Escherichia coli Phylogenetic and Antimicrobial Pattern as an Indicator of Anthropogenic Impact on Threatened Freshwater Mussels
Abstract
:1. Introduction
2. Results
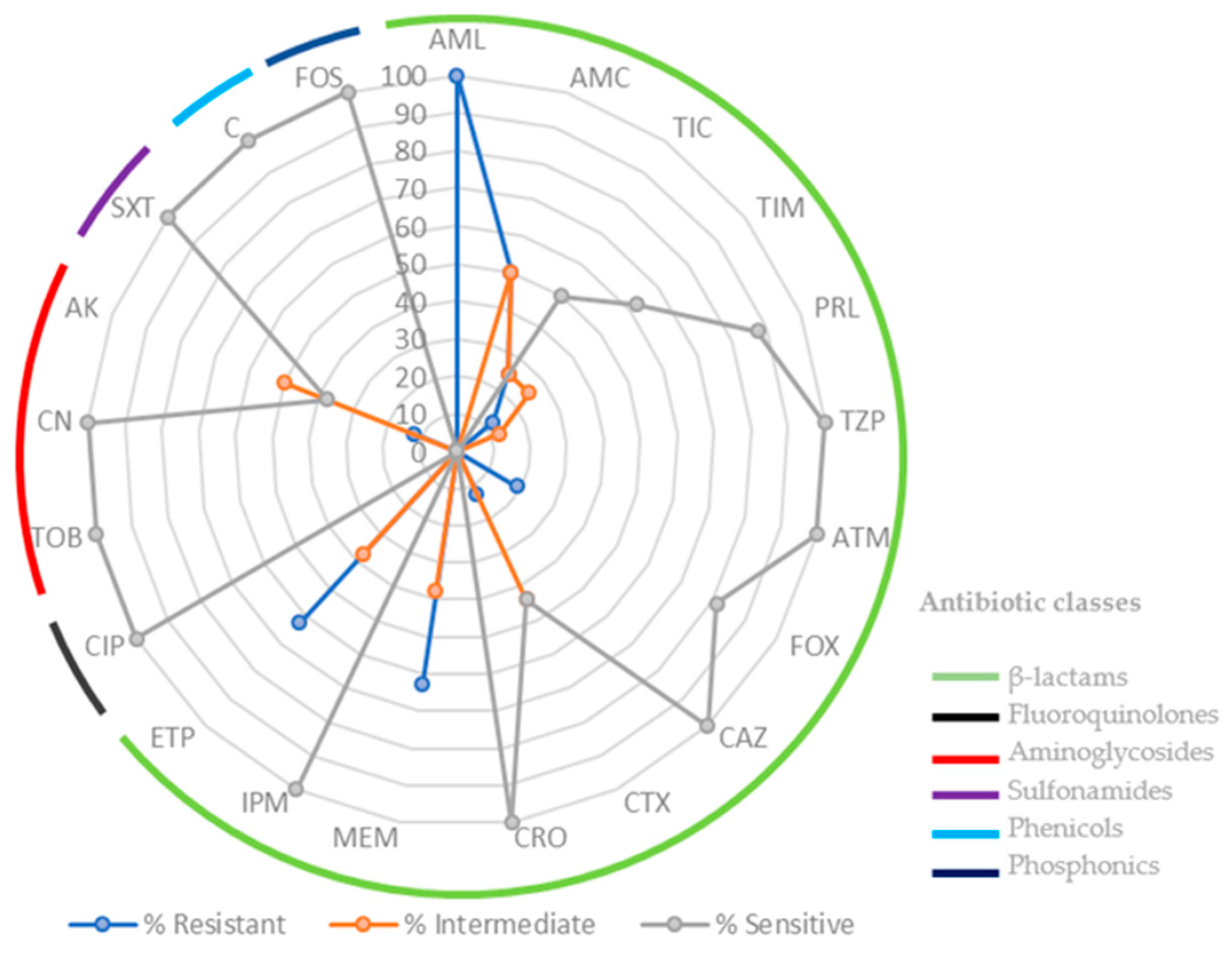
2.1. Bacterial Isolates
2.2. Molecular Characterization of E. coli Isolates
3. Materials and Methods
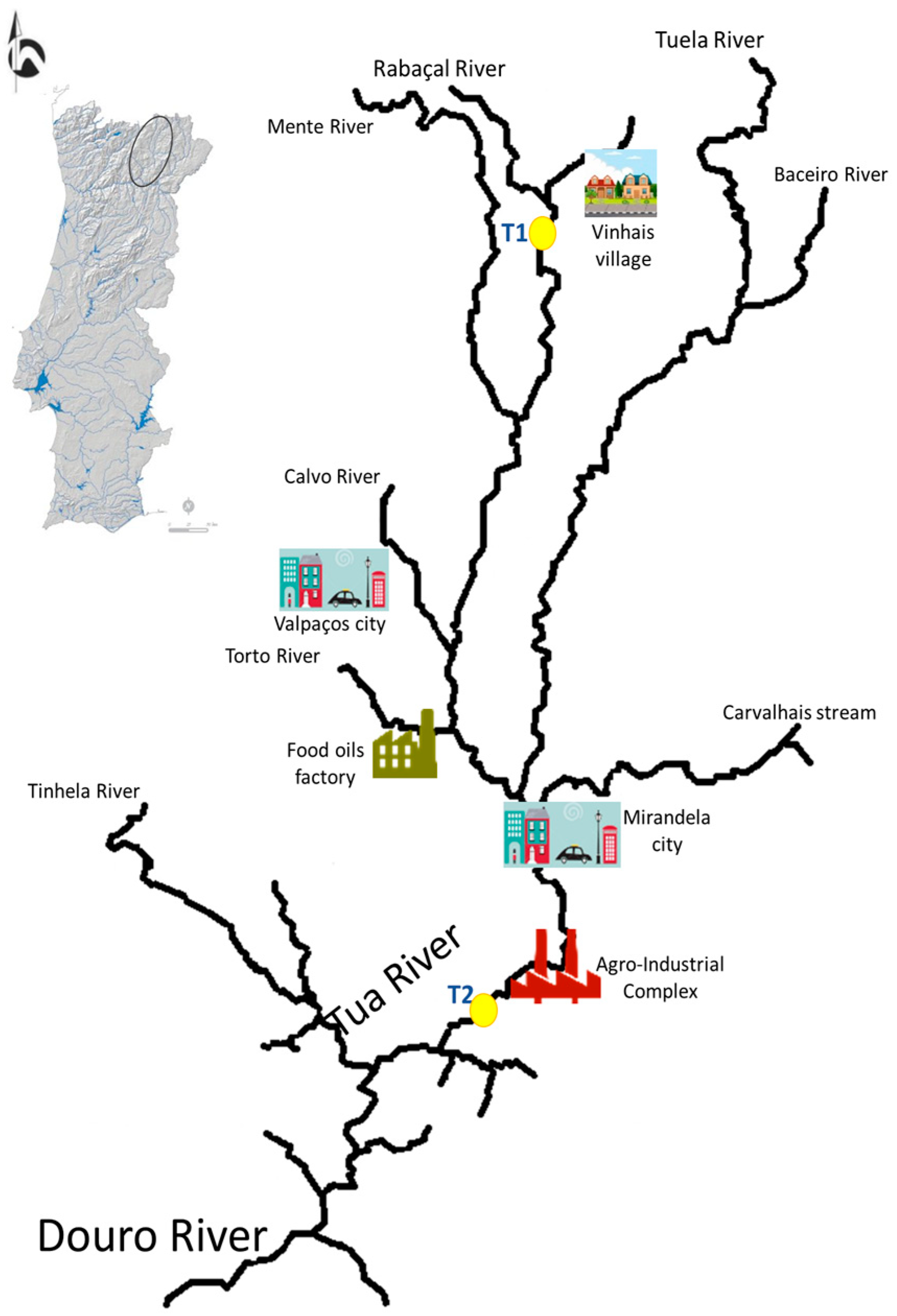
3.1. Study Area and Freshwater Mussel Sampling
3.2. Bacterial Isolates
3.3. Kirby-Bauer Disk Diffusion Susceptibility Test
3.4. Molecular Characterization of E. coli Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes-Lima, M.; Burlakova, L.; Karatayev, A.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of freshwater bivalves at the global scale: Diversity, threats and research needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef]
- Nogueira, J.; Lopes-Lima, M.; Beja, P.; Filipe, A.F.; Froufe, E.; Gonçalves, D.V.; Silva, J.P.; Sousa, R.; Teixeira, A.; Varandas, S.; et al. Identifying freshwater priority areas for cross-taxa interactions. Sci. Total Environ. 2023, 864, 161073. [Google Scholar] [CrossRef] [PubMed]
- Ilarri, M.; Amorim, L.; Souza, A.T.; Sousa, R.G. Physical legacy of freshwater bivalves: Effects of habitat complexity on the taxonomical and functional diversity of invertebrates. Sci. Total Environ. 2018, 634, 1398–1405. [Google Scholar] [CrossRef]
- Gomes, S.; Fernandes, C.; Monteiro, S.; Cabecinha, E.; Teixeira, A.; Varandas, S.; Saavedra, M.J. The Role of Aquatic Ecosystems (River Tua, Portugal) as Reservoirs of Multidrug-Resistant Aeromonas spp. Water 2021, 13, 698. [Google Scholar] [CrossRef]
- Araújo, R.; Reis, J.; Machordom, A.; Toledo, C.; Madeira, M.J.; Gómez, I.; Velasco Marcos, J.C.; Morales, J.; Barea, J.M.; Ondina, P.; et al. Las náyades de la península Ibérica. In Iberus, Sociedad Española de Malacología; Spanish Society of Malacology: Madrid, Spain, 2009; Volume 27, pp. 7–72. Available online: http://hdl.handle.net/10261/45260 (accessed on 24 June 2023).
- Kappell, A.D.; DeNies, M.S.; Ahuja, N.H.; Ledeboer, N.A.; Newton, R.J.; Hristova, K.R. Detection of multidrug resistant Escherichia coli in the urban waterways of Milwaukee, WI. Front. Microbiol. 2015, 6, 336. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Suzuki, S.; Pruden, A.; Virta, M.; Zhang, T. Antibiotic resistance in aquatic systems. Front. Microbiol. 2017, 8, 14. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic resistance in the environment: A link to the clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Dias, C.; Mota, V.; Martinez-Murcia, A.; Saavedra, M.J. Antimicrobial resistance patterns of Aeromonas spp. isolated from ornamental fish. J. Aquac. Res. Dev. 2012, 3, 131. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a Potential Hotspot for Antibiotic Resistance Dissemination by Horizontal Gene Transfer Events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018, 6, 2–6. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method re-visited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Fernandes, C.; Teixeira, T.; Álvarez, X.; Varandas, S. Multiresistant bacteria: Invisible enemies of freshwater mussels. Environ. Pollut. 2022, 295, 118671. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef]
- Andrès, S.; Baudrimont, M.; Lapaquellerie, Y.; Ribeyre, F.; Maillet, N.; Latouche, C.; Boudou, A. Field transplantation of the freshwater bivalve Corbicula fluminea along a polymetallic contamination gradient (River Lot, France): I. Geochemical characteristics of the sampling sites and cadmium and zinc bioaccumulation kinetics. Environ. Toxicol. Chem. 1999, 18, 2462–2471. [Google Scholar] [CrossRef]
- Bighiu, M.A.; Haldén, A.N.; Goedkoop, W.; Ottoson, J. Assessing microbial contamination and antibiotic resistant bacteria using zebra mussels (Dreissena polymorpha). Sci. Total Environ. 2019, 650, 2141–2149. [Google Scholar] [CrossRef]
- Meletis, G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2015, 3, 15–21. [Google Scholar] [CrossRef]
- Wambugu, P.; Habtu, M.; Impwi, P.; Matiru, V.; Kiiru, J. Antimicrobial susceptibility profiles among Escherichia coli strains isolated from Athi River water in Machakos County, Kenya. Adv. Microbiol. 2015, 5, 711. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Leon, A.; Garcia-Omil, C.; Dalama, J.; Rodriguez-Souto, R.; Martinez-Urtaza, J.; Gonzalez-Escalona, N. Detection of colistin resistance mcr-1 gene in Salmonella enterica serovar Rissen isolated from mussels, Spain, 2012 to 2016. Eurosurveillance 2019, 24, 1900200. [Google Scholar] [CrossRef]
- Odonkor, S.T.; Addo, K.K. Prevalence of Multidrug-Resistant Escherichia coli Isolated from Drinking Water Sources. Int. J. Microbiol. 2018, 7204013. [Google Scholar] [CrossRef]
- Anjum, M.F.; Schmitt, H.; Börjesson, S.; Berendonk, T.U.; WAWES Network. The potential of using E. coli as an indicator for the surveillance of antimicrobial resistance (AMR) in the environment. Curr. Opin. Microbiol. 2021, 64, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lupo, A.; Coyne, S.; Berendonk, T. Origin and Evolution of Antibiotic Resistance: The Common Mechanisms of Emergence and Spread in Water Bodies. Front. Microbiol. 2012, 3, 18. [Google Scholar] [CrossRef]
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef]
- Shivakumaraswamy, S.K.; Deekshit, V.K.; Vittal, R.; Akhila, D.S.; Mundanda, D.M.; Mohan Raj, J.R.; Chakraborty, A.; Karunasagar, I. Phenotypic & genotypic study of antimicrobial profile of bacteria isolates from environmental samples. Indian J. Med. Res. 2019, 149, 232–239. [Google Scholar] [CrossRef]
- Vignaroli, C.; Di Sante, L.; Leoni, F.; Chierichetti, S.; Ottaviani, D.; Citterio, B.; Biavasco, F. Multidrug-resistant and epidemic clones of Escherichia coli from natural beds of Venus clam. Food Microbiol. 2016, 59, 1–6. [Google Scholar] [CrossRef]
- Giacometti, F.; Pezzi, A.; Galletti, G.; Tamba, M.; Merialdi, G.; Piva, S.; Serraino, A.; Rubini, S. Antimicrobial resistance patterns in Salmonella enterica subsp. enterica and Escherichia coli isolated from bivalve molluscs and marine environment. Food Control 2021, 121, 107590. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, 7209. [Google Scholar] [CrossRef]
- Woolnough, D.A.; Bellamy, A.; Hummel, S.L.; Annis, M. Environmental exposure of freshwater mussels to contaminants of emerging concern: Implications for species conservation. J. Great Lakes Res. 2020, 46, 1625–1638. [Google Scholar] [CrossRef]
- Chandurvelan, R.; Marsden, I.D.; Glover, C.N.; Gaw, S. Assessment of a mussel as a metal bioindicator of coastal contamination: Relationships between metal bioaccumulation and multiple biomarker responses. Sci. Total Environ. 2015, 511, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, P.; Prearo, M.; Anselmi, S.; Menconi, V.; Bertoli, M.; Dondo, A.; Pizzul, E.; Renzi, M. Use of the Zebra Mussel Dreissena polymorpha (Mollusca, Bivalvia) as a Bioindicator of Microplastics Pollution in Freshwater Ecosystems: A Case Study from Lake Iseo (North Italy). Water 2021, 13, 434. [Google Scholar] [CrossRef]
- Sousa, R.; Ferreira, A.; Carvalho, F.; Lopes-Lima, M.; Varandas, S.; Teixeira, A.; Gallardo, B. Small hydropower plants as a threat to the endangered pearl mussel Margaritifera margaritifera. Sci. Total Environ. 2020, 719, 137361. [Google Scholar] [CrossRef]
- Mezzelani, M.; Fattorini, D.; Gorbi, S.; Nigro, M.; Regoli, F. Human pharmaceuticals in marine mussels: Evidence of sneaky environmental hazard along Italian coasts. Mar. Environ. Res. 2020, 162, 105137. [Google Scholar] [CrossRef] [PubMed]
- Zieritz, A.; Sousa, R.; Aldridge, D.C.; Douda, K.; Esteves, E.; Ferreira-Rodríguez, N.; Mageroy, J.H.; Nizzoli, D.; Osterling, M.; Reis, J.; et al. A global synthesis of ecosystem services provided and disrupted by freshwater bivalve molluscs. Biol. Rev. 2022, 97, 1967–1998. [Google Scholar] [CrossRef] [PubMed]
| Isolates | arpA 400 bp | chuA 288 bp | yjaA 211 bp | TspE4.C2 152 bp | Phylogroup (%) |
|---|---|---|---|---|---|
| Pt1; Pt3; Pt4; Pt7 | + | + | + | - | E or Clade I (40%) |
| Pt8; Pt9 | + | - | - | + | B1 (20%) |
| Pt2; Pt5; Pt6 | + | - | - | - | A (30%) |
| Pt10 | + | + | - | + | D or E (10%) |
| Mg1; Mg2; Mg3; Mg4; Mg5; Mg6 | + | + | - | - | D or E (100%) |
| Primer Name | Primer Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| chuA | F: 5-ATGGTACCGGACGAACCAAC-3 R: 5-TGCCGCCAGTACCAAAGACA-3 | 288 |
| yjaA | F: 5-CAAACGTGAAGTGTCAGGAG-3 R: 5-AATGCGTTCCTCAACCTGTG-3 | 211 |
| TspE4C2 | F: 5-CACTATTCGTAAGGTCATCC-3 R: 5-AGTTTATCGCTGCGGGTCGC-3 | 152 |
| arpA | F: 5-AACGCTATTCGCCAGCTTGC-3 R: 5-TCTCCCCATACCGTACGCTA-3 | 400 |
| arpA (group E) | F: 5-GATTCCATCTTGTCAAAATATGCC-3 R: 5GAAAAGAAAAAGAATTCCCAAGAG | 301 |
| pA (group C) | F: 5-AGTTTTATGCCCAGTGCGAG-3 R: 5-TCTGCGCCGGTCACGCCC-3 | 219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varandas, S.; Fernandes, C.; Cabecinha, E.; Gomes, S.; da Silva, G.J.; Saavedra, M.J. Escherichia coli Phylogenetic and Antimicrobial Pattern as an Indicator of Anthropogenic Impact on Threatened Freshwater Mussels. Antibiotics 2023, 12, 1401. https://doi.org/10.3390/antibiotics12091401
Varandas S, Fernandes C, Cabecinha E, Gomes S, da Silva GJ, Saavedra MJ. Escherichia coli Phylogenetic and Antimicrobial Pattern as an Indicator of Anthropogenic Impact on Threatened Freshwater Mussels. Antibiotics. 2023; 12(9):1401. https://doi.org/10.3390/antibiotics12091401
Chicago/Turabian StyleVarandas, Simone, Conceição Fernandes, Edna Cabecinha, Sónia Gomes, Gabriela Jorge da Silva, and Maria José Saavedra. 2023. "Escherichia coli Phylogenetic and Antimicrobial Pattern as an Indicator of Anthropogenic Impact on Threatened Freshwater Mussels" Antibiotics 12, no. 9: 1401. https://doi.org/10.3390/antibiotics12091401
APA StyleVarandas, S., Fernandes, C., Cabecinha, E., Gomes, S., da Silva, G. J., & Saavedra, M. J. (2023). Escherichia coli Phylogenetic and Antimicrobial Pattern as an Indicator of Anthropogenic Impact on Threatened Freshwater Mussels. Antibiotics, 12(9), 1401. https://doi.org/10.3390/antibiotics12091401










