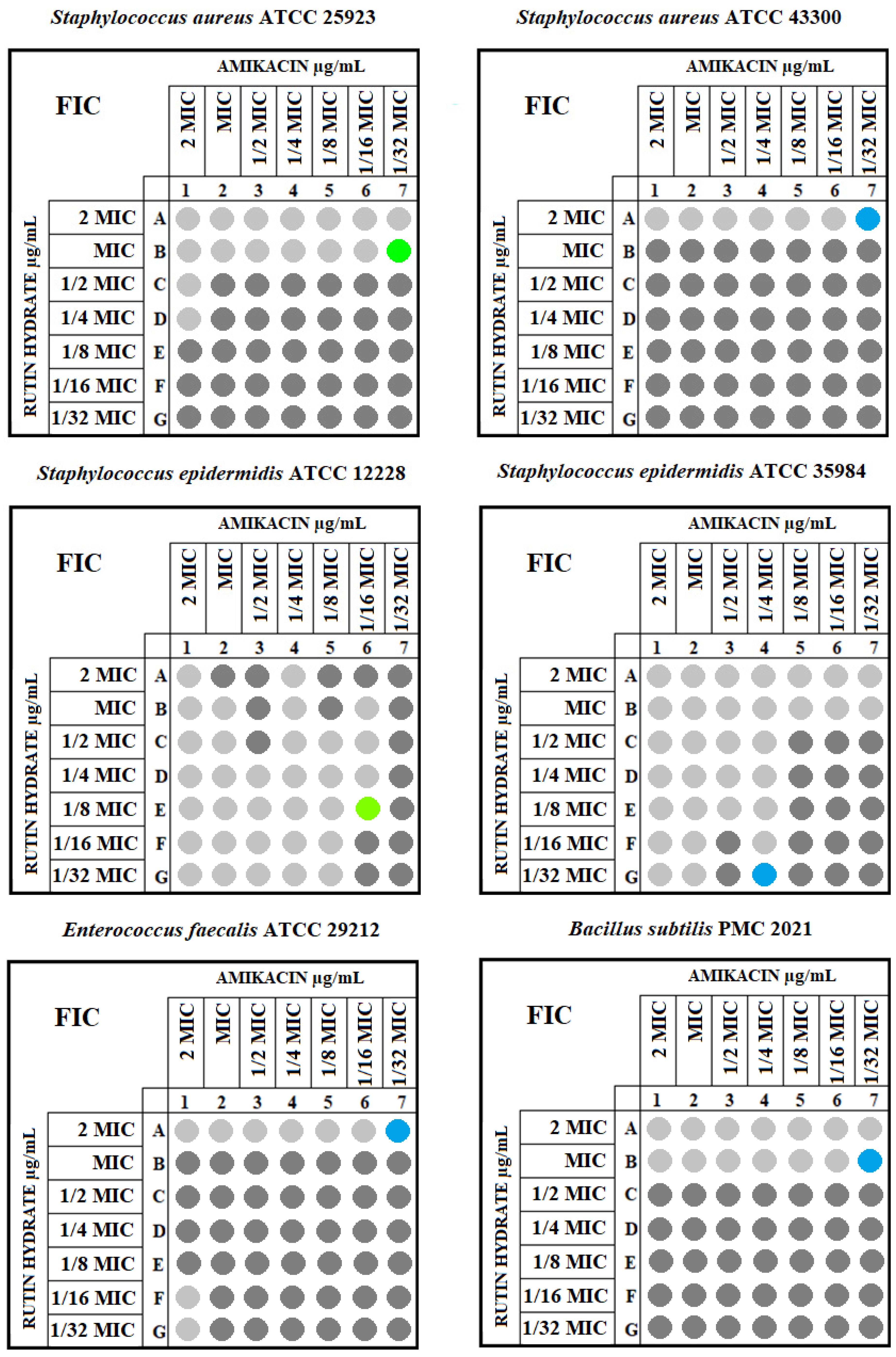
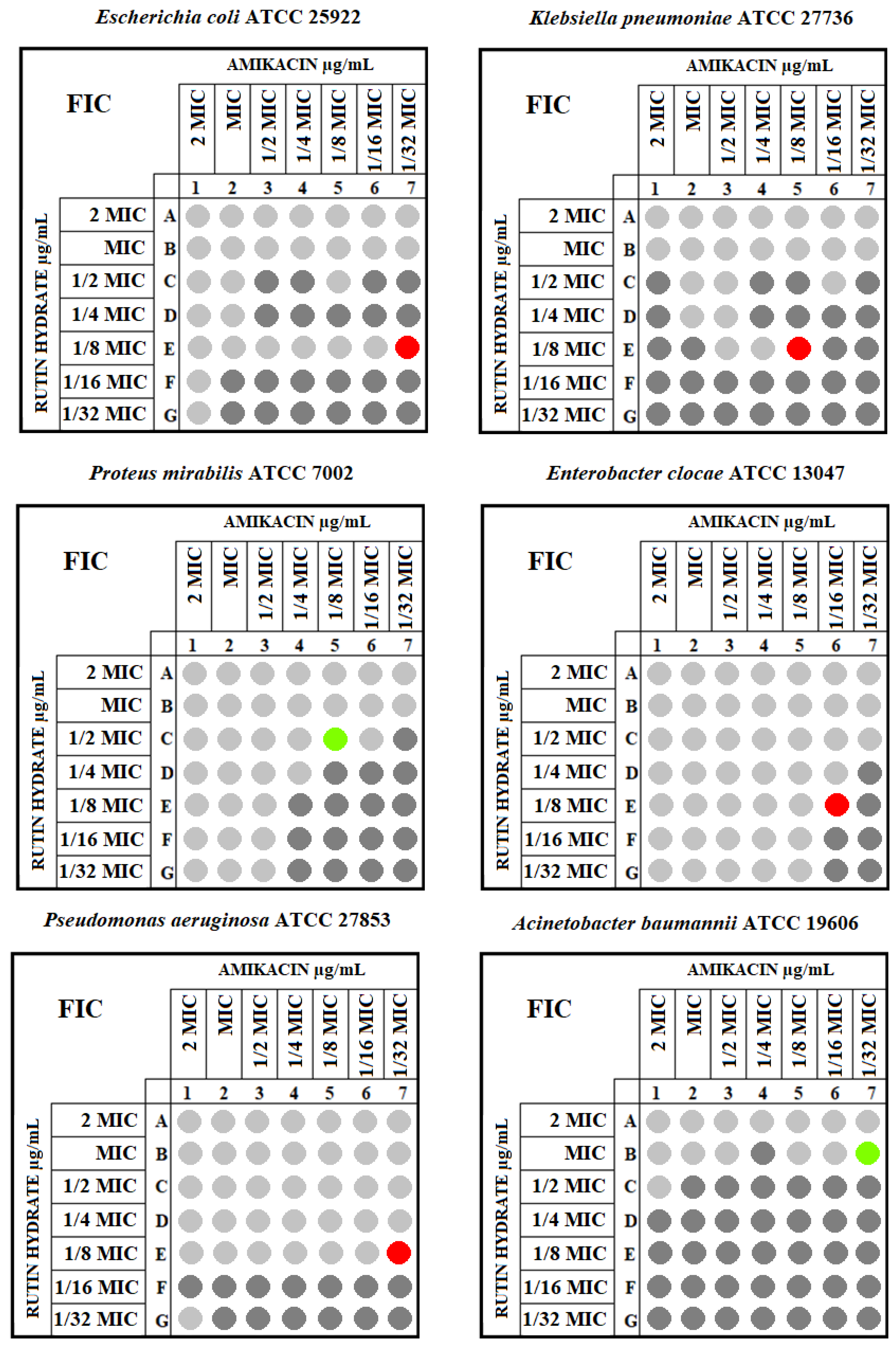
| Strain | Gram | MIC of Rutin Hydrate [μg/mL] | MIC of Amikacin [μg/mL] | MIC of Amikacin with Rutin Hydrate [μg/mL] | FIC Index | Interaction |
|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | Positive | 512 | 1 | 0.0625 | 0.563 | additive |
| Staphylococcus aureus ATCC 43300 | Positive | 512 | 64 | 1 | 1.016 | indifference |
| Staphylococcus epidermidis ATCC 12228 | Positive | 512 | 0.5 | 0.25 | 0.656 | additive |
| Staphylococcus epidermidis ATCC 35984 | Positive | 128 | 4 | 4 | 1.013 | indifference |
| Enterococcus faecalis ATCC 29212 | Positive | 256 | 4 | 0.125 | 1.031 | indifference |
| Bacillus subtilis PMC 2021 | Positive | 256 | 2 | 0.0625 | 2.031 | indifference |
| Escherichia coli ATCC 25922 | Negative | 512 | 8 | 0.125 | 0.078 | synergism |
| Klebsiella pneumoniae ATCC 27736 | Negative | 256 | 64 | 4 | 0.188 | synergism |
| Proteus mirabilis ATCC 7002 | Negative | 256 | 8 | 2 | 0.75 | additive |
| Enterobacter clocae ATCC 13047 | Negative | 128 | 2 | 0.5 | 0.5 | synergism |
| Pseudomonas aeruginosa ATCC 27853 | Negative | 1024 | 16 | 0.5 | 0.094 | synergism |
| Acinetobacter baumannii ATCC 19606 | Negative | 1024 | 64 | 2 | 0.532 | additive |
| MIC of Rutin Hydrate [μg/mL] | MIC of Amikacin [μg/mL] | MIC of Amikacin with Rutin Hydrate [μg/mL] | ||||
|---|---|---|---|---|---|---|
| Gram-Positive Strains | Gram-Negative Strains | Gram-Positive Strains | Gram-Negative Strains | Gram-Positive Strains | Gram-Negative Strains | |
| Median | 384 | 384 | 3 | 12 | 0.19 | 1.25 |
| Standard deviation | 170.13 | 400.02 | 25.23 | 29 | 0.38 | 1.46 |
| Lower quartile | 256 | 256 | 1 | 8 | 0.06 | 0.5 |
| Upper quartile | 512 | 1024 | 4 | 64 | 0.62 | 2 |
| Mann–Whitney U test | p = 0.676 | p = 0.105 | p = 0.226 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklasińska-Majdanik, M.; Kępa, M.; Wąsik, T.J.; Zapletal-Pudełko, K.; Klim, M.; Wojtyczka, R.D. The Direction of the Antibacterial Effect of Rutin Hydrate and Amikacin. Antibiotics 2023, 12, 1469. https://doi.org/10.3390/antibiotics12091469
Miklasińska-Majdanik M, Kępa M, Wąsik TJ, Zapletal-Pudełko K, Klim M, Wojtyczka RD. The Direction of the Antibacterial Effect of Rutin Hydrate and Amikacin. Antibiotics. 2023; 12(9):1469. https://doi.org/10.3390/antibiotics12091469
Chicago/Turabian StyleMiklasińska-Majdanik, Maria, Małgorzata Kępa, Tomasz J. Wąsik, Karolina Zapletal-Pudełko, Magdalena Klim, and Robert D. Wojtyczka. 2023. "The Direction of the Antibacterial Effect of Rutin Hydrate and Amikacin" Antibiotics 12, no. 9: 1469. https://doi.org/10.3390/antibiotics12091469
APA StyleMiklasińska-Majdanik, M., Kępa, M., Wąsik, T. J., Zapletal-Pudełko, K., Klim, M., & Wojtyczka, R. D. (2023). The Direction of the Antibacterial Effect of Rutin Hydrate and Amikacin. Antibiotics, 12(9), 1469. https://doi.org/10.3390/antibiotics12091469










