Risk Factors and Pathogens of Wound Infection in Burn Inpatients from East China
Abstract
:1. Introduction
2. Results
2.1. Patient Demographics and Clinical Details
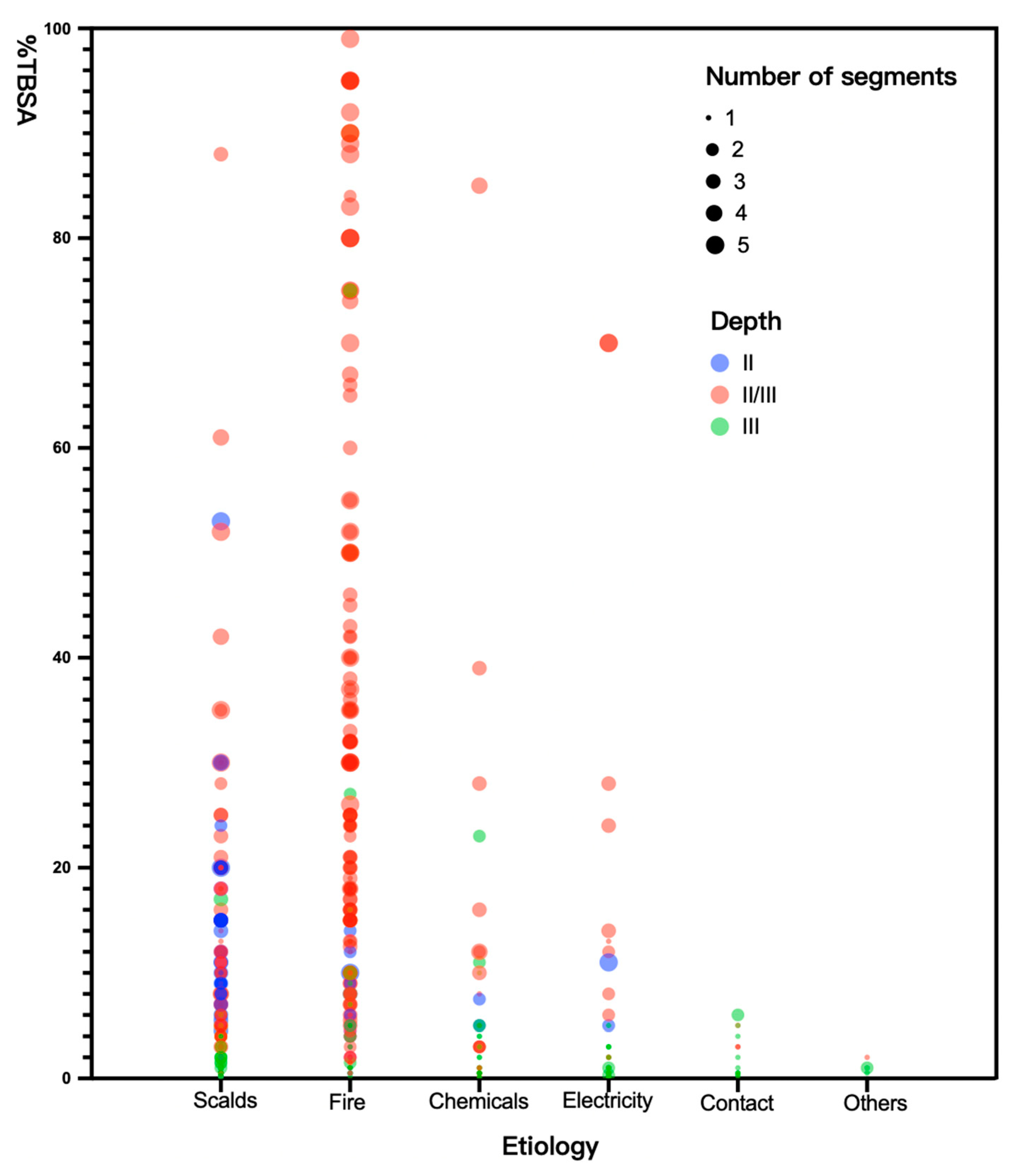
2.2. Burn Injury Event
2.2.1. Related Incidence and Risk Factors
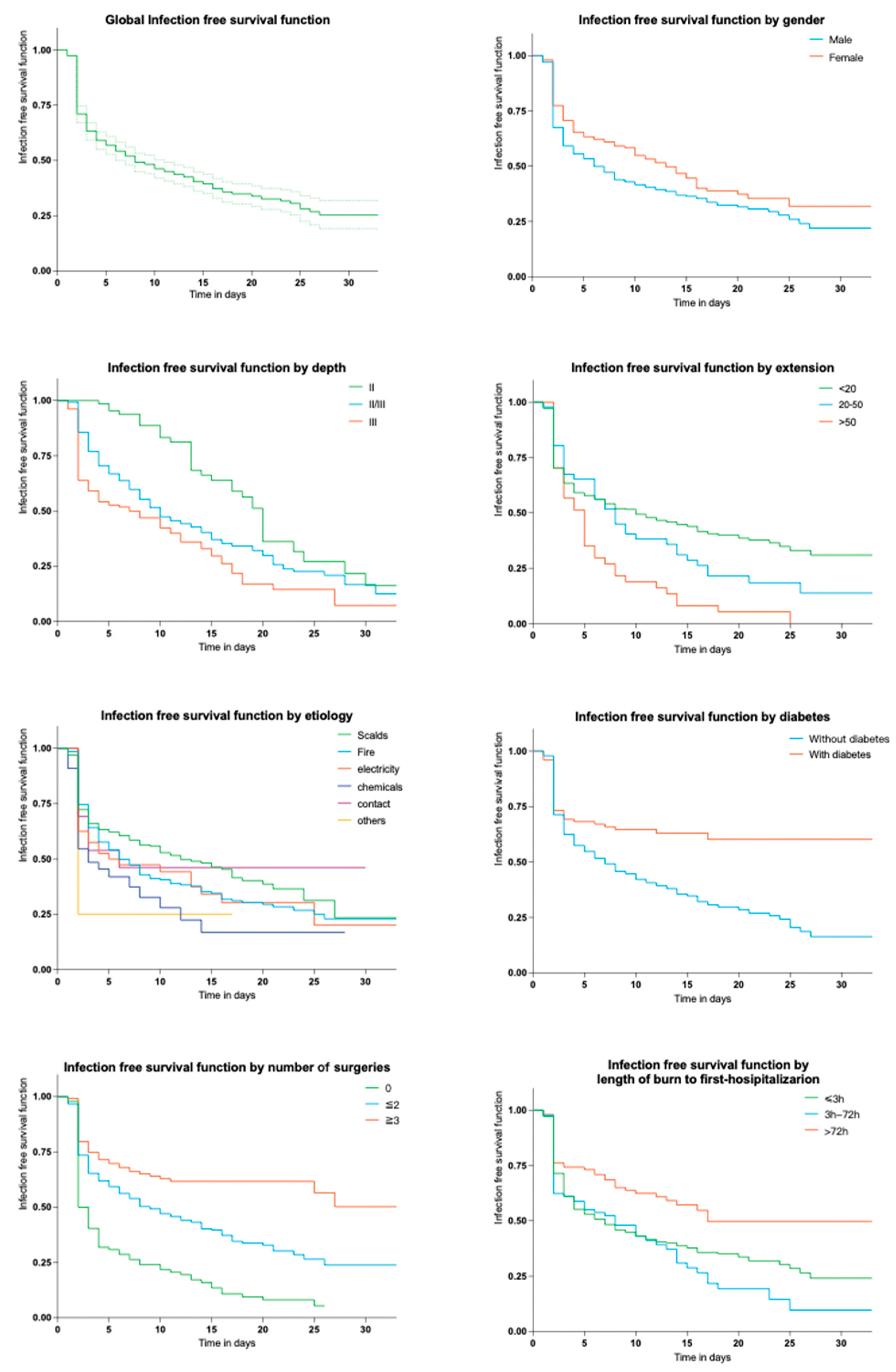
2.2.2. Time of Appearance of First Infection
2.3. Microorganisms
2.3.1. Related Incidence and Antibiotic Resistance
2.3.2. Time of Appearance of Infection
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
- Patients for non-burn reasons such as scar plastic surgery or chronic persistent infections;
- Patients who had been taking immunosuppressive drugs such as glucocorticoids for a long time or had serious autoimmune diseases;
- Patients who were uncooperative due to mental disorders or special circumstances and insisted on being discharged against medical advice or treatment voluntarily;
- Patients with inadequate clinical data.
4.2. Definitions
4.3. Microbiology
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haagsma, J.A.; Graetz, N.; Bolliger, I.; Naghavi, M.; Higashi, H.; Mullany, E.C.; Abera, S.F.; Abraham, J.P.; Adofo, K.; Alsharif, U.; et al. The global burden of injury: Incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj. Prev. 2016, 22, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Leilei, D.; Pengpeng, Y.; A Haagsma, J.; Ye, J.; Yuan, W.; Yuliang, E.; Xiao, D.; Xin, G.; Cuirong, J.; Linhong, W.; et al. The burden of injury in China, 1990–2017: Findings from the Global Burden of Disease Study 2017. Lancet Public Heal. 2019, 4, e449–e461. [Google Scholar] [CrossRef]
- Alp, E.; Coruh, A.; Gunay, G.K.; Yontar, Y.; Doganay, M. Risk Factors for Nosocomial Infection and Mortality in Burn Patients. J. Burn. Care Res. 2012, 33, 379–385. [Google Scholar] [CrossRef]
- Gomez, R.; Murray, C.K.; Hospenthal, D.R.; Cancio, L.C.; Renz, E.M.; Holcomb, J.B.; Wade, C.E.; Wolf, S.E. Causes of Mortality by Autopsy Findings of Combat Casualties and Civilian Patients Admitted to a Burn Unit. J. Am. Coll. Surg. 2009, 208, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Erol, S.; Altoparlak, U.; Akcay, M.N.; Celebi, F.; Parlak, M. Changes of microbial flora and wound colonization in burned patients. Burns 2004, 30, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Stanojcic, M.P.; Abdullahi, A.M.; Rehou, S.M.; Parousis, A.M.; Jeschke, M.G. Pathophysiological Response to Burn Injury in Adults. Ann. Surg. 2018, 267, 576–584. [Google Scholar] [CrossRef]
- Uppal, S.K.; Ram, S.; Kwatra, B.; Garg, S.; Gupta, R. Comparative evaluation of surface swab and quantitative full thickness wound biopsy culture in burn patients. Burns 2007, 33, 460–463. [Google Scholar] [CrossRef]
- Alrawi, M.; Crowley, T.; Pape, S. Bacterial colonisation of the burn wound: A UK experience. J. Wound Care 2014, 23, 274–277. [Google Scholar] [CrossRef]
- El Hamzaoui, N.; Barguigua, A.; Larouz, S.; Maouloua, M. Epidemiology of burn wound bacterial infections at a Meknes hospital, Morocco. New Microbes New Infect. 2020, 38, 100764. [Google Scholar] [CrossRef]
- Cato, L.D.; Al-Tarrah, K.; Moiemen, N. Changes in Burn Wound Microbiology Profile Over 14 Years of an Adult Tertiary Burn Center. J. Burn. Care Res. 2021, 44, 293–301. [Google Scholar] [CrossRef]
- Rafik, A.; Chabbak, H.; Jouhri, K.; Diouri, M.; Bahechar, N.; Chlihi, A.-S. Nosocomial Infections in a Morocco Burn Unit. OALib 2015, 02, 1–5. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Xu, L.; Hu, Y.; Sang, Y.; Zhang, G.; Dai, H. Epidemiology and outcomes of bloodstream infections in severe burn patients: A six-year retrospective study. Antimicrob. Resist. Infect. Control. 2021, 10, 1–8. [Google Scholar] [CrossRef]
- Strassle, P.D.; Williams, F.N.; Weber, D.J.; Sickbert-Bennett, E.E.; Lachiewicz, A.M.; Napravnik, S.; Jones, S.W.; Cairns, B.A.; van Duin, D. Risk Factors for Healthcare-Associated Infections in Adult Burn Patients. Infect. Control. Hosp. Epidemiology 2017, 38, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, S.L.; Ramírez, C.E.; Viviescas, A.F.; Abril, A.F.; Flórez, D.F.; Sosa, C.D. Evaluation of Burn Wound Infection in a Referral Center in Colombia. Indian J. Plast. Surg. 2022, 55, 075–080. [Google Scholar] [CrossRef]
- Ayaz, M.; Karami, M.Y.; Deilami, I.; Moradzadeh, Z. Effects of Early Versus Delayed Excision and Grafting on Restoring the Functionality of Deep Burn-Injured Hands: A Double-Blind, Randomized Parallel Clinical Trial. J. Burn. Care Res. 2019, 40, 451–456. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; Van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Kaita, Y.; Otsu, A.; Tanaka, Y.; Yoshikawa, K.; Matsuda, T.; Yamaguchi, Y. Epidemiology of bloodstream infections and surface swab cultures in burn patients. Acute Med. Surg. 2022, 9, e752. [Google Scholar] [CrossRef] [PubMed]
- Karyoute, S. Burn wound infection in 100 patients treated in the burn unit at Jordan University Hospital. Burns 1989, 15, 117–119. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.-H.; Huangfu, Y.-C.; Wang, W.-K.; Liu, Y.; Ni, Y.-X.; Han, L.-Z. Molecular epidemiologic analysis of Staphylococcus aureus isolated from four burn centers. Burns 2012, 38, 738–742. [Google Scholar] [CrossRef]
- van Duin, D.; Strassle, P.D.; DiBiase, L.M.; Lachiewicz, A.M.; Rutala, W.A.; Eitas, T.; Maile, R.; Kanamori, H.; Weber, D.J.; Cairns, B.A.; et al. Timeline of health care–associated infections and pathogens after burn injuries. Am. J. Infect. Control. 2016, 44, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Hettiaratchy, S.; Papini, R. Initial management of a major burn: II—assessment and resuscitation. BMJ 2004, 329, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA; Baltimore, MD, USA, 2021. [Google Scholar]
- National Nosocomial Infections Surveillance System Manual; Centers for Disease Control and Prevention: Atlanta, Georgia, 1993.


| Characteristic | Total (n = 580) | BWI (n = 348) | Incidence | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | aOR (95% CI) | p-Value | ||||
| Age(years, median, IQR) | 39.5 (23–56) | 41 (24–57) | 60.0% | 1.00 (1.00–1.01) | 0.552 | ||
| Age ≥ 60 | 120 (20.7%) | 71 (20.4%) | 59.2% | 0.99 (0.95–1.03) | 0.631 | ||
| Ward | |||||||
| I | 318 (54.8%) | 189 (54.3%) | 59.4% | - | - | ||
| II | 262 (45.2%) | 159 (45.7%) | 60.7% | 1.05 (0.75–1.47) | 0.759 | ||
| Gender | |||||||
| Male | 372 (64.1%) | 240 (69.0%) | 64.5% | - | - | ||
| Female | 208 (35.9%) | 108 (31.0%) | 51.9% | 1.68 (1.19–2.38) | 0.003 | 0.68 (0.46–1.00) | 0.048 |
| Depth | |||||||
| II | 66 (11.4%) | 35 (10.0%) | 53.0% | - | - | ||
| II/III | 278 (47.9%) | 184 (52.9%) | 66.2% | 0.94 (0.52–1.62) | 0.814 | 2.39 (1.20–4.78) | 0.014 |
| III | 236 (40.7%) | 129 (37.1%) | 54.7% | 1.62 (1.14–2.32) | 0.008 | 2.84 (1.43–5.67) | <0.001 |
| Anatomical segments | |||||||
| Limbs | 535 (92.2%) | 325 (93.4%) | 60.8% | 1.48 (0.81–2.72) | 0.207 | ||
| Torso | 217 (37.4%) | 150 (43.1%) | 69.1% | 1.87 (1.31–2.66) | 0.001 | ||
| Head and neck | 246 (42.4%) | 163 (46.8%) | 66.3% | 1.58 (1.12–2.23) | 0.008 | ||
| Hip | 70 (12.1%) | 59 (17.0%) | 84.3% | 4.10 (2.11–7.99) | <0.001 | ||
| Perineum | 53 (9.1%) | 45 (12.9%) | 84.9% | 4.16 (1.92–9.00) | <0.001 | ||
| TBSA (%) | |||||||
| <20 | 458 (79.0%) | 247 (71.0%) | 55.3% | - | - | ||
| 20–50 | 85 (14.7%) | 65 (18.7%) | 76.5% | 3.32 (1.57–7.02) | 0.002 | 7.41 (3.00–18.28) | <0.001 |
| >50 | 37 (6.4%) | 36 (10.3%) | 97.3% | 29.06 (3.95–18.77) | 0.001 | 26.43 (7.88–49.48) | <0.001 |
| Total | 5 (1–16) | 5 (1–16) | 60.0% | 1.05 (1.03–1.06) | <0.001 | ||
| Inhalation injury | 149 (25.7%) | 107 (30.8%) | 71.8% | 2.01 (1.34–3.01) | <0.001 | ||
| Hypovolemia | 167 (28.8%) | 128 (36.8%) | 76.7% | 2.88 (1.92–4.33) | <0.001 | ||
| Etiology | |||||||
| Scalds | 289 (49.8%) | 149 (42.8%) | 51.6% | - | - | ||
| Fire | 201 (34.7%) | 138 (39.7%) | 68.7% | 2.06 (1.41–3.00) | <0.001 | ||
| Chemicals | 40 (6.9%) | 27 (7.8%) | 67.5% | 2.51 (1.13–5.58) | 0.024 | ||
| Electricity | 33 (5.7%) | 24 (6.9%) | 72.7% | 1.95 (0.97–3.93) | 0.61 | ||
| Contact | 13 (2.2%) | 7 (2.0%) | 53.9% | 1.10 (0.36–3.34) | 0.872 | ||
| Others | 4 (0.7%) | 3 (0.9%) | 75.0% | 2.82 (0.29–27.42) | 0.372 | ||
| Diabetes | 101 (17.4%) | 69 (19.8%) | 68.3% | 1.54 (0.97–2.43) | 0.065 | ||
| Surgery | |||||||
| 0 | 94 (16.2%) | 49 (14.1%) | 52.1% | - | - | ||
| 1/2 | 363 (62.6%) | 194 (55.8%) | 53.4% | 1.05 (0.67–1.66) | 0.82 | 0.13 (0.06–0.28) | <0.001 |
| ≥3 | 123 (21.2%) | 105 (30.2%) | 85.4% | 5.36 (2.82–10.19) | <0.001 | 0.04 (0.03–0.10) | <0.001 |
| Total | 2 (1–2) | 2 (1–2) | 60.0% | 1.60 (1.38–1.85) | <0.001 | ||
| Duration from burn to first hospitalization (h) | |||||||
| ≤3 h | 382 (65.9%) | 250 (71.8%) | 65.5% | - | - | ||
| 3 h–72 h | 85 (14.7%) | 42 (12.1%) | 49.4% | 1.86 (1.19–2.89) | 0.006 | 4.21 (2.30–7.70) | <0.001 |
| >72 h | 101 (17.4%) | 51 (14.7%) | 50.5% | 0.96 (0.54–1.71) | 0.883 | 5.03 (2.30–11.02) | <0.001 |
| Total | 2 (2–12) | 2 (2–4) | 60.0% | 1.00 (1.00–1.00) | 0.368 | ||
| Hospital length of stay, days (median, IQR) | 17(10–26) | 15 (9.75–22) | 60.0% | 0.97(0.96–0.98) | <0.001 | 0.97(0.96–0.99) | 0.002 |
| First Isolated Pathogen | Frequency (N/%) | Time Between Admission and First Positive Swab Culture |
|---|---|---|
| Gram-positive | 343 (52.9%) | 3 (2–7) |
| S. aureus | 102 (15.7%) | 2 (2–7) |
| MRSA | 52 (8.0%) | 2 (1–9) |
| MSSA | 50 (7.7%) | 1 (1–2) |
| S. epidermidis | 92 (14.2%) | 3 (2–6) |
| Enterococcus faecalis | 44 (6.8%) | 4 (2–7) |
| S. hemolyticus | 38 (5.9%) | 5 (2–10) |
| Others | 67 (10.3%) | - |
| Gram-negative | 269 (41.5%) | 10 (4–17) |
| K. pneumoniae | 69 (10.6%) | 14 (6–18) |
| A. baumanii | 69(10.6%) | 9 (5–15.5) |
| P. aeruginosa | 55 (8.5%) | 12 (5–22) |
| Escherichia coli | 13 (2.0%) | 6 (2–11) |
| Others | 63 (9.7%) | - |
| Fungi | 37 (5.7%) | 16 (9.75–22.5) |
| Total | 6 (2–14) |
| Antibiotics | K. pneumoniae (N = 69) | A. baumanii (N = 69) | P. aeruginosa (N = 55) | Escherichia coli (N = 13) | ||||
|---|---|---|---|---|---|---|---|---|
| n | Resistance (%) | n | Resistance (%) | n | Resistance (%) | n | Resistance (%) | |
| Cefazolin | 63 | 91.3% | 69 | 100.0% | - | - | 10 | 76.9% |
| Cefuroxime | 64 | 92.8% | 67 | 97.1% | - | - | 8 | 61.5% |
| Ceftazidime | 58 | 84.1% | 65 | 94.2% | 16 | 29.1% | 4 | 30.8% |
| Ceftriaxone | 62 | 89.9% | 68 | 98.6% | - | - | 8 | 61.5% |
| Cefepime | 57 | 82.6% | 64 | 92.8% | 18 | 32.7% | 4 | 30.8% |
| Aztreonam | 58 | 84.1% | - | - | 13 | 23.6% | 2 | 15.4% |
| Imipenem | 51 | 73.9% | 65 | 94.2% | 41 | 74.6% | 1 | 7.7% |
| Meropenem | 51 | 73.9% | 65 | 94.2% | 39 | 70.9% | 2 | 15.4% |
| Ampicillin-sulbactam | 60 | 87.0% | 61 | 88.4% | - | - | 4 | 30.8% |
| Piperacilin-tazobactam | 54 | 78.3% | 65 | 94.2% | 19 | 34.6% | 1 | 7.7% |
| Ticarcillin-clavulanate | 55 | 79.7% | 65 | 94.2% | 39 | 70.9% | 1 | 7.7% |
| Cefoperazone-sulbactam | 55 | 79.7% | 33 | 47.8% | 38 | 69.1% | 1 | 7.7% |
| Amikacin | 50 | 72.5% | 52 | 75.4% | 37 | 67.3% | 1 | 7.7% |
| Tobramycin | 50 | 72.5% | 59 | 85.5% | 38 | 69.1% | 3 | 23.1% |
| Ciprofloxacin | 61 | 88.4% | 65 | 94.2% | 40 | 72.7% | 8 | 61.5% |
| Levofloxacin | 61 | 88.4% | 61 | 88.4% | 41 | 74.6% | 7 | 53.9% |
| Doxycycline | 57 | 82.6% | 24 | 34.8% | - | - | 7 | 53.9% |
| Minocycline | 54 | 78.3% | 3 | 4.4% | - | - | 6 | 46.2% |
| Tigecycline | - | - | 2 | 2.9% | - | - | 0 | 0.0% |
| Colistin | 11 | 16.0% | 2 | 2.9% | 2 | 3.6% | 1 | 7.7% |
| Fosfomycin | 53 | 76.8% | 55 | 79.7% | - | - | 3 | 23.1% |
| Trimethoprim-sulfamethoxazole | 59 | 85.5% | 66 | 95.7% | - | - | 9 | 69.2% |
| Antibiotics | S. aureus (N = 102) | MRSA (N = 52) | MSSA (N = 50) | |||
|---|---|---|---|---|---|---|
| n | Resistance | n | Resistance | n | Resistance | |
| Penicillin | 94 | 92.2% | 52 | 100.0% | 42 | 84.0% |
| Oxacillin | 53 | 52.0% | 52 | 100.0% | 1 | 2.0% |
| Gentamicin | 13 | 12.8% | 12 | 23.1% | 1 | 2.0% |
| Ciprofloxacin | 23 | 22.6% | 15 | 28.9% | 8 | 16.0% |
| Levofloxacin | 24 | 23.5% | 15 | 28.9% | 9 | 18.0% |
| Moxifloxacin | 22 | 21.6% | 15 | 28.9% | 7 | 14.0% |
| Clindamycin | 40 | 39.2% | 25 | 48.1% | 15 | 30.0% |
| Erythromycin | 45 | 44.1% | 28 | 53.9% | 17 | 100.0% |
| Rifampin | 6 | 5.9% | 6 | 11.5% | 0 | 0.0% |
| Vancomycin | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Linezolid | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Trimethoprim-sulfamethoxazole | 13 | 12.8% | 10 | 19.2% | 3 | 6.0% |
| Quinupristin-dalfopristin | 1 | 1.0% | 1 | 1.9% | 0 | 0.0% |
| Tetracycline | 25 | 24.51% | 21 | 40.4% | 4 | 8.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Xiao, S.; Wang, X.; Wang, X.; Han, L. Risk Factors and Pathogens of Wound Infection in Burn Inpatients from East China. Antibiotics 2023, 12, 1432. https://doi.org/10.3390/antibiotics12091432
Zhou S, Xiao S, Wang X, Wang X, Han L. Risk Factors and Pathogens of Wound Infection in Burn Inpatients from East China. Antibiotics. 2023; 12(9):1432. https://doi.org/10.3390/antibiotics12091432
Chicago/Turabian StyleZhou, Siqi, Shuzhen Xiao, Xuedong Wang, Xuefeng Wang, and Lizhong Han. 2023. "Risk Factors and Pathogens of Wound Infection in Burn Inpatients from East China" Antibiotics 12, no. 9: 1432. https://doi.org/10.3390/antibiotics12091432
APA StyleZhou, S., Xiao, S., Wang, X., Wang, X., & Han, L. (2023). Risk Factors and Pathogens of Wound Infection in Burn Inpatients from East China. Antibiotics, 12(9), 1432. https://doi.org/10.3390/antibiotics12091432






