The Significance of Bayesian Pharmacokinetics in Dosing for Critically Ill Patients: A Primer for Clinicians Using Vancomycin as an Example
Abstract
1. Introduction
2. Altered PKs in Critical Illness
2.1. Changes in Volume of Distribution (Vd)
2.2. Changes in Drug Elimination
3. Antibiotic Dosing Approaches and Variability
3.1. Fixed Dosing
3.2. Covariate-Based Dosing
3.3. Dosing Based on Therapeutic Drug Monitoring (TDM)
3.4. Nomogram-Based Dosing
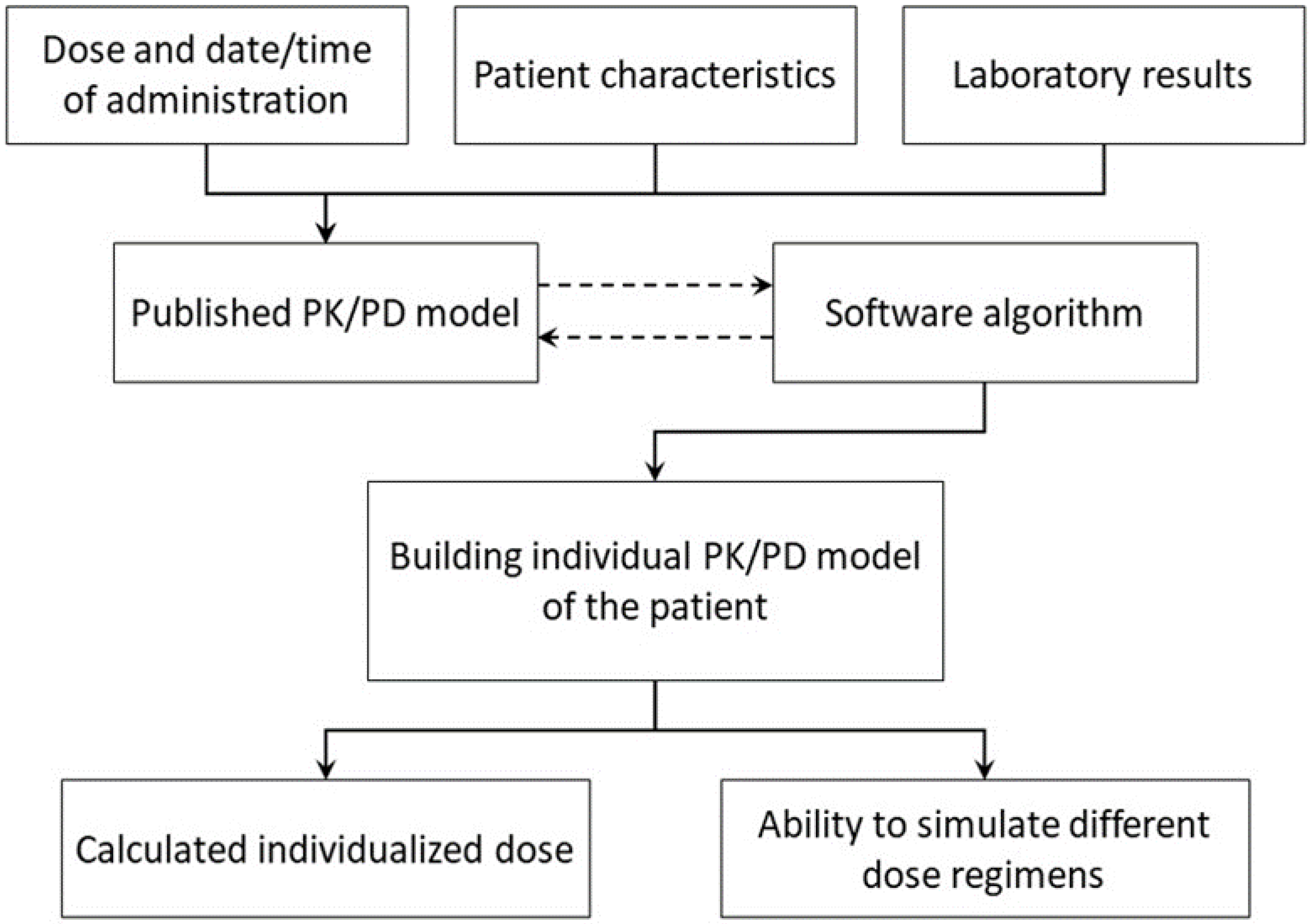
3.5. Bayesian Dosing
4. Application of the Bayesian method in Vancomycin Dosing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thabit, A.K.; Crandon, J.L.; Nicolau, D.P. Antimicrobial resistance: Impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin. Pharmacother. 2015, 16, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- Pakyz, A.L.; MacDougall, C.; Oinonen, M.; Polk, R.E. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch. Intern. Med. 2008, 168, 2254–2260. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Chahine, E.B.; Dougherty, J.A.; Thornby, K.-A.; Guirguis, E.H. Antibiotic approvals in the last decade: Are we keeping up with resistance? Ann. Pharmacother. 2022, 56, 441–462. [Google Scholar] [CrossRef]
- Carlier, M.; Carrette, S.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Does consistent piperacillin dosing result in consistent therapeutic concentrations in critically ill patients? A longitudinal study over an entire antibiotic course. Int. J. Antimicrob. Agents 2014, 43, 470–473. [Google Scholar] [CrossRef]
- Sime, F.B.; Roberts, M.S.; Peake, S.L.; Lipman, J.; Roberts, J.A. Does beta-lactam pharmacokinetic variability in critically ill patients justify therapeutic drug monitoring? A systematic review. Ann. Intensive Care 2012, 2, 35. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health-Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [PubMed]
- Theuretzbacher, U. Pharmacokinetic and pharmacodynamic issues for antimicrobial therapy in patients with cancer. Clin. Infect. Dis. 2012, 54, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, B.; Jullien, V.; Vinsonneau, C.; Tod, M. Influence of burns on pharmacokinetics and pharmacodynamics of drugs used in the care of burn patients. Clin. Pharmacokinet. 2008, 47, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Rey, E.; Tréluyer, J.-M.; Pons, G. Drug disposition in cystic fibrosis. Clin. Pharmacokinet. 1998, 35, 313–329. [Google Scholar] [CrossRef]
- Nyström, P.-O. The systemic inflammatory response syndrome: Definitions and aetiology. J. Antimicrob. Chemother. 1998, 41 (Suppl. A), 1–7. [Google Scholar] [CrossRef]
- Gosling, P.; Sanghera, K.; Dickson, G. Generalized vascular permeability and pulmonary function in patients following serious trauma. J. Trauma 1994, 36, 477–481. [Google Scholar] [CrossRef]
- Jamal, J.-A.; Economou, C.J.; Lipman, J.; Roberts, J.A. Improving antibiotic dosing in special situations in the ICU: Burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr. Opin. Crit. Care 2012, 18, 460–471. [Google Scholar] [CrossRef]
- McKindley, D.S.; Fabian, T.C.; Boucher, B.A.; Croce, M.A.; Proctor, K.G. Antibiotic pharmacokinetics following fluid resuscitation from traumatic shock. Arch. Surg. 1995, 130, 1321–1329. [Google Scholar] [CrossRef]
- Macedo, E.; Bouchard, J.; Soroko, S.H.; Chertow, G.M.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Mehta, R.L. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit. Care 2010, 14, R82. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Implications of augmented renal clearance in critically ill patients. Nat. Rev. Nephrol. 2011, 7, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Yiew, X.T.; Bateman, S.W.; Hahn, R.G.; Bersenas, A.M.; Muir, W.W. Understanding volume kinetics: The role of pharmacokinetic modeling and analysis in fluid therapy. Front. Vet. Sci. 2020, 7, 587106. [Google Scholar] [CrossRef]
- Joynt, G.; Lipman, J.; Gomersall, C.; Young, R.; Wong, E.; Gin, T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 2001, 47, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, A.; Ortega, A.; Idoate, A.; Giráldez, J.; Brugarolas, A. Effects of hepatic function on vancomycin pharmacokinetics in patients with cancer. Ther. Drug Monit. 2000, 22, 250–257. [Google Scholar] [CrossRef]
- Etzel, J.; Nafziger, A.; Bertino, J. Variation in the pharmacokinetics of gentamicin and tobramycin in patients with pleural effusions and hypoalbuminemia. Antimicrob. Agents Chemother. 1992, 36, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Mangin, O.; Urien, S.; Mainardi, J.-L.; Fagon, J.-Y.; Faisy, C. Vancomycin pharmacokinetic and pharmacodynamic models for critically ill patients with post-sternotomy mediastinitis. Clin. Pharmacokinet. 2014, 53, 849–861. [Google Scholar] [CrossRef]
- Dresser, L.; Granton, J.; Fan, E. Pharmacokinetic Alterations Associated with Critical Illness. Clin. Pharmacokinet. 2023, 62, 209–220. [Google Scholar]
- Dakdouki, G.K.; Al-Awar, G.N. Cefepime-induced encephalopathy. Int. J. Infect. Dis. 2004, 8, 59–61. [Google Scholar] [CrossRef]
- Abanades, S.; Nolla, J.; Rodríguez-Campello, A.; Pedro, C.; Valls, A.; Farré, M. Reversible coma secondary to cefepime neurotoxicity. Ann. Pharmacother. 2004, 38, 606–608. [Google Scholar] [CrossRef]
- Bresson, J.; Paugam-Burtz, C.; Josserand, J.; Bardin, C.; Mantz, J.; Pease, S. Cefepime overdosage with neurotoxicity recovered by high-volume haemofiltration. J. Antimicrob. Chemother. 2008, 62, 849–850. [Google Scholar] [CrossRef]
- Jones, E.; McMullin, C.; Hedges, A.; Lovering, A.; White, L.; Reeves, D.; MacGowan, A. The pharmacokinetics of intravenous ciprofloxacin 400 mg 12 hourly in patients with severe sepsis: The effect of renal function and intra-abdominal disease. J. Antimicrob. Chemother. 1997, 40, 121–124. [Google Scholar] [CrossRef][Green Version]
- van Zanten, A.R.; Polderman, K.H.; van Geijlswijk, I.M.; van der Meer, G.Y.; Schouten, M.A.; Girbes, A.R. Ciprofloxacin pharmacokinetics in critically ill patients: A prospective cohort study. J. Crit. Care 2008, 23, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Rohwedder, R.W.; Bergan, T.; Thorsteinsson, S.B.; Scholl, H. Transintestinal elimination of ciprofloxacin. Diagn. Microbiol. Infect. Dis. 1990, 13, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, H.; Wolters, J. The metabolic disposition of flucloxacillin in patients with impaired kidney function. Eur. J. Clin. Pharmacol. 1982, 22, 429–434. [Google Scholar] [CrossRef]
- Eyler, R.F.; Mueller, B.A. Antibiotic dosing in critically ill patients with acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 226–235. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Antibacterial dosing in intensive care. Clin. Pharmacokinet. 2006, 45, 755–773. [Google Scholar] [CrossRef]
- Roberts, D.M.; Roberts, J.A.; Roberts, M.S.; Liu, X.; Nair, P.; Cole, L.; Lipman, J.; Bellomo, R. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: A multicentre pharmacokinetic study. Crit. Care Med. 2012, 40, 1523–1528. [Google Scholar] [CrossRef]
- Bergner, R.; Hoffmann, M.; Riedel, K.-D.; Mikus, G.; Henrich, D.M.; Haefeli, W.E.; Uppenkamp, M.; Walter-Sack, I. Fluconazole dosing in continuous veno-venous haemofiltration (CVVHF): Need for a high daily dose of 800 mg. Nephrol. Dial. Transplant. 2006, 21, 1019–1023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic initial β-lactam concentrations in select critically ill patients. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Shorr, A.F.; Boots, R.J.; Lipman, J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: Identifying at-risk patients. Crit. Care 2013, 17, R35. [Google Scholar] [CrossRef]
- Udy, A.A.; Jarrett, P.; Stuart, J.; Lassig-Smith, M.; Starr, T.; Dunlop, R.; Wallis, S.C.; Roberts, J.A.; Lipman, J. Determining the mechanisms underlying augmented renal drug clearance in the critically ill: Use of exogenous marker compounds. Crit. Care 2014, 18, 657. [Google Scholar] [CrossRef]
- Claus, B.O.; Hoste, E.A.; Colpaert, K.; Robays, H.; Decruyenaere, J.; De Waele, J.J. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J. Crit. Care 2013, 28, 695–700. [Google Scholar] [CrossRef]
- Crass, R.L.; Rodvold, K.A.; Mueller, B.A.; Pai, M.P. Renal dosing of antibiotics: Are we jumping the gun? Clin. Infect. Dis. 2019, 68, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Nejat, M.; Pickering, J.W.; Walker, R.J.; Westhuyzen, J.; Shaw, G.M.; Frampton, C.M.; Endre, Z.H. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit. Care 2010, 14, R85. [Google Scholar] [CrossRef] [PubMed]
- Leem, A.Y.; Park, M.S.; Park, B.H.; Jung, W.J.; Chung, K.S.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Kim, Y.S. Value of serum cystatin C measurement in the diagnosis of sepsis-induced kidney injury and prediction of renal function recovery. Yonsei Med. J. 2017, 58, 604–612. [Google Scholar] [CrossRef]
- Linné, E.; Elfström, A.; Åkesson, A.; Fisher, J.; Grubb, A.; Pettilä, V.; Vaara, S.T.; Linder, A.; Bentzer, P. Cystatin C and derived measures of renal function as risk factors for mortality and acute kidney injury in sepsis—A post-hoc analysis of the FINNAKI cohort. J. Crit. Care 2022, 72, 154148. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, G.; Sakamoto, T.; Kimura, M.; Ukishima, Y.; Sonoda, A.; Mori, N.; Kato, Y.; Maeda, T.; Kagawa, Y. Serum cystatin C as a better marker of vancomycin clearance than serum creatinine in elderly patients. Clin. Biochem. 2007, 40, 485–490. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Ni, H.; Jin, N. Serum cystatin C is associated with renal function recovery in critically ill patients undergoing continuous renal replacement therapy. Nephron Clin. Pract. 2013, 122, 86–92. [Google Scholar] [CrossRef]
- Tahir, N.A.M.; Saffian, S.M.; Islahudin, F.H.; Gafor, A.H.A.; Makmor-Bakry, M. A meta-analysis on the performance of cystatin C-versus creatinine-based eGFR equations in predicting vancomycin clearance. J. Korean Med. Sci. 2020, 35, e306. [Google Scholar] [CrossRef]
- Thomson, A.; Staatz, C.; Tobin, C.; Gall, M.; Lovering, A. Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J. Antimicrob. Chemother. 2009, 63, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Donagher, J.; Martin, J.H.; Barras, M.A. Individualised medicine: Why we need Bayesian dosing. Intern. Med. J. 2017, 47, 593–600. [Google Scholar] [CrossRef]
- Beumer, J.H.; Chu, E.; Allegra, C.; Tanigawara, Y.; Milano, G.; Diasio, R.; Kim, T.W.; Mathijssen, R.H.; Zhang, L.; Arnold, D. Therapeutic drug monitoring in oncology: IATDMCT recommendations for 5-fluorouracil therapy. Clin. Pharmacol. Ther. 2019, 105, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Smythe, M.A.; Priziola, J.; Dobesh, P.P.; Wirth, D.; Cuker, A.; Wittkowsky, A.K. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 165–186. [Google Scholar] [CrossRef] [PubMed]
- van Lent-Evers, N.A.; Mathôt, R.A.; Geus, W.P.; van Hout, B.A.; Vinks, A.A. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: A cost-effectiveness analysis. Ther. Drug Monit. 1999, 21, 63–73. [Google Scholar] [CrossRef]
- Roberts, J.A.; Norris, R.; Paterson, D.L.; Martin, J.H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 2012, 73, 27–36. [Google Scholar] [CrossRef]
- Kang, J.-S.; Lee, M.-H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Avent, M.; Rogers, B.; Cheng, A.; Paterson, D. Current use of aminoglycosides: Indications, pharmacokinetics and monitoring for toxicity. Intern. Med. J. 2011, 41, 441–449. [Google Scholar] [CrossRef]
- Burton, M.E. Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Avent, M.; Rogers, B. Optimising antimicrobial therapy through the use of Bayesian dosing programs. Int. J. Clin. Pharm. 2019, 41, 1121–1130. [Google Scholar] [CrossRef]
- Nicolau, D.P.; Freeman, C.D.; Belliveau, P.P.; Nightingale, C.H.; Ross, J.W.; Quintiliani, R. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob. Agents Chemother. 1995, 39, 650–655. [Google Scholar] [CrossRef]
- Mohan, M.; Batty, K.T.; Cooper, J.A.; Wojnar-Horton, R.E.; Ilett, K.F. Comparison of gentamicin dose estimates derived from manual calculations, the Australian ‘Therapeutic Guidelines: Antibiotic’nomogram and the SeBA-GEN and DoseCalc software programs. Br. J. Clin. Pharmacol. 2004, 58, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Robson, J.M.; Wagener, M.M.; Peters, M. Monitoring of serum aminoglycoside levels with once-daily dosing. Pathology 1998, 30, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.; Philippe, M.; Rushing, T.; Fu, X.; van Guilder, M.; Bayard, D.; Schumitzky, A.; Bleyzac, N.; Goutelle, S. Accurately achieving target busulfan exposure in children and adolescents with very limited sampling and the BestDose software. Ther. Drug Monit. 2016, 38, 332–342. [Google Scholar] [CrossRef]
- Duffull, S.; Kirkpatrick, C.; Begg, E. Comparison of two Bayesian approaches to dose-individualization for once-daily aminoglycoside regimens. Br. J. Clin. Pharmacol. 1997, 43, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Neely, M.; Rodvold, K.A.; Lodise, T.P. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv. Drug Deliv. Rev. 2014, 77, 50–57. [Google Scholar] [CrossRef]
- Neely, M.N.; Kato, L.; Youn, G.; Kraler, L.; Bayard, D.; van Guilder, M.; Schumitzky, A.; Yamada, W.; Jones, B.; Minejima, E. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob. Agents Chemother. 2018, 62, e02042-17. [Google Scholar] [CrossRef]
- Heil, E.L.; Nicolau, D.P.; Farkas, A.; Roberts, J.A.; Thom, K.A. Pharmacodynamic target attainment for cefepime, meropenem, and piperacillin-tazobactam using a pharmacokinetic/pharmacodynamic-based dosing calculator in critically ill patients. Antimicrob. Agents Chemother. 2018, 62, e01008–e01018. [Google Scholar] [CrossRef]
- Matthews, I.; Kirkpatrick, C.; Holford, N. Quantitative justification for target concentration intervention–parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br. J. Clin. Pharmacol. 2004, 58, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.; Jelliffe, R. Practical therapeutic drug management in HIV-infected patients: Use of population pharmacokinetic models supplemented by individualized Bayesian dose optimization. J. Clin. Pharmacol. 2008, 48, 1081–1091. [Google Scholar] [CrossRef]
- Burgard, M.; Sandaradura, I.; van Hal, S.J.; Stacey, S.; Hennig, S. Evaluation of tobramycin exposure predictions in three Bayesian forecasting programmes compared with current clinical practice in children and adults with cystic fibrosis. Clin. Pharmacokinet. 2018, 57, 1017–1027. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotscahfer, J.C.; Moellering, R.C., Jr.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Aljutayli, A.; Thirion, D.J.G.; Bonnefois, G.; Nekka, F. Pharmacokinetic equations versus Bayesian guided vancomycin monitoring: Pharmacokinetic model and model-informed precision dosing trial simulations. Clin. Transl. Sci. 2022, 15, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Alsowaida, Y.S.; Kubiak, D.W.; Dionne, B.; Kovacevic, M.P.; Pearson, J.C. Vancomycin Area under the Concentration-Time Curve Estimation Using Bayesian Modeling versus First-Order Pharmacokinetic Equations: A Quasi-Experimental Study. Antibiotics 2022, 11, 1239. [Google Scholar] [CrossRef]
- Wrishko, R.E.; Levine, M.; Khoo, D.; Abbott, P.; Hamilton, D. Vancomycin Pharmacokinetics and Bayesian Estimation in Pediatric Patients. Ther. Drug Monit. 2000, 22, 522–531. [Google Scholar] [CrossRef]
- Wysocki, M.; Delatour, F.; Faurisson, F.o.; Rauss, A.; Pean, Y.; Misset, B.; Thomas, F.; Timsit, J.-F.o.; Similowski, T.; Mentec, H. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: Prospective multicenter randomized study. Antimicrob. Agents Chemother. 2001, 45, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Berthaud, R.; Benaboud, S.; Hirt, D.; Genuini, M.; Oualha, M.; Castelle, M.; Briand, C.; Artru, S.; Norsa, L.; Boyer, O. Early Bayesian dose adjustment of vancomycin continuous infusion in children in a randomized controlled trial. Antimicrob. Agents Chemother. 2019, 63, e01102–e01119. [Google Scholar] [CrossRef] [PubMed]
| Dosing Method | Advantages | Disadvantages |
|---|---|---|
| Fixed dosing |
|
|
| Covariate dosing |
|
|
| Therapeutic drug monitoring |
|
|
| Nomogram |
|
|
| Bayesian dosing |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnezary, F.S.; Almutairi, M.S.; Gonzales-Luna, A.J.; Thabit, A.K. The Significance of Bayesian Pharmacokinetics in Dosing for Critically Ill Patients: A Primer for Clinicians Using Vancomycin as an Example. Antibiotics 2023, 12, 1441. https://doi.org/10.3390/antibiotics12091441
Alnezary FS, Almutairi MS, Gonzales-Luna AJ, Thabit AK. The Significance of Bayesian Pharmacokinetics in Dosing for Critically Ill Patients: A Primer for Clinicians Using Vancomycin as an Example. Antibiotics. 2023; 12(9):1441. https://doi.org/10.3390/antibiotics12091441
Chicago/Turabian StyleAlnezary, Faris S., Masaad Saeed Almutairi, Anne J. Gonzales-Luna, and Abrar K. Thabit. 2023. "The Significance of Bayesian Pharmacokinetics in Dosing for Critically Ill Patients: A Primer for Clinicians Using Vancomycin as an Example" Antibiotics 12, no. 9: 1441. https://doi.org/10.3390/antibiotics12091441
APA StyleAlnezary, F. S., Almutairi, M. S., Gonzales-Luna, A. J., & Thabit, A. K. (2023). The Significance of Bayesian Pharmacokinetics in Dosing for Critically Ill Patients: A Primer for Clinicians Using Vancomycin as an Example. Antibiotics, 12(9), 1441. https://doi.org/10.3390/antibiotics12091441






