Isolation and Characterisation of Human-Derived blaKPC-3-Producing Salmonella enterica Serovar Rissen in 2018
Abstract
1. Introduction
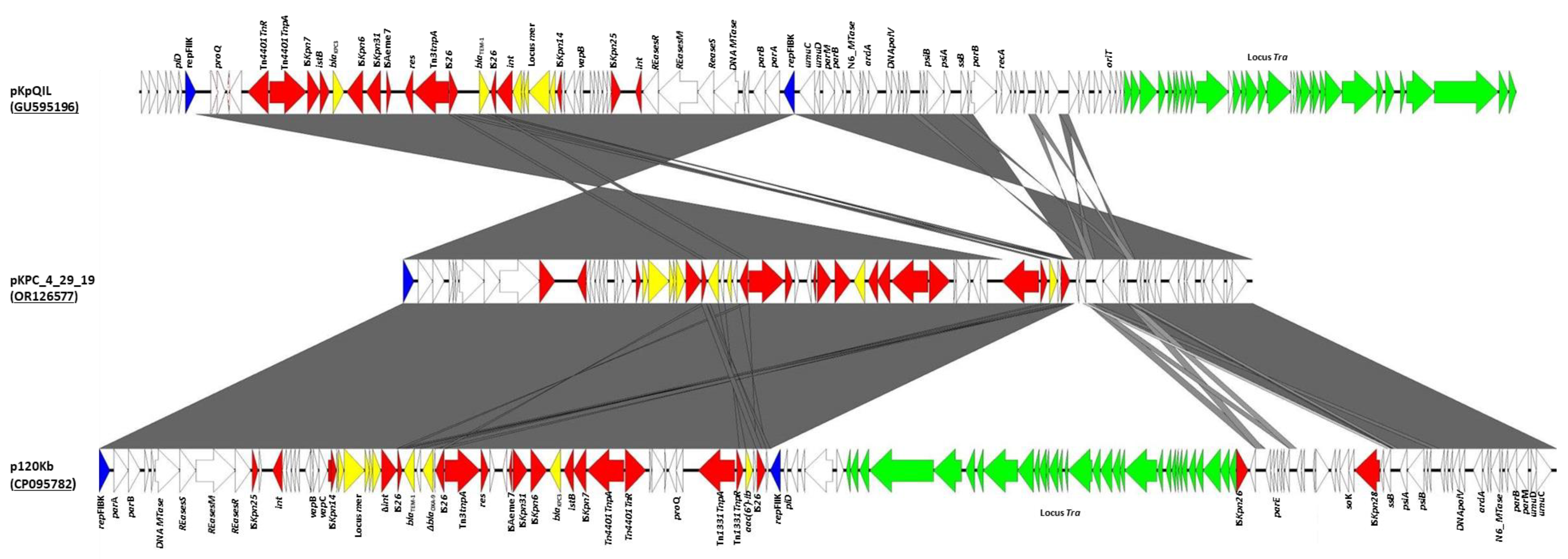
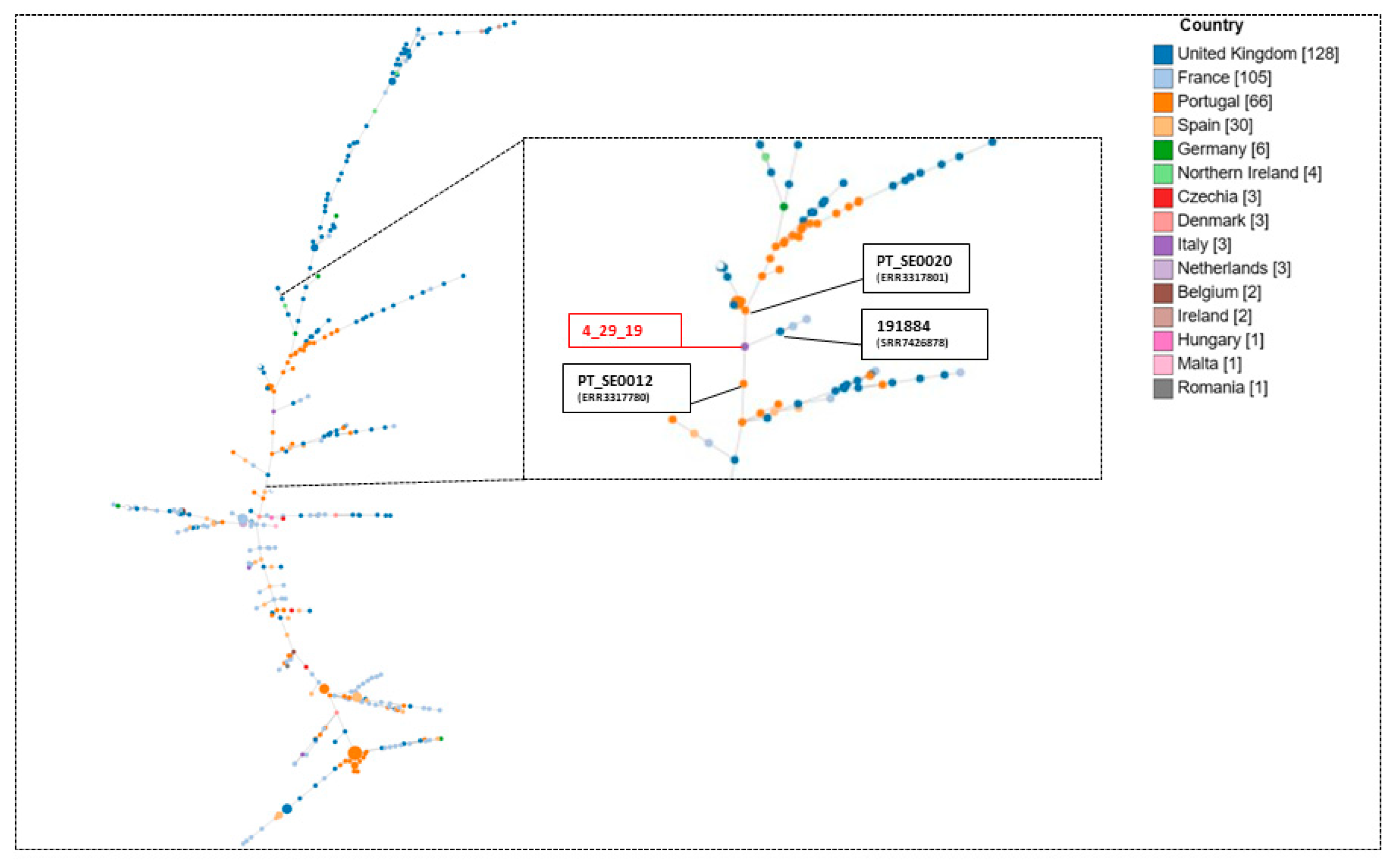
2. Results
3. Discussion
4. Materials and Methods
4.1. Phenotypic Analysis
4.2. Genomic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant Gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef]
- Peirano, G.; Chen, L.; Kreiswirth, B.N.; Pitout, J.D.D. Emerging antimicrobial-resistant high-risk Klebsiella pneumoniae clones ST307 and ST147. Antimicrob. Agents Chemother. 2020, 64, e01148-20. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, A.; Chmelnitsky, I.; Ofek, I.; Carmeli, Y.; Navon-Venezia, S. Plasmid pKpQIL encoding KPC-3 and TEM-1 confers carbapenem resistance in an extremely drug-resistant epidemic Klebsiella pneumoniae strain. J. Antimicrob. Chemother. 2010, 65, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. The Difficult-to-Control Spread of Carbapenemase Producers among Enterobacteriaceae Worldwide. Clin. Microbiol. Infect. 2014, 20, 821–830. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Villa, L.; Feudi, C.; Fortini, D.; Brisse, S.; Passet, V.; Bonura, C.; Endimiani, A.; Mammina, C.; Ocampo, A.M.; Jimenez, J.N.; et al. Diversity, Virulence, and Antimicrobial Resistance of the KPC producing Klebsiella pneumoniae ST307 Clone. Microb. Genom. 2017, 3, e000110. [Google Scholar] [CrossRef] [PubMed]
- Gootz, T.D.; Lescoe, M.K.; Dib-Hajj, F.; Dougherty, B.A.; He, W.; Della-Latta, P.; Huard, R.C. Genetic Organization of Transposase Regions Surrounding blaKPC Carbapenemase Genes on Plasmids from Klebsiella Strains Isolated in a New York City Hospital. Antimicrob. Agents Chemother. 2009, 53, 1998–2004. [Google Scholar] [CrossRef]
- Chen, L.; Chavda, K.D.; Melano, R.G.; Jacobs, M.R.; Koll, B.; Hong, T.; Rojtman, A.D.; Levi, M.H.; Bonomo, R.A.; Kreiswirtha, B.N. Comparative Genomic Analysis of Kpc-Encoding pKpQIL-like Plasmids and Their Distribution in New Jersey and New York Hospitals. Antimicrob. Agents Chemother. 2014, 58, 2871–2877. [Google Scholar] [CrossRef]
- Kopotsa, K.; Osei Sekyere, J.; Mbelle, N.M. Plasmid Evolution in Carbapenemase-Producing Enterobacteriaceae: A Review. Ann. N. Y. Acad. Sci. 2019, 1457, 61–91. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Nordmann, P.; Poirel, L. Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Fontana, H.; Cardoso, B.; Esposito, F.; de Lima, A.V.; Sampaio, J.L.M.; Lincopan, N. Small IncQ1 Plasmid Encoding KPC-2 Expands to Invasive Nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2021, 65, e0155221. [Google Scholar] [CrossRef]
- Jure, M.A.; Duprilot, M.; Musa, H.E.; López, C.; De Castillo, M.C.; Weill, F.X.; Arlet, G.; Decré, D. Emergence of KPC-2-Producing Salmonella enterica Serotype Schwarzengrund in Argentina. Antimicrob. Agents Chemother. 2014, 58, 6335–6336. [Google Scholar] [CrossRef][Green Version]
- Leavitt, A.; Chmelnitsky, I.; Carmeli, Y.; Navon-Venezia, S. Complete Nucleotide Sequence of KPC-3-Encoding Plasmid pKpQIL in the Epidemic Klebsiella pneumoniae Sequence Type 258. Antimicrob. Agents Chemother. 2010, 54, 4493–4496. [Google Scholar] [CrossRef]
- García-Fernández, A.; Villa, L.; Carta, C.; Venditti, C.; Giordano, A.; Venditti, M.; Mancini, C.; Carattoli, A. Klebsiella pneumoniae ST258 Producing KPC-3 Identified in Italy Carries Novel Plasmids and OmpK36/OmpK35 Porin Variants. Antimicrob. Agents Chemother. 2012, 56, 2143–2145. [Google Scholar] [CrossRef]
- Low, W.W.; Wong, J.L.C.; Beltran, L.C.; Seddon, C.; David, S.; Kwong, H.S.; Bizeau, T.; Wang, F.; Peña, A.; Costa, T.R.D.; et al. Mating pair stabilization mediates bacterial conjugation species specificity. Nat. Microbiol. 2022, 7, 1016–1027. [Google Scholar] [CrossRef]
- Villa, L.; Capone, A.; Fortini, D.; Dolejska, M.; Rodriguez, I.; Taglietti, F.; De Paolis, P.; Petrosillo, N.; Carattoli, A. Reversion to Susceptibility of a Carbapenem-Resistant Clinical Isolate of Klebsiella pneumoniae Producing KPC-3. J. Antimicrob. Chemother. 2013, 68, 2482–2486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The Versatile β-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same Species, Different Diseases: How and Why Typhoidal and Non-Typhoidal Salmonella enterica Serovars Differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Xu, H.; Chu, C.; Wang, J.; Jiao, X.; Li, Q. Whole-Genome Sequencing Analysis Reveals Pig as the Main Reservoir for Persistent Evolution of Salmonella enterica Serovar Rissen Causing Human Salmonellosis. Food Res. Int. 2022, 154, 111007. [Google Scholar] [CrossRef]
- Peruzy, M.F.; Proroga, Y.T.R.; Capuano, F.; Mancusi, A.; Montone, A.M.I.; Cristiano, D.; Balestrieri, A.; Murru, N. Occurrence and Distribution of Salmonella Serovars in Carcasses and Foods in Southern Italy: Eleven-Year Monitoring (2011–2021). Front. Microbiol. 2022, 13, 1005035. [Google Scholar] [CrossRef]
- Mellon, G.; Delanoe, C.; Roux, A.L.; Heym, B.; Dubourg, O.; Hardy, P.; Chevallier, B.; Perronne, C.; Rouveix, E.; Salomon, J. Non-typhi Salmonella enterica urinary tract infections. Méd. Mal. Infect. 2017, 47, 389–393. [Google Scholar] [CrossRef]
- Magnet, S.; Courvalin, P.; Lambert, T. Activation of the Cryptic Aac(6)-Iy Aminoglycoside Resistance Gene of Salmonella by a Chromosomal Deletion Generating a Transcriptional Fusion. J. Bacteriol. 1999, 181, 6650–6655. [Google Scholar] [CrossRef]
- Wang, M.; Qazi, I.H.; Wang, L.; Zhou, G.; Han, H. Salmonella Virulence and Immune Escape. Microorganisms 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Lynne, A.M. Food Animal-Associated Salmonella Challenges: Pathogenicity and Antimicrobial Resistance. J. Anim. Sci. 2008, 86, E173–E187. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Amadesi, S.; Lazzarotto, T.; Ambretti, S. Genome characterization of a Klebsiella pneumoniae co-producing OXA-181 and KPC-121 resistant to ceftazidime/avibactam, meropenem/vaborbactam, imipenem/relebactam and cefiderocol isolated from a critically ill patient. J. Glob. Antimicrob. Resist. 2022, 30, 262–264. [Google Scholar] [CrossRef]
- Warburg, G.; Hidalgo-Grass, C.; Partridge, S.R.; Tolmasky, M.E.; Temper, V.; Moses, A.E.; Block, C.; Strahilevitz, J. A carbapenem-resistant Klebsiella pneumoniae epidemic clone in Jerusalem: Sequence type 512 carrying a plasmid encoding aac(6)-Ib. J. Antimicrob. Chemother. 2012, 67, 898–901. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Zhou, A.; Li, J.; Xu, Z.; Ni, J.; Guo, J.; Yao, Y.F.; Wu, W. Whole-Genome Comparative and Pathogenicity Analysis of Salmonella enterica subsp. enterica Serovar Rissen. G3 2020, 10, 2159–2170. [Google Scholar] [CrossRef]
- García-Fierro, R.; Montero, I.; Bances, M.; González-Hevia, M.Á.; Rodicio, M.R. Antimicrobial Drug Resistance and Molecular Typing of Salmonella enterica Serovar Rissen from Different Sources. Microb. Drug Resist. 2016, 22, 211–217. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar] [CrossRef]
- Pornsukarom, S.; Patchanee, P.; Erdman, M.; Cray, P.F.; Wittum, T.; Lee, J.; Gebreyes, W.A. Comparative Phenotypic and Genotypic Analyses of Salmonella Rissen That Originated from Food Animals in Thailand and United States. Zoonoses Public Health 2015, 62, 151–158. [Google Scholar] [CrossRef]
- Egan, D.A.; Naughton, V.; Dooley, J.S.; Naughton, P.J. Detection of Salmonella enterica Serovar Rissen in Slaughter Pigs in Northern Ireland. Adv. Microbiol. 2017, 7, 513–522. [Google Scholar] [CrossRef][Green Version]
- Silveira, L.; Pinto, M.; Isidro, J.; Pista, Â.; Themudo, P.; Vieira, L.; Machado, J.; Gomes, J.P. Multidrug-Resistant Salmonella enterica Serovar Rissen Clusters Detected in Azores Archipelago, Portugal. Int. J. Genom. 2019, 2019, 1860275. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2019–2020. EFSA J. 2022, 20, e07209. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2018/2019. EFSA J. 2021, 19, e06490. [Google Scholar] [CrossRef]
- Di Pilato, V.; Errico, G.; Monaco, M.; Giani, T.; Del Grosso, M.; Antonelli, A.; David, S.; Lindh, E.; Camilli, R.; Aanensen, D.M.; et al. The changing epidemiology of carbapenemase-producing Klebsiella pneumoniae in Italy: Toward polyclonal evolution with emergence of high-risk lineages. J. Antimicrob. Chemother. 2021, 76, 355–361. [Google Scholar] [CrossRef]
- Doumith, M.; Findlay, J.; Hirani, H.; Hopkins, K.L.; Livermore, D.M.; Dodgson, A.; Woodford, N. Major Role of pKpQIL-like Plasmids in the Early Dissemination of KPC-Type Carbapenemases in the UK. J. Antimicrob. Chemother. 2017, 72, 2241–2248. [Google Scholar] [CrossRef]
- Stohr, J.J.J.M.; Kluytmans-Van Den Bergh, M.F.Q.; Weterings, V.A.T.C.; Rossen, J.W.A.; Kluytmans, J.A.J.W. Distinguishing Bla KPC Gene-Containing IncF Plasmids from Epidemiologically Related and Unrelated Enterobacteriaceae Based on Short-and Long-Read Sequence Data. Antimicrob. Agents Chemother. 2021, 65, e00147-21. [Google Scholar] [CrossRef]
- Patil, S.; Liu, X.; Chen, H.; Francisco, N.M.; Wen, F.; Chen, Y. Genetic Characterization of Colistin-Resistant Salmonella enterica ST34 Co-Harbouring Plasmid-Borne mcr-1, blaCTX-M-15 and blaKPC-2 Recovered from a Paediatric Patient in Shenzhen, China. Infect. Drug Resist. 2022, 15, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Miriagou, V.; Tzouvelekis, L.S.; Rossiter, S.; Tzelepi, E.; Angulo, F.J.; Whichard, J.M. Imipenem Resistance in a Salmonella Clinical Strain Due to Plasmid-Mediated Class A Carbapenemase KPC-2. Antimicrob. Agents Chemother. 2003, 47, 1297–1300. [Google Scholar] [CrossRef]
- Melgarejo-T, N.; Martinez, M.; Franco, R.; Falcón, M.; Álvarez, M.; Ortiz, H.; Irala, J. First Isolation of Salmonella Javiana with KPC-2 in Paraguay. Rev. De Salud Publica Del Parag. 2017, 7, 51–56. [Google Scholar] [CrossRef]
- Rodríguez, E.; Bautista, A.; Barrero, L. First Report of a Salmonella enterica Serovar Typhimurium Isolate with Carbapenemase (KPC-2) in Colombia. Antimicrob. Agents Chemother. 2014, 58, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
| Antibiotic | MIC Value | MIC Interpretation * |
|---|---|---|
| Amikacin | >16 | R |
| Ampicillin | >8 | R |
| Amoxicillin–clavulanic acid | >32 | R |
| Ceftazidime | >32 | R |
| Cefotaxime | >32 | R |
| Cefoxitin | 16 | R |
| Colistin | ≤2 | S |
| Ciprofloxacin | ≤0.06 | S |
| Cefepime | >8 | R |
| Ceftazidime/Avibactam | ≤2 | S |
| Nitrofurantoin | ≤64 | S |
| Fosfomycin | ≤16 | S |
| Gentamicin | ≤2 | S |
| Imipenem | 8 | R |
| Meropenem | 4 | I |
| Ertapenem | >1 | R |
| Trimethoprim/sulfamethoxazole | ≤2/38 | S |
| Tigecycline | ≤1 | S |
| Virulence Factors Classes | Virulence Factors | Genes |
|---|---|---|
| Fimbrial adherence determinants | Agf (thin aggregative fimbriae/curli) | csgABCDEFG; steAC |
| Lpf (long polar fimbriae) | lpfABCDE | |
| Type 1 fimbriae | fimCDFHI | |
| Non-fimbrial adherence determinants | SinH | sinH |
| MisL | misL | |
| Iron uptake | Enterobactin | entCDEFHS; fepABCDG |
| Magnesium uptake | Magnesium uptake/transporter | mgtABCLRS |
| Iron and manganese transport | Periplasmic-binding protein | sitABCD |
| Macrophage inducible gene | Antimicrobial peptide resistance protein Mig-14 | mig14 |
| Motility | Flagella | cheABYWRVZ; flgABCDEFGHIJKL |
| Secretion system | T3SS (SPI-1 encoded) | invACEFGHR; orgABC; prgHIJK; sicAP; sipABCD |
| T3SS-1 translocated effectors | avrA; sspABH2; sopABDF | |
| T3SS (SPI-2 encoded) | ssaUTSRQPONVMLKJIGDE; sseABCDE; sscAB | |
| T3SS-2 translocated effectors | pipABB2; sifAB; sopD2E2; sseFGJ | |
| Serum resistance | OmpA (outer membrane protein A) | ompA |
| Others | Lipooligosaccharide | gmhB/lpcA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortini, D.; García-Fernández, A.; Lucarelli, C.; Dionisi, A.M.; Arena, S.; Owczarek, S.; Equestre, M.; Carattoli, A.; Sacco, F.; Rossi, S.; et al. Isolation and Characterisation of Human-Derived blaKPC-3-Producing Salmonella enterica Serovar Rissen in 2018. Antibiotics 2023, 12, 1377. https://doi.org/10.3390/antibiotics12091377
Fortini D, García-Fernández A, Lucarelli C, Dionisi AM, Arena S, Owczarek S, Equestre M, Carattoli A, Sacco F, Rossi S, et al. Isolation and Characterisation of Human-Derived blaKPC-3-Producing Salmonella enterica Serovar Rissen in 2018. Antibiotics. 2023; 12(9):1377. https://doi.org/10.3390/antibiotics12091377
Chicago/Turabian StyleFortini, Daniela, Aurora García-Fernández, Claudia Lucarelli, Anna Maria Dionisi, Sergio Arena, Slawomir Owczarek, Michele Equestre, Alessandra Carattoli, Federica Sacco, Stefano Rossi, and et al. 2023. "Isolation and Characterisation of Human-Derived blaKPC-3-Producing Salmonella enterica Serovar Rissen in 2018" Antibiotics 12, no. 9: 1377. https://doi.org/10.3390/antibiotics12091377
APA StyleFortini, D., García-Fernández, A., Lucarelli, C., Dionisi, A. M., Arena, S., Owczarek, S., Equestre, M., Carattoli, A., Sacco, F., Rossi, S., Ortenzi, R., Primavilla, S., & Villa, L. (2023). Isolation and Characterisation of Human-Derived blaKPC-3-Producing Salmonella enterica Serovar Rissen in 2018. Antibiotics, 12(9), 1377. https://doi.org/10.3390/antibiotics12091377







