In Vitro Evaluation of Antiviral Activities of Funicone-like Compounds Vermistatin and Penisimplicissin against Canine Coronavirus Infection
Abstract
1. Introduction
2. Results
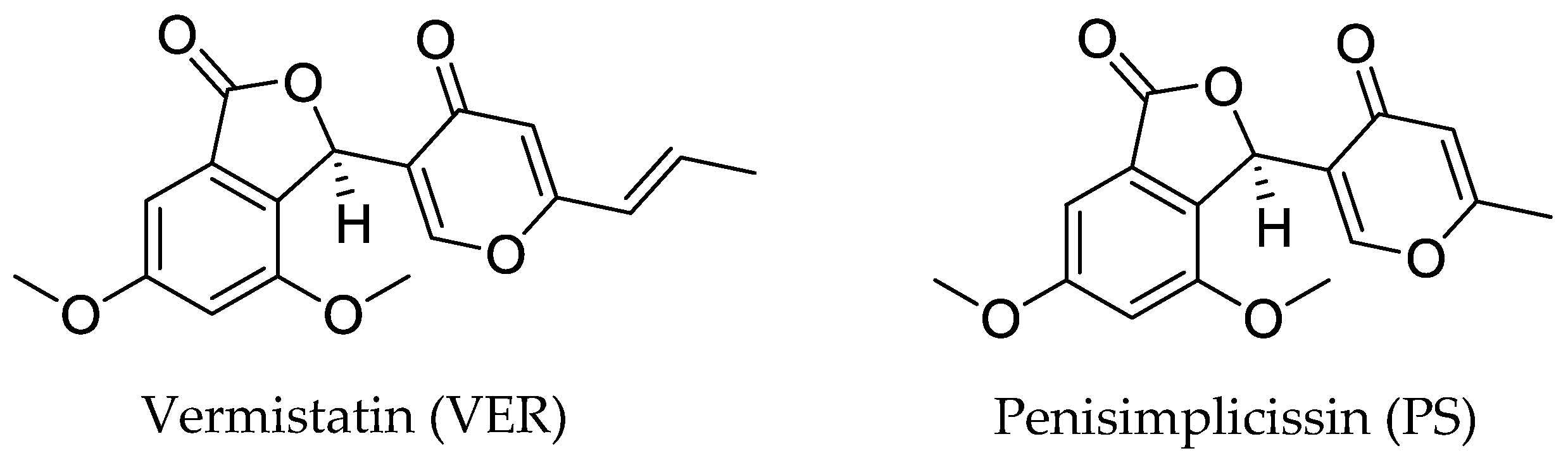
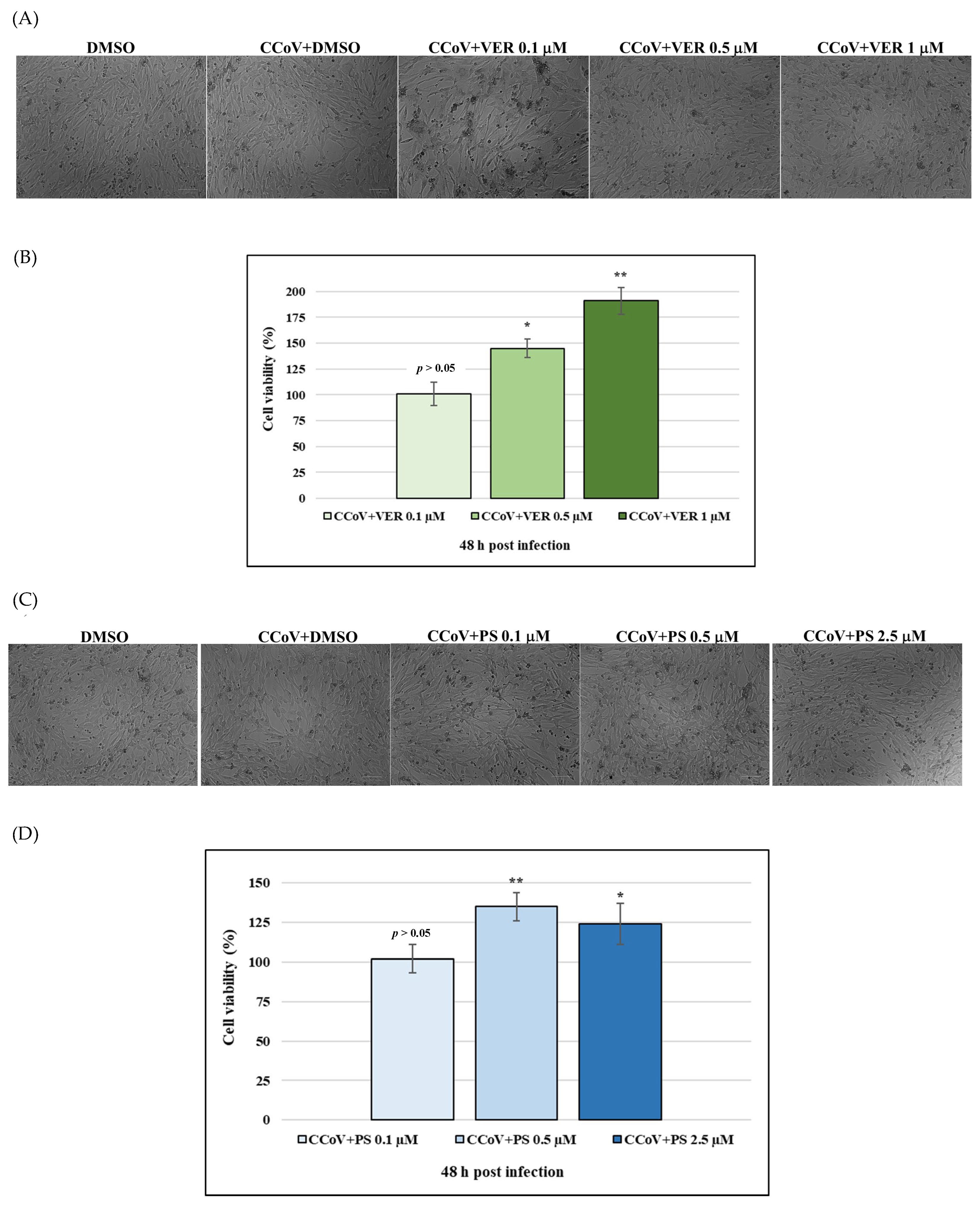
2.1. Funicone-like Compounds VER and PS Have Antiviral Activity against CCoV Infection
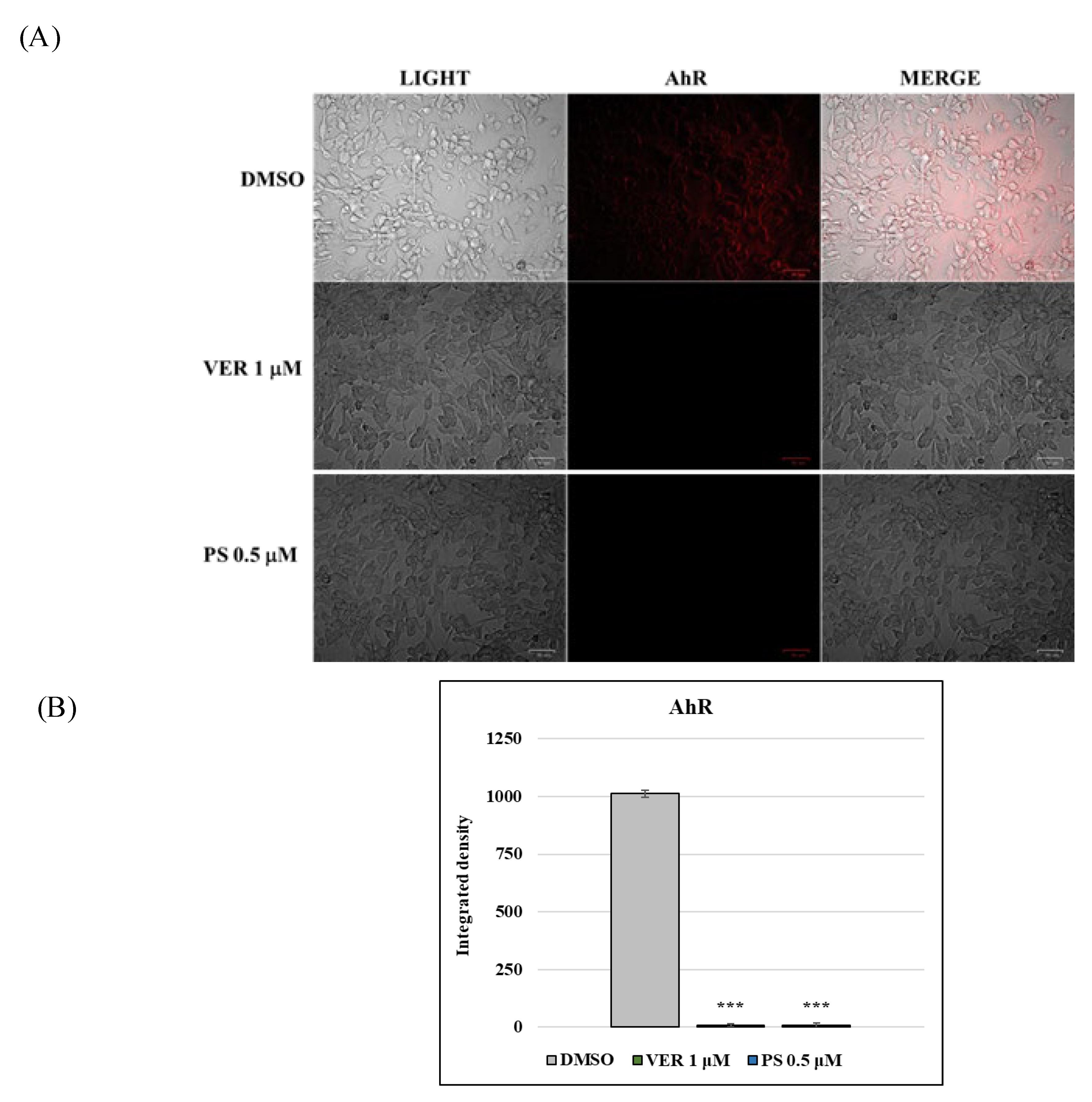
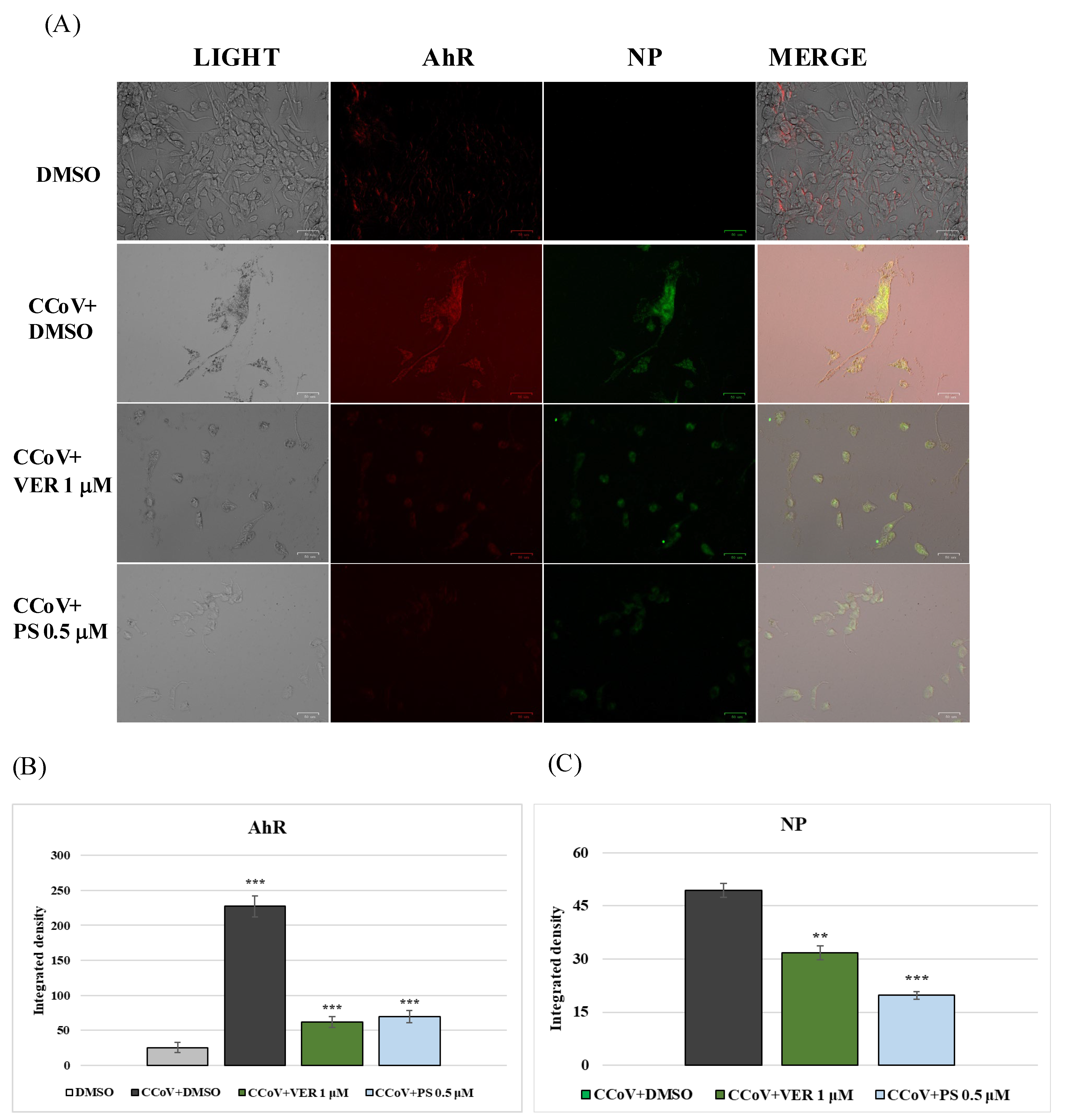
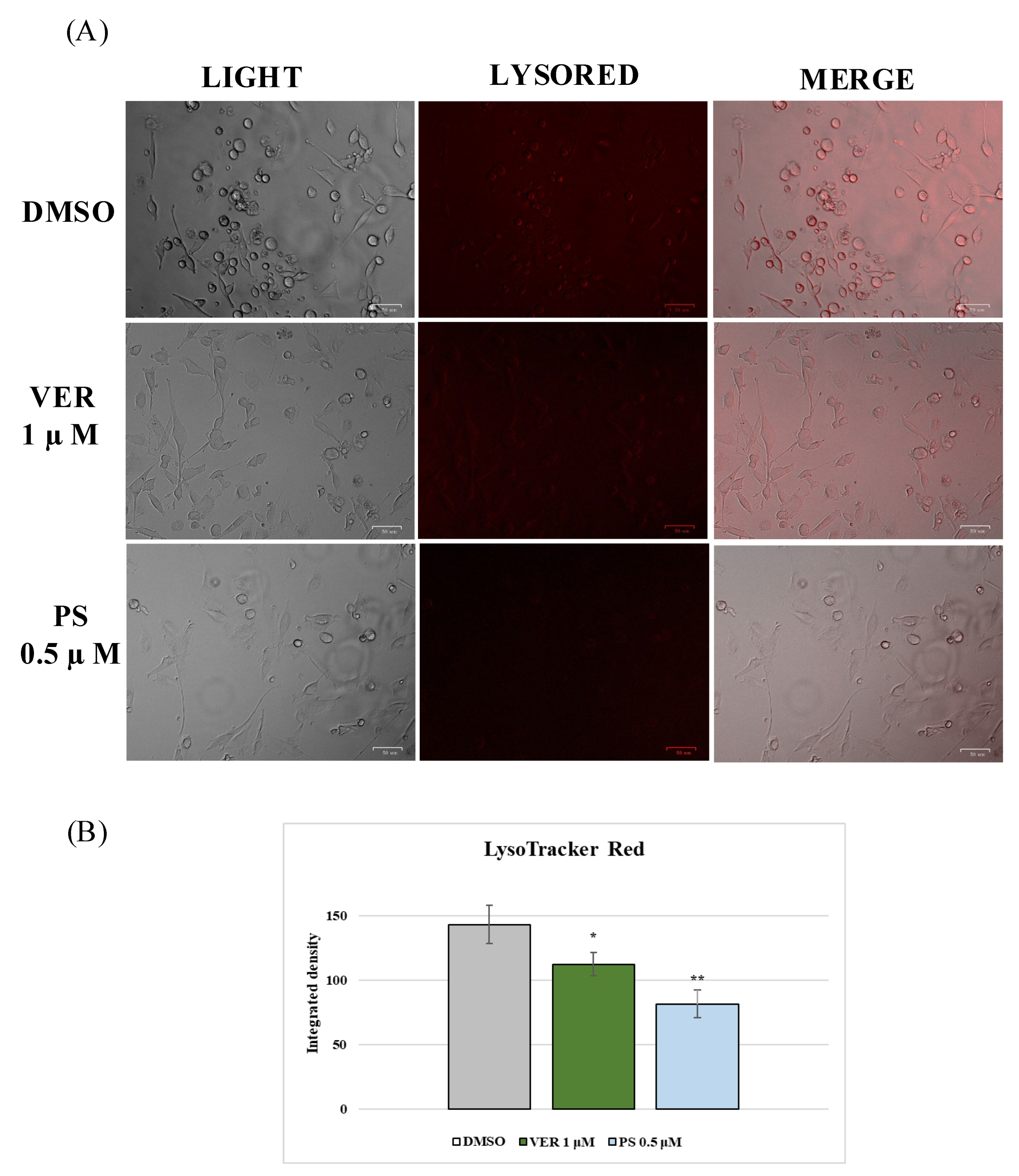
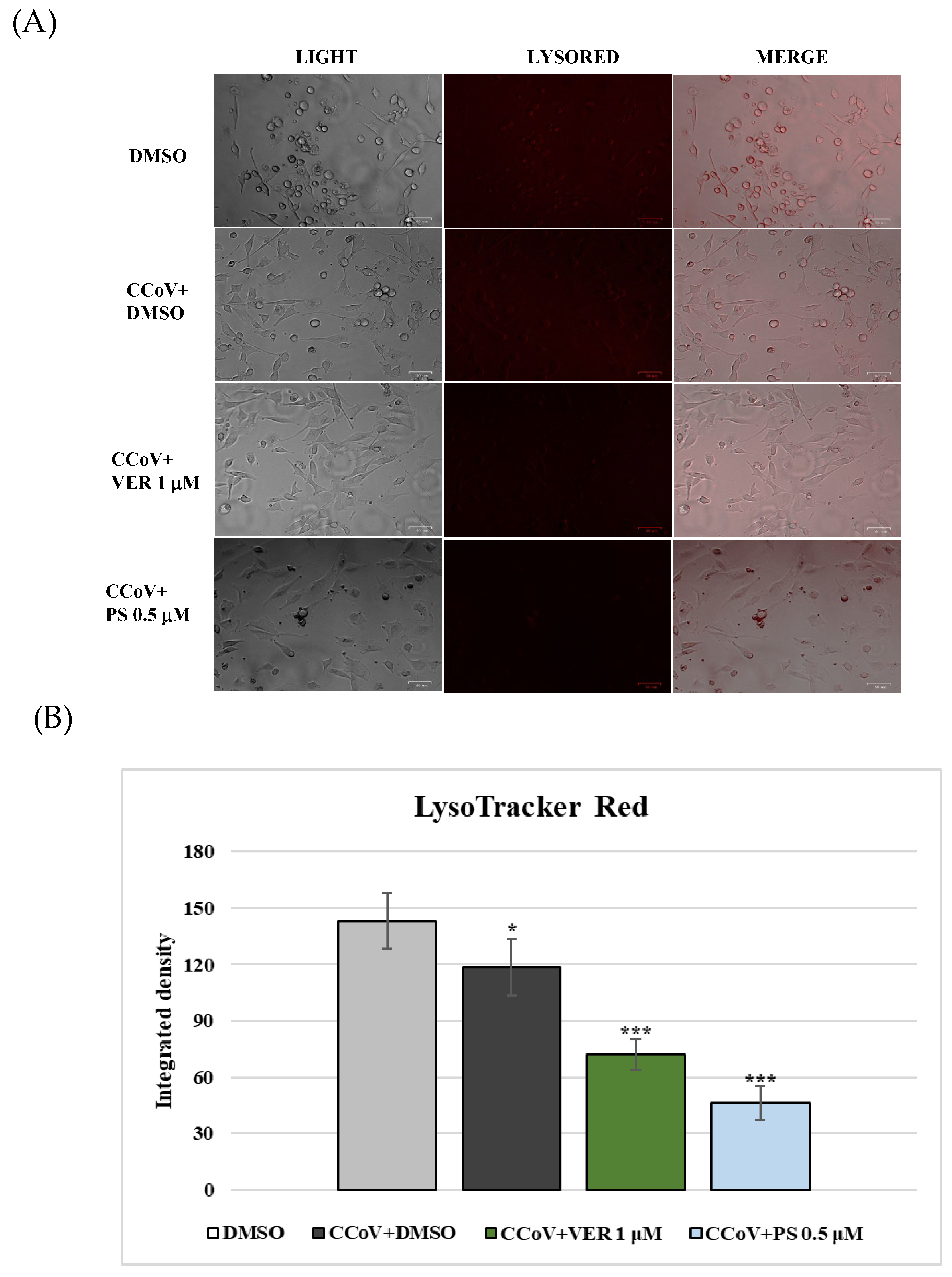
2.2. AhR and Lysosomes Are Involved in Anti-CCoV Activity of VER and PS
3. Discussion
4. Materials and Methods
4.1. Production and Isolation of Funicone-like Compounds
4.2. Cell Cultures and Virus Infection
4.3. Cell Viability
4.4. Examination of Cell Morphology
4.5. LysoRed Staining
4.6. Immunofluorescence (IF) Staining
4.7. Virus Production
4.8. Viral Nucleic Acids Extraction Procedures
4.9. Real-Time RT-PCR for CCoV Quantification
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bills, G.F.; Gloer, J.B. Biologically active secondary metabolites from the fungi. Fungal Kingd. 2017, 4, 1087–1119. [Google Scholar]
- Chepkirui, C.; Stadler, M. The genus Diaporthe: A rich source of diverse and bioactive metabolites. Mycol. Prog. 2017, 16, 477–494. [Google Scholar] [CrossRef]
- Toghueo, R.M.K. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism-from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar]
- Shankar, A.; Sharma, K.K. Fungal secondary metabolites in food and pharmaceuticals in the era of multi-omics. Appl. Microbiol. Biotechnol. 2022, 106, 3465–3488. [Google Scholar]
- Nicoletti, R.; Bellavita, R.; Falanga, A. The outstanding chemodiversity of marine-derived Talaromyces. Biomol. 2023, 13, 1021. [Google Scholar]
- Zhai, M.M.; Li, J.; Jiang, C.X.; Shi, Y.P.; Di, D.L.; Crews, P.; Wu, Q.X. The bioactive secondary metabolites from Talaromyces species. Nat. Prod. Bioprospect. 2016, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Andolfi, A.; Salvatore, M.M. Endophytic fungi of the genus Talaromyces and plant health. In Microbial Endophytes and Plant Growth; Accademic Press: London, UK, 2023; pp. 183–213. ISBN 978-0-323-90620-3. [Google Scholar]
- Lei, L.R.; Gong, L.Q.; Jin, M.Y.; Wang, R.; Liu, R.; Gao, J.; Liu, M.D.; Huang, L.; Wang, G.Z.; Wang, D.; et al. Research advances in the structures and biological activities of secondary metabolites from Talaromyces. Front. Microbiol. 2022, 13, 984801. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; DellaGreca, M.; Andolfi, A. New insights into chemical and biological properties of funicone-like compounds. Toxins 2022, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Komai, S.; Hosoe, T.; Itabashi, T.; Nozawa, K.; Okada, K.; de Campos Takaki, G.M.; Chikamori, M.; Yaguchi, T.; Fukushima, K.; Miyaji, M.; et al. A new funicone derivative isolated from Talaromyces flavus IFM52668. Mycotoxins 2004, 54, 15–19. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhuang, Y.; Kong, F.; Zhang, C.; Zhu, W. Phenolic polyketides from the co-cultivation of marine-derived Penicillium sp. WC-29-5 and Streptomyces fradiae 007. Mar. Drugs 2014, 12, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Komai, S.I.; Hosoe, T.; Itabashi, T. New vermistatin derivatives isolated from Penicillium simplicissimum. Heterocycles 2005, 65, 2771–2776. [Google Scholar]
- Xia, X.K.; Huang, H.R.; She, Z.G.; Cai, J.W.; Lan, L.; Zhang, J.Y.; Fu, L.W.; Vrijmoed, L.L.P.; Lin, Y.C. Structural and biological properties of vermistatin and two new vermistatin derivatives isolated from the marine-mangrove endophytic Guignardia sp. No. 4382. Helv. Chim. Acta 2007, 90, 1925–1931. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Tang, D.Q.; Zhang, F.; Wang, H.Y.; Chen, G.Y. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JS1-2. J. Antibiot. 2019, 72, 779–782. [Google Scholar]
- Nakajima, S.; Watashi, K.; Kamisuki, S.; Tsukuda, S.; Takemoto, K.; Matsuda, M.; Suzuki, R.; Aizaki, H.; Sugawara, F.; Wakita, T. Specific inhibition of hepatitis C virus entry into host hepatocytes by fungi-derived sulochrin and its derivatives. Biochem. Biophys. Res. Commun. 2013, 440, 515–520. [Google Scholar] [CrossRef]
- Fiorito, F.; Cerracchio, C.; Salvatore, M.M.; Serra, F.; Pucciarelli, A.; Amoroso, M.G.; Nicoletti, R.; Andolfi, A. Antiviral property of the fungal metabolite 3-O-methylfunicone in bovine Herpesvirus 1 infection. Microorganisms 2022, 10, 188. [Google Scholar] [CrossRef]
- Cerracchio, C.; Iovane, V.; Salvatore, M.M.; Amoroso, M.G.; Dakroub, H.; DellaGreca, M.; Nicoletti, R.; Andolfi, A.; Fiorito, F. Effectiveness of the fungal metabolite 3-O-methylfunicone towards canine coronavirus in a canine fibrosarcoma cell line (A72). Antibiotics 2022, 11, 1594. [Google Scholar] [CrossRef]
- Pratelli, A. Genetic evolution of canine coronavirus and recent advances in prophylaxis. Vet. Res. 2006, 37, 191–200. [Google Scholar] [CrossRef][Green Version]
- Pratelli, A.; Tempesta, M.; Elia, G.; Martella, V.; Decaro, N.; Buonavoglia, C. The knotty biology of canine coronavirus: A worrying model of coronaviruses’ danger. Res. Vet. Sci. 2022, 144, 190–195. [Google Scholar] [CrossRef]
- Buonavoglia, C.; Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Desario, C.; Castagnaro, M.; Tempesta, M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006, 12, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Cordonnier, N.; Demeter, Z.; Egberink, H.; Elia, G.; Grellet, A.; Le Poder, S.; Mari, V.; Martella, V.; Ntafis, V.; et al. European surveillance for pantropic canine coronavirus. J. Clin. Microbiol. 2013, 51, 83–88. [Google Scholar] [CrossRef]
- Alfano, F.; Fusco, G.; Mari, V.; Occhiogrosso, L.; Miletti, G.; Brunetti, R.; Galiero, G.; Desario, C.; Cirilli, M.; Decaro, N. Circulation of pantropic canine coronavirus in autochthonous and imported dogs, Italy. Transbound. Emerg. Dis. 2020, 67, 1991–1999. [Google Scholar]
- Vlasova, A.N.; Diaz, A.; Damtie, D.; Xiu, L.; Toh, T.H.; Lee, J.S.Y.; Saif, L.J.; Gray, G.C. Novel canine coronavirus isolated from a hospitalized patient with pneumonia in East Malaysia. Clin. Infect. Dis. 2022, 74, 446–454. [Google Scholar]
- Lednicky, J.A.; Tagliamonte, M.S.; White, S.K.; Blohm, G.M.; Alam, M.M.; Iovine, N.M.; Salemi, M.; Mavian, C.; Morris, J.G. Isolation of a novel recombinant canine coronavirus from a visitor to Haiti: Further evidence of transmission of coronaviruses of zoonotic origin to humans. Clin. Infect. Dis. 2022, 75, e1184–e1187. [Google Scholar] [PubMed]
- Vlasova, A.N.; Toh, T.H.; Lee, J.S.Y.; Poovorawan, Y.; Davis, P.; Azevedo, M.S.P.; Lednicky, J.A.; Saif, L.J.; Gray, G.C. Animal alphacoronaviruses found in human patients with acute respiratory illness in different countries. Emerg. Microbes Infect. 2022, 11, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, A.; Tempesta, M.; Elia, G.; Martella, V.; Decaro, N.; Buonavoglia, C.; Buonavoglia, A.; Lanave, G.; Tempesta, M.; Camero, M.; et al. One world, one health, one virology of the mysterious labyrinth of coronaviruses: The canine coronavirus affair. Res. Vet. Sci. 2021, 2, e646–e647. [Google Scholar]
- Salvatore, M.M.; DellaGreca, M.; Nicoletti, R.; Salvatore, F.; Vinale, F.; Naviglio, D.; Andolfi, A. Talarodiolide, a new 12-membered macrodiolide, and GC/MS investigation of culture filtrate and mycelial extracts of Talaromyces pinophilus. Molecules 2018, 23, 950. [Google Scholar] [CrossRef]
- Bank, H.L. Assessment of islet cell viability using fluorescent dyes. Diabetologia 1987, 30, 812–816. [Google Scholar] [CrossRef]
- Cerracchio, C.; Serra, F.; Amoroso, M.G. Canine coronavirus activates aryl hydrocarbon receptor during in vitro infection. Viruses 2022, 14, 2437. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses: Methods and Protocols; Humana: New York, NY, USA, 2015; pp. 1–23. ISBN 978-1-4939-2437-0. [Google Scholar]
- Kenney, S.P.; Wang, Q.; Vlasova, A.; Jung, K.; Saif, L. Naturally occurring animal coronaviruses as models for studying highly pathogenic human coronaviral disease. Vet. Pathol. 2021, 58, 438–452. [Google Scholar] [PubMed]
- Torti, M.F.; Giovannoni, F.; Quintana, F.J.; García, C.C. The aryl hydrocarbon receptor as a modulator of anti-viral immunity. Front. Immunol. 2021, 12, 624293. [Google Scholar] [PubMed]
- Yang, T.; Feng, Y.L.; Chen, L.; Vaziri, N.D.; Zhao, Y.Y. Dietary natural flavonoids treating cancer by targeting aryl hydrocarbon receptor. Crit. Rev. Toxicol. 2019, 49, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, T. Hypothesis: Emerging roles for aryl hydrocarbon receptor in orchestrating CoV-2-related inflammation. Cells 2022, 11, 648. [Google Scholar]
- Tang, B.S.F.; Chan, K.H.; Cheng, V.C.C.; Yuen, K.Y. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by SARS coronavirus and human coronavirus 229E. Hong Kong Med. J. 2005, 15, 23–26. [Google Scholar]
- Grunewald, M.E.; Shaban, M.G.; Mackin, S.R.; Fehr, A.R.; Perlman, S. Murine coronavirus infection activates the aryl hydrocarbon receptor in an indoleamine 2,3-dioxygenase-independent manner, contributing to cytokine modulation and proviral TCDD-inducible-PARP expression. J. Virol. 2020, 94, e01743-19. [Google Scholar] [CrossRef]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Dávola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef]
- Zhao, L.; Yao, L.; Chen, R.; He, J.; Lin, T.; Qiu, S.; Chen, G.; Chen, H.; Qiu, S.X. Pinosrtobin from plants and propolis against human coronavirus HCoV-OC43 by modulating host AHR/CYP1A1 pathway and lipid metabolism. Antivir. Res. 2023, 212, 105570. [Google Scholar] [CrossRef]
- Shi, J.; Du, T.; Wang, J.; Tang, C.; Lei, M.; Yu, W.; Yang, Y.; Ma, Y.; Huang, P.; Chen, H.; et al. Aryl hydrocarbon receptor is a proviral host factor and a candidate pan-SARS-CoV-2 therapeutic target. Sci. Adv. 2023, 9, eadf0211. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, P.; Huang, Y.W. Lysosomal ion channels involved in cellular entry and uncoating of enveloped viruses: Implications for therapeutic strategies against SARS-CoV-2. Cell Calcium 2021, 94, 102360. [Google Scholar]
- Pereira, G.J.d.S.; Leão, A.H.F.F.; Erustes, A.G.; Morais, I.B.d.M.; Vrechi, T.A.d.M.; Zamarioli, L.D.S.; Pereira, C.A.S.; Marchioro, L.d.O.; Sperandio, L.P.; Lins, Í.V.F.; et al. Pharmacological modulators of autophagy as a potential strategy for the treatment of COVID-19. Int. J. Mol. Sci. 2021, 22, 4067. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell 2020, 183, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ladilov, Y. Targeting the sAC-dependent cAMP pool to prevent SARS-Cov-2 infection. Cells 2020, 9, 1962. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, K.; Chen, C.Z.; Bostwick, R.; Rasmussen, L.; Tran, B.N.; Cheng, Y.S.; Xu, M.; Pradhan, M.; Henderson, M.; Zhu, W.; et al. The SARS-CoV-2 cytopathic effect is blocked by lysosome alkalizing small molecules. ACS Infect. Dis. 2021, 7, 1389–1408. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Luo, D.; Liao, H.; Li, S. Coronavirus usurps the autophagy-lysosome pathway and induces membranes rearrangement for infection and pathogenesis. Front. Microbiol. 2022, 13, 846543. [Google Scholar] [CrossRef] [PubMed]
- Blaess, M.; Kaiser, L.; Sommerfeld, O.; Csuk, R.; Deigner, H.P. Drugs, metabolites, and lung accumulating small lysosomotropic molecules: Multiple targeting impedes SARS-COV-2 infection and progress to COVID-19. Int. J. Mol. Sci. 2021, 22, 1797. [Google Scholar]
- Sun, M.H.; Li, X.H.; Xu, Y.; Xu, Y.; Pan, Z.N.; Sun, S.C. Citrinin exposure disrupts organelle distribution and functions in mouse oocytes. Environ. Res. 2020, 185, 109476. [Google Scholar] [CrossRef]
- Alessandrini, F.; Pezzè, L.; Ciribilli, Y. LAMPs: Shedding light on cancer biology. Semin. Oncol. 2017, 44, 239–253. [Google Scholar]
- Wang, Y.; Xing, C.H.; Chen, S.; Sun, S.C. Zearalenone exposure impairs organelle function during porcine oocyte meiotic maturation. Theriogenology 2022, 177, 22–28. [Google Scholar] [CrossRef]
- Gao, W.; Jiang, L.; Ge, L.; Chen, M.; Geng, C.; Yang, G.; Li, Q.; Ji, F.; Yan, Q.; Zou, Y.; et al. Sterigmatocystin-induced oxidative DNA damage in human liver-derived cell line through lysosomal damage. Toxicol. Vitr. 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Jiang, L.P.; Liu, X.F.; Wang, D.; Yang, G.; Geng, C.Y.; Li, Q.; Zhong, L.F.; Sun, Q.; Chen, M. The role of oxidative stress in citreoviridin-induced DNA damage in human liver-derived HepG2 cells. Environ. Toxicol. 2015, 30, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Connolly, L.; Frizzell, C.; Elliott, C.T. Cytotoxic assessment of the regulated, co-existing mycotoxins aflatoxin B1, fumonisin B1 and ochratoxin, in single, binary and tertiary mixtures. Toxicon 2014, 90, 70–81. [Google Scholar] [PubMed]
- Binn, L.N.; Marchwicki, R.H.; Stephenson, E.H. Establishment of a canine cell line: Derivation, characterization, and viral spectrum. Am. J. Vet. Res. 1980, 41, 855–860. [Google Scholar] [PubMed]
- De Martino, L.; Marfé, G.; Longo, M.; Fiorito, F.; Montagnaro, S.; Iovane, V.; Decaro, N.; Pagnini, U. Bid cleavage, cytochrome c release and caspase activation in canine coronavirus-induced apoptosis. Vet. Microbiol. 2010, 141, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Marfè, G.; Tafani, M.; Fiorito, F.; Pagnini, U.; Iovane, G.; de Martino, L. Involvement of FOXO transcription factors, TRAIL-FasL/Fas, and Sirtuin proteins family in canine coronavirus type II-induced apoptosis. PLoS ONE 2011, 6, e27313. [Google Scholar]
- Ruggieri, A.; Di Trani, L.; Gatto, I.; Franco, M.; Vignolo, E.; Bedini, B.; Elia, G.; Buonavoglia, C. Canine coronavirus induces apoptosis in cultured cells. Vet. Microbiol. 2007, 121, 64–72. [Google Scholar] [CrossRef]
- Fiorito, F.; Marfè, G.; Granato, G.E.; Ciarcia, R.; De Blasio, E.; Tafani, M.; Florio, S.; De Martino, L.; Muzi, G.; Pagnini, U.; et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modifies expression and nuclear/cytosolic localization of bovine herpesvirus 1 immediate-early protein (bICP0) during infection. J. Cell. Biochem. 2010, 111, 333–342. [Google Scholar] [CrossRef]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimarães, J.E. Critical evaluation of techniques to detect and measure cell death—Study in a model of UV radiation of the leukaemic cell line HL60. Anal. Cell. Pathol. 1999, 19, 139–151. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar]
- Zakeri, Z.; Lockshin, R.A. Cell death: History and future. Adv. Exp. Med. Biol. 2008, 615, 1–11. [Google Scholar]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Sulforaphane alleviates cadmium-induced toxicity in human mesenchymal stem cells through POR and TNFSF10 genes expression. Biomed. Pharmacother. 2019, 115, 108896. [Google Scholar] [PubMed]
- Altamura, G.; Power, K.; Martano, M.; degli Uberti, B.; Galiero, G.; De Luca, G.; Maiolino, P.; Borzacchiello, G. Felis catus papillomavirus type-2 E6 binds to E6AP, promotes E6AP/p53 binding and enhances p53 proteasomal degradation. Sci. Rep. 2018, 8, 17529. [Google Scholar] [PubMed]
- Tofani, S.; Ianiro, G.; De Sabato, L.; Monini, M.; Angeloni, G.; Ponterio, E.; D’Agostino, C.; Di Bari, M.A.; Valeri, M.; Di Bartolo, I. Detection and whole genome sequencing of murine norovirus in animal facility in Italy. Anim. Biotechnol. 2021, 33, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Baert, L.; Wobus, C.E.; Van Coillie, E.; Thackray, L.B.; Debevere, J.; Uyttendaele, M. Detection of murine norovirus 1 by using plaque assay, transfection assay, and real-time reverse transcription-PCR before and after heat exposure. Appl. Environ. Microbiol. 2008, 74, 543–546. [Google Scholar]
- Amoroso, M.G.; Serra, F.; Esposito, C.; D’alessio, N.; Ferrara, G.; Cioffi, B.; Anzalone, A.; Pagnini, U.; De Carlo, E.; Fusco, G.; et al. Prevalence of infection with porcine circovirus types 2 and 3 in the wild boar population in the campania region (Southern italy). Animals 2021, 11, 3215. [Google Scholar] [CrossRef]
- Decaro, N.; Elia, G.; Martella, V.; Campolo, M.; Mari, V.; Desario, C.; Lucente, M.S.; Lorusso, E.; Kanellos, T.; Gibbons, R.H.; et al. Immunity after natural exposure to enteric canine coronavirus does not provide complete protection against infection with the new pantropic CB/05 strain. Vaccine 2010, 28, 724–729. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerracchio, C.; Salvatore, M.M.; Del Sorbo, L.; Serra, F.; Amoroso, M.G.; DellaGreca, M.; Nicoletti, R.; Andolfi, A.; Fiorito, F. In Vitro Evaluation of Antiviral Activities of Funicone-like Compounds Vermistatin and Penisimplicissin against Canine Coronavirus Infection. Antibiotics 2023, 12, 1319. https://doi.org/10.3390/antibiotics12081319
Cerracchio C, Salvatore MM, Del Sorbo L, Serra F, Amoroso MG, DellaGreca M, Nicoletti R, Andolfi A, Fiorito F. In Vitro Evaluation of Antiviral Activities of Funicone-like Compounds Vermistatin and Penisimplicissin against Canine Coronavirus Infection. Antibiotics. 2023; 12(8):1319. https://doi.org/10.3390/antibiotics12081319
Chicago/Turabian StyleCerracchio, Claudia, Maria Michela Salvatore, Luca Del Sorbo, Francesco Serra, Maria Grazia Amoroso, Marina DellaGreca, Rosario Nicoletti, Anna Andolfi, and Filomena Fiorito. 2023. "In Vitro Evaluation of Antiviral Activities of Funicone-like Compounds Vermistatin and Penisimplicissin against Canine Coronavirus Infection" Antibiotics 12, no. 8: 1319. https://doi.org/10.3390/antibiotics12081319
APA StyleCerracchio, C., Salvatore, M. M., Del Sorbo, L., Serra, F., Amoroso, M. G., DellaGreca, M., Nicoletti, R., Andolfi, A., & Fiorito, F. (2023). In Vitro Evaluation of Antiviral Activities of Funicone-like Compounds Vermistatin and Penisimplicissin against Canine Coronavirus Infection. Antibiotics, 12(8), 1319. https://doi.org/10.3390/antibiotics12081319









