Engineering 3D-Printed Advanced Healthcare Materials for Periprosthetic Joint Infections
Abstract
1. Introduction
2. Periprosthetic Joint Infections
3. Parenteral Locally Applied Implants
- Preformed implants, also known as solid implants. They must be placed through a surgical procedure and can be biodegradable or non-biodegradable. The latter requires another surgery for removal. Their main advantage is their capacity for long-term and sustained-release drug delivery. Release time can be controlled by the material and the drug-loading technique utilized, such as coating or encapsulation [14,15,35].
- In situ-forming implants consist of liquids or semisolids in which the drug is dispersed or dissolved. After SC or IM implantation through a needle, it turns into a solid reservoir at the injection site. Compared to preformed implants, these IDDSs are easier to manufacture and administer, being less painful for patients. In situ-forming implants can be divided into three different groups: in situ cross-linked polymer systems, in situ polymer precipitation, and thermally induced gelling systems [14,15,35].
- (i)
- (ii)
- Controlled drug delivery via a diffusion process. In this case, the drug diffuses from the core of the implant towards the medium in which it is implanted. The drug release is not easily regulated or modified after implantation as this is a passive diffusion process. For this reason, it is key to evaluate the initial parameters such as the material chosen, the implant shape and geometry, and the drug formulation; all of these in combination will dictate the final drug release kinetics [9,14,35,37].
- (iii)
4. Three-Dimensional Printing Technologies
4.1. Vat Photopolymerization
4.2. Binder Jetting
4.3. Material Extrusion
- Fused deposition modeling (FDM), where a heater on the material reservoir melts the material. Its advantages include the potential for low-cost manufacturing, the ability to employ any powdered feedstock, and a high build rate in comparison to other 3D printing processes. However, pellets or powders can also be used for FDM. This 3D printing technique is widely used due to the possibility of creating complex structures, which makes it ideal for complex scaffolds or formulations combining different release profiles and the high quality, speed, and reduced cost of the printing process [52].
- Pressure-assisted microsyringe (PAM) or semi-solid extrusion (SSE), consisting of a syringe extruder for depositing viscous or semi-liquid material. The extrusion is achieved via the action of a pressurized-air or mechanical piston. The key factors in PAM 3D printing are the viscosity, viscoelasticity, and apparent elastic limit of the materials [53].
4.4. Powder Bed Fusion
5. Materials
5.1. Polymers
5.2. Photopolymers
5.3. Metals
| Metal | Alloying Elements | Advantages | Fabrication Techniques | Application | Ref |
|---|---|---|---|---|---|
| Titanium | Al, Nb, V | Corrosion resistance, high specific strength, low density, microarchitecture, osteointegration | SLM, EBM, SLS | Joint replacement, dental implants, fracture fixation, spinal fusion implants, spinal disc replacements | [91,97,98] |
| Stainless Steel | Mn, Ni, Ti, Si, Mo, Se, Cr, N | Mechanical strength, non-magnetic, corrosion resistance, fatigue strength | SLM, SLS, binder jetting | Artificial bone, artificial joints, dental implants, fracture fixation, stents, hip stems, spinal implants, cables | [91,97,99] |
| Iron | Mn, Pd | Ease of manufacturing, mechanical reliability, high fracture strength, high ductility, and high hardness | Binder jetting, extrusion-based 3DP, SLM | Temporary cardiovascular stents and bone tissue engineering | [97] |
| Magnesium | Al, Zn, Mn, Y, Nd | Biodegradable, mechanical properties similar to human bone and fast degradation | SLM, WAAM, binder jetting, extrusion-based deposition | Orthopedic applications, cardiovascular stents, and bone tissue engineering | [91,97] |
| Zinc | Mg, Al, Sr | Biodegradable | FDM | Wound closure devices, orthopedic devices, and cardiovascular stents | [97] |
| Cobalt | Cr, Fe, Ni, Si, Mg, Mo | Mechanical strength, durability, corrosion resistance, fatigue strength, wear resistance | SLM | Joint replacements, stents, pacemaker conductor wires, spinal disc replacements, dental bridgework | [91,97,99] |
5.4. Natural Materials
5.5. Ceramics
6. Three-Dimensionally Printed IDDSs for PJIs
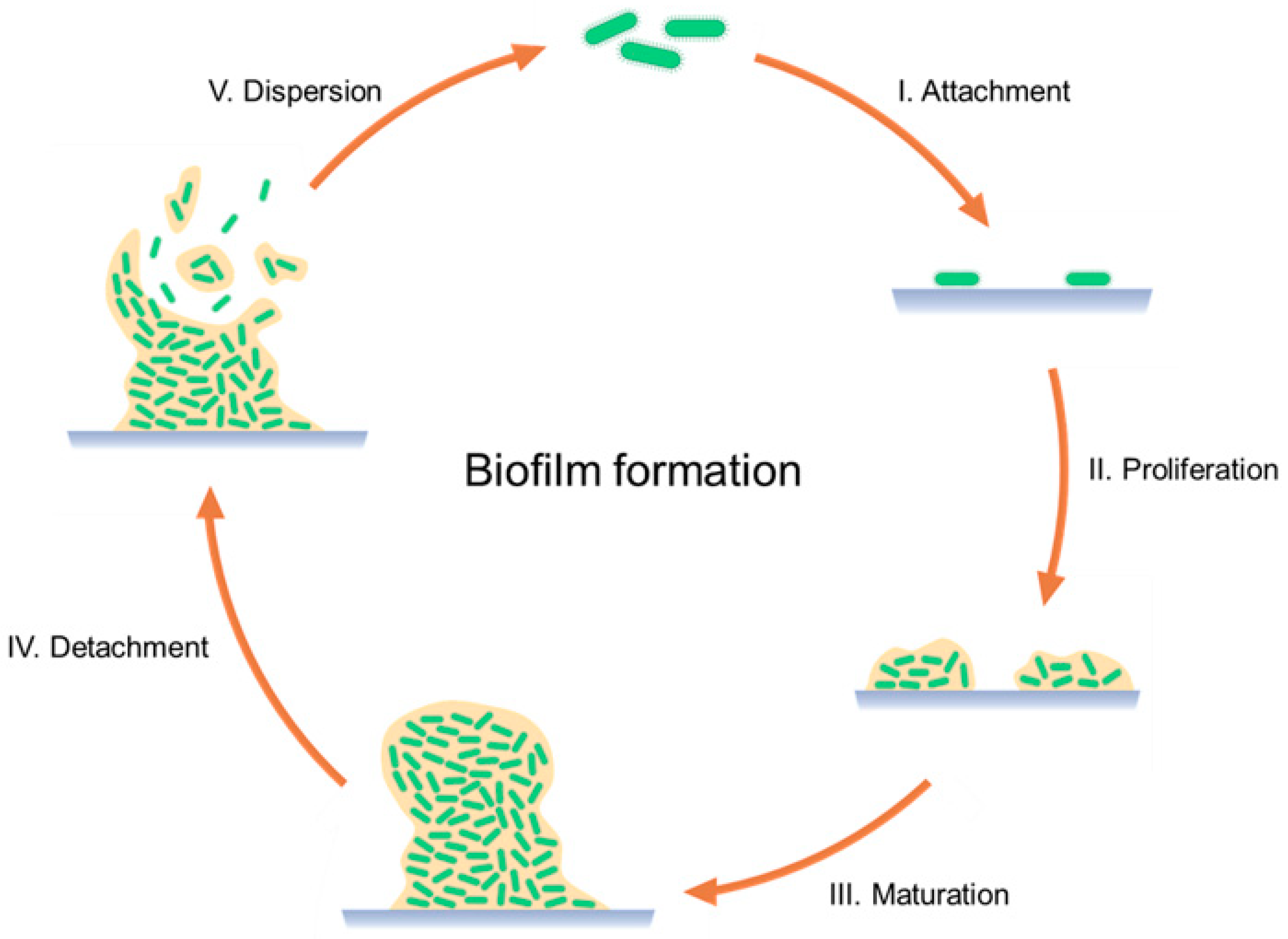
- The use of 3D-printed scaffolds loaded or coated with antimicrobials, and usually in combination with bone regeneration treatments (Table 6). Inzana et al. [123] compared the antibiotic delivery efficacy of rifampicin- and vancomycin-laden calcium phosphate scaffolds with poly(methyl methacrylate) (PMMA) bone cement, one of the traditional treatments of PIJs. The scaffolds via 3D-printed with binder jetting and showed a higher reduction in pathogenic burden and osteolytic bone resorption. Deng et al. [124] used FDM for the fabrication of polyetheretherketone (PEEK) scaffolds with Ag-modified surfaces. PEEK is a substitute material for bone regeneration, and in combination with Ag nanoparticles, showed optimal osteoblast adhesion and differentiation combined with an antimicrobial effect. Poly-L-lactic acid (PLLA)/ pearl scaffolds, printed using PAM, were mixed with a solution of rifampicin/moxifloxacin-poly lactic-co-glycolic acid (PLGA) microspheres (RM-P) before printing. The scaffolds promoted bone cell adhesion, proliferation, and differentiation and bone defect repair, and showed an anti-infection effect [125]. Zhou et al. [126] developed a PCL scaffold coated with polydopamine (PDA). The coating was used for the adsorption of PLGA microspheres loaded with vancomycin, exhibiting sustained drug release (>4 weeks) with a high antibacterial effect. Moreover, the PCL/PDA scaffold showed higher cell adhesion and proliferation in comparison with plain PCL scaffolds. Topsakal et al. [127] compared the cytocompatibility and the mechanical and antimicrobial properties of four different types of scaffold: (i) polyvinyl alcohol (PVA); (ii) PVA and gold nanoparticles (AuNP); (iii) PVA and ampicillin (AMP); and (iv) PVA/AuNP/AMP. The best outcomes were obtained using PVA/AuNP/AMP scaffolds, resulting in good biocompatibility, osteoinduction, and antimicrobial properties. Liu et al. [128] used tantalum for developing scaffolds, a material already used in arthroplasty. However, this material does not possess antibacterial properties. Porous tantalum scaffolds with chitosan and vancomycin coatings were developed. This combination was shown to prevent bacterial adhesion and biofilm formation. Additionally, the scaffold structure allowed for the generation of a mineralized matrix and osteogenic gene expression. Yang et al. [129] evaluated antibiofilm hydroxypropyl trimethyl ammonium chloride chitosan (HACC)/HA/PLGA scaffolds. The scaffolds showed optimal antimicrobial and osteoconductive in vitro properties resulting in high anti-infection and bone regeneration capabilities in different infected bone defect models. Zhang et al. [130] used PAM for the fabrication of scaffolds with controlled dual-stage release to achieve an antibacterial effect while promoting bone regeneration. β-TCP and PLGA were used as scaffold materials, combined with loaded graphene oxide nanosheets and an osteogenic peptide (p24), which showed an increase in antibacterial sensitivity and osteogenic differentiation.
- The combination of 3D printing with orthopedic implants by creating porous structures or microchannels inside the implants for drug loading, or with different coating methods (Table 7). Hassanin et al. [58] evaluated the optimal conditions of inner reservoirs in drug-delivering Ti-6Al-4V implants via SLM with different internal reservoirs and releasing microchannels (MC). The best hollow implants were those with an MC of 271 μm in diameter, a horizontal surface roughness of 4.4 μm, a vertical surface roughness (Ra) of 9.2 μm, and 1.4% build porosity. Allen [131] developed cobalt–chrome spacers, 3D-printed via SLM, with different antibiotic-eluting reservoir designs. The geometry of the reservoirs affected the API release profile, which could be modulated, resulting in a reduction in the biofilm formation on the spacer surface. Additionally, the spacers had improved mechanical properties in comparison to PMMA spacers. Kim et al. [132] designed a 3D-printed liner for knee arthroplasty. The material used was PLA as the liner material, with different infills in the 3D printing process for creating reservoirs, which were filled with a solution of tetracycline. This liner showed controllable antibiotic release with improved mechanical properties, characterized by higher strength and less brittleness than PMMA, adapted to the patient’s anatomy. To avoid bacterial adhesion in the porous surface of DMLS titanium implants, Guan et al. [133] added antibacterial multilayers to the surface of the 3D-printed implants. This coating consisted of a first phase-transited lysozyme layer and minocycline-loaded multilayers of HA and CS. This IDDS inhibited bacterial adhesion while preserving osteoblast viability and functionality. Griseti et al. [134] compared the bacterial inhibition of 3D-printed porous titanium, tantalum, antibiotic-loaded bone cement, and a smooth titanium alloy. For drug loading, a soaking solution of vancomycin was used in which implants were soaked for one hour. Three-dimensionally printed porous titanium showed higher bacterial inhibition during the first three days in comparison to the other materials. A photopolymer, photocured rigid polyurethane (RPU 60), was used for 3D printing with CLIP spacers with reservoirs for drug release. The channels were loaded with calcium sulfate embedded with gentamicin. This study showed that the reservoir length, diameter, geometry, and quantity modulated the drug release. The longest drug release and antimicrobial effect were achieved with the smallest diameter (0.5 mm), lowest porosity (one channel per side), and greatest length (7 mm) [135]. Instead of directly 3D printing the implant, Maver et al. [136] printed an antimicrobial coating consisting of a hydrogel made of carboxymethyl cellulose, nanofibrillated cellulose, and alginate with clindamycin to be placed on stainless steel and titanium substrates. The 3D-printed coating presented a uniform distribution of clindamycin, with optimal moisture absorption and biodegradability after 7 days. Moreover, no toxicity in osteoblasts was observed along with the antibacterial effects, with an initial burst release combined with a sustained release. Wu et al. [137] 3D printed an antimicrobial hydrogel of chitosan and gelatine on the surface of titanium implants. This coating layer showed an antimicrobial effect against different species of bacteria. Moreover, the hydrogel coating layer allowed for cell adhesion and bone growth, promoting the osteointegration of the prosthesis.
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seoane-Viano, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef]
- Dodziuk, H. Applications of 3D printing in healthcare. Kardiochirurgia Torakochirurgia Pol. Pol. J. Cardio-Thorac. Surg. 2016, 13, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Cerda, J.R.; Yuste, I.; Luciano, F.C.; Anaya, B.J.; Serrano, D.R. 3D Printing Technologies for Personalized Drug Delivery. In Emerging Drug Delivery and Biomedical Engineering Technologies: Transforming Therapy; Lamprou, D., Ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Konta, A.A.; Garcia-Pina, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef]
- Palo, M.; Hollander, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef]
- Uniformity of Dosage Units. United States Pharmacopeia 24/National Formulary 19; United States Pharmacopeial Convention: Rockville, MD, USA, 1999. [Google Scholar]
- Manchanda, S.; Das, N.; Chandra, A.; Bandyopadhyay, S.; Chaurasia, S. Chapter 2—Fabrication of advanced parenteral drug-delivery systems. In Drug Delivery Systems; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2020; pp. 47–84. [Google Scholar]
- Bose, S. Parenteral Drug Delivery for Older Patients. In Developing Drug Products in an Aging Society; Stegemann, S., Ed.; AAPS Advances in the Pharmaceutical Sciences Series & Springer: Berlin/Heidelberg, Germany, 2016; Volume 26. [Google Scholar]
- Gulati, N.; Gupta, H. Parenteral drug delivery: A review. Recent Pat. Drug Deliv. Formul. 2011, 5, 133–145. [Google Scholar] [CrossRef]
- Broadhead, J.; Gibson, M. Parenteral Dosage Form. In Pharmaceutical Preformulation and Formulation; Gibson, M., Ed.; Informa Healthcare: New York, NY, USA, 2009; Volume 199. [Google Scholar]
- Vhora, I.; Khatri, N.; Misra, A. Applications of Polymers in Parenteral Drug Delivery. In Applications of Polymers in Drug Delivery; Misra, A., Shahiwala, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 221–261. [Google Scholar]
- Tejashree, A.; Ghadge, S.D.; Chavare, D.A.; Kulkarni, S.K. A Review on Parenteral Implants. Int. J. Res. Rev. Pharm. Appl. Sci. 2014, 4, 1056–1072. [Google Scholar]
- Kempe, S.; Mader, K. In situ forming implants—An attractive formulation principle for parenteral depot formulations. J. Control. Release Off. J. Control. Release Soc. 2012, 161, 668–679. [Google Scholar] [CrossRef]
- Available online: https://www.precedenceresearch.com/orthopedic-implant-marke (accessed on 10 March 2023).
- Mühlhofer, H.M.; Deiss, L.; Mayer-Kuckuk, P.; Pohlig, F.; Harrasser, N.; Lenze, U.; Gollwitzer, H.; Suren, C.; Prodinger, P.; VON Eisenhart-Rothe, R.; et al. Increased Resistance of Skin Flora to Antimicrobial Prophylaxis in Patients Undergoing Hip Revision Arthroplasty. In Vivo 2017, 31, 673–676. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebse, R. The EBJIS definition of periprosthetic joint infection. Bone Jt. J. 2021, 103-B, 18–25. [Google Scholar] [CrossRef]
- Blanco, J.F.; Diaz, A.; Melchor, F.R.; da Casa, C.; Pescador, D. Risk factors for periprosthetic joint infection after total knee arthroplasty. Arch. Orthop. Trauma Surg. 2020, 140, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e1483. [Google Scholar] [CrossRef] [PubMed]
- Hip and knee replacement. In Health at a Glance 2017: OECD Indicators; OECD Publishing: Paris, France, 2017.
- Franco, M. Epidemiología de la Infección de Prótesis Articular en España en la última Década. Análisis de la Evolución de la Etiología en el Tiempo. Ph.D. Thesis, Universidad Autónoma de Barcelona, Barcelona, France, 2017. [Google Scholar]
- Tansey, R.; Mirza, Y.; Sukeik, M.; Shaath, M.; Haddad, F.S. Definition of Periprosthetic Hip and Knee Joint Infections and the Economic Burden. Open Orthop. J. 2016, 10, 662–668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garrote-Garrote, M.; Del-Moral-Luque, J.A.; Checa-Garcia, A.; Valverde-Canovas, J.F.; Campelo-Gutierrez, C.; Martinez-Martin, J.; Gil-de-Miguel, A.; Rodriguez-Caravaca, G. Prophylactic antibiotherapy in hip arthroplasty. Cohort study. Rev. Esp. Quimioter. 2018, 31, 118–122. [Google Scholar]
- Berberich, C.; Josse, J.; Ruiz, P.S. Patients at a high risk of PJI: Can we reduce the incidence of infection using dual antibiotic-loaded bone cement? Arthroplasty 2022, 4, 41. [Google Scholar] [CrossRef]
- Hossain, F.; Patel, S.; Haddad, F.S. Midterm assessment of causes and results of revision total knee arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.J.; Cho, W.S.; Choi, N.Y.; Kim, T.K.; Kleos Korea Research, G. Causes, risk factors, and trends in failures after TKA in Korea over the past 5 years: A multicenter study. Clin. Orthop. Relat. Res. 2014, 472, 316–326. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Banerjee, S.; Cherian, J.J.; Bozic, K.J.; Mont, M.A. The Economic Impact of Periprosthetic Infections After Total Hip Arthroplasty at a Specialized Tertiary-Care Center. J. Arthroplast. 2016, 31, 1422–1426. [Google Scholar] [CrossRef]
- Taha, M.; Abdelbary, H.; Ross, F.P.; Carli, A.V. New Innovations in the Treatment of PJI and Biofilms-Clinical and Preclinical Topics. Curr. Rev. Musculoskelet. Med. 2018, 11, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Goswami, K.; Li, W.T.; Tan, T.L.; Yayac, M.; Wang, S.H.; Parvizi, J. Is Treatment of Periprosthetic Joint Infection Improving Over Time? J. Arthroplast. 2020, 35, 1696–1702 e1691. [Google Scholar] [CrossRef] [PubMed]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2019, 34, S337–S342. [Google Scholar] [CrossRef]
- Lu, J.; Han, J.; Zhang, C.; Yang, Y.; Yao, Z. Infection after total knee arthroplasty and its gold standard surgical treatment: Spacers used in two-stage revision arthroplasty. Intractable Rare Dis. Res. 2017, 6, 256–261. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.C. Current advances in sustained-release systems for parenteral drug delivery. Expert Opin. Drug Deliv. 2005, 2, 1039–1058. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release Off. J. Control. Release Soc. 2014, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Sinn Aw, M.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef]
- Lakshmanan, S.; Gupta, G.K.; Avci, P.; Chandran, R.; Sadasivam, M.; Jorge, A.E.; Hamblin, M.R. Physical energy for drug delivery; poration, concentration and activation. Adv. Drug Deliv. Rev. 2014, 71, 98–114. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Wallis, M.; Al-Dulimi, Z.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D printing for enhanced drug delivery: Current state-of-the-art and challenges. Drug Dev. Ind. Pharm. 2020, 46, 1385–1401. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, L.; Li, Z.; Wang, S.; Shi, J.; Tang, W.; Li, N.; Yang, J. The recent development of vat photopolymerization: A review. Addit. Manuf. 2021, 48, 102423. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Bao, Y.; Paunovic, N.; Leroux, J.-C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv. Funct. Mater. 2022, 32, 2109864. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Ayres, T.J.; Sama, S.R.; Joshi, S.B.; Manoghran, G.P. Influence of resin infiltrants on mechanical and thermal performance in plaster binder jetting additive manufacturing. Addit. Manuf. 2019, 30, 100885. [Google Scholar]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Binder Jetting. In Additive Manufacturing Technologies; Springer: Cham, The Netherlands, 2021; pp. 237–252. [Google Scholar]
- Ziaee, M.; Crane, N.B. Binder jetting: A review of process, materials, and methods. Addit. Manuf. 2019, 28, 781–801. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Material Extrusion. In Additive Manufacturing Technologies; Springer: Cham, The Netherlands, 2021; pp. 171–201. [Google Scholar]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Optimization of semisolid extrusion (pressure-assisted microsyringe)-based 3D printing process for advanced drug delivery application. Ann. 3d Print. Med. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Powder Bed Fusion. In Additive Manufacturing Technologies; Springer: Cham, The Netherlands, 2021; pp. 125–170. [Google Scholar]
- Sun, S.; Brandt, M.; Easton, M. 2—Powder bed fusion processes: An overview. In Laser Additive Manufacturing; Brandt, M., Ed.; Woodhead Publishing Series in Electronic and Optical Materials: Sawston, UK, 2017; pp. 55–77. [Google Scholar]
- Singh, D.D.; Mahender, T.; Reddy, A.R. Powder bed fusion process: A brief review. Mater. Today Proc. 2021, 46, 350–355. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Hassanin, H.; Finet, L.; Cox, S.C.; Jamshidi, P.; Grover, L.M.; Shepherd, D.E.T.; Addison, O.; Attallah, M.M. Tailoring selective laser melting process for titanium drug-delivering implants with releasing micro-channels. Addit. Manuf. 2018, 20, 144–155. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef]
- Shishkovsky, I.V.; Kuznetsovc, M.V.; Morozov, Y.G. Porous Titanium and Nitinol Implants Synthesized by SHS/SLS: Microstructural and Histomorphological Analyses of Tissue Reactions. Int. J. Self-Propagating High-Temp. Synth 2010, 19, 157–167. [Google Scholar] [CrossRef]
- Galati, M.; Iuliano, L. A literature review of powder-based electron beam melting focusing on numerical simulations. Addit. Manuf. 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Ivone, R.; Yang, Y.; Shen, J. Recent Advances in 3D Printing for Parenteral Applications. AAPS J. 2021, 23, 87. [Google Scholar] [CrossRef]
- Geevarghese, R.; Sajjadi, S.S.; Hudecki, A.; Sajjadi, S.; Jalal, N.R.; Madrakian, T.; Ahmadi, M.; Wlodarczyk-Biegun, M.K.; Ghavami, S.; Likus, W.; et al. Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation. Int. J. Mol. Sci. 2022, 23, 16185. [Google Scholar] [CrossRef]
- Stewart, S.A.; Dominguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larraneta, E. Development of a Biodegradable Subcutaneous Implant for Prolonged Drug Delivery Using 3D Printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 1–16. [Google Scholar] [CrossRef]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A.; Mishra, N. Polymers in Drug Delivery. J. Biosci. Med. 2016, 4, 69–84. [Google Scholar] [CrossRef]
- Stewart, S.A.; Dominguez-Robles, J.; Donnelly, R.F.; Larraneta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Biodegradable Polymers for Controlled Release. Available online: https://healthcare.evonik.com/en/drugdelivery/parenteral-drug-delivery/parenteral-excipients/bioresorbable-polymers/standard-polymers (accessed on 17 March 2023).
- Rivera-Hernandez, G.; Antunes-Ricardo, M.; Martinez-Morales, P.; Sanchez, M.L. Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef] [PubMed]
- Basa, B.; Jakab, G.; Kallai-Szabo, N.; Borbas, B.; Fulop, V.; Balogh, E.; Antal, I. Evaluation of Biodegradable PVA-Based 3D Printed Carriers during Dissolution. Materials 2021, 14, 1350. [Google Scholar] [CrossRef]
- Kumar, M.T.; Rajeswari, C.; Balasubramaniam, J.; Pandit, J.K.; Kant, S. In vitro and in vivo characterization of scleral implant of indomethacin: Role of plasticizer and cross-linking time. Drug Deliv. 2003, 10, 269–275. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef]
- Long, J.; Gholizadeh, H.; Lu, J.; Bunt, C.; Seyfoddin, A. Application of Fused Deposition Modelling (FDM) Method of 3D Printing in Drug Delivery. Curr. Pharm. Des. 2017, 23, 433–439. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Wan, C. Poly (glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Versatility of polyethylene glycol (PEG) in designing solid-solid phase change materials (PCMs) for thermal management and their application to innovative technologies. J. Mater. Chem. A 2017, 5, 18379–18396. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Yeo, T.; Ko, Y.-G.; Kim, E.J.; Kwon, O.K.; Chung, H.Y.; Kwon, O.H. Promoting bone regeneration by 3D-printed poly(glycolic acid)/hydroxyapatite composite scaffolds. J. Ind. Eng. Chem. 2021, 94, 343–351. [Google Scholar] [CrossRef]
- Younes, H.M. Photopolymerization of Polymeric Composites in Drug Delivery, Tissue Engineering, and Other Biomedical Applications. In Polymer Nanocomposites in Biomedical Engineering. Lecture Notes in Bioengineering; Sadasivuni, K., Ponnamma, D., Rajan, M., Ahmed, B., Al-Maadeed, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Chiulan, I.; Heggset, E.B.; Voicu, S.I.; Chinga-Carrasco, G. Photopolymerization of Bio-Based Polymers in a Biomedical Engineering Perspective. Biomacromolecules 2021, 22, 1795–1814. [Google Scholar] [CrossRef]
- Cody, D.; Casey, A.; Naydenova, I.; Mihaylova, E. A Comparative Cytotoxic Evaluation of Acrylamide and Diacetone Acrylamide to Investigate Their Suitability for Holographic Photopolymer Formulations. Int. J. Polym. Sci. 2013, 2013, 564319. [Google Scholar] [CrossRef]
- Li, W.; Mille, L.S.; Robledo, J.A.; Uribe, T.; Huerta, V.; Zhang, Y.S. Recent Advances in Formulating and Processing Biomaterial Inks for Vat Polymerization-Based 3D Printing. Adv. Healthc. Mater. 2020, 9, e2000156. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, F.; Lim, C.Y.; Chi, H.; Chen, D.; Wang, F.; Jiao, X. High-Performance Nano-Photoinitiators with Improved Safety for 3D Printing. ACS Appl. Mater. Interfaces 2017, 9, 32418–32423. [Google Scholar] [CrossRef] [PubMed]
- Vehse, M.; Petersen, S.; Sternberg, K.; Schmitz, K.-P.; Seitz, H. Drug Delivery From Poly(ethylene glycol) Diacrylate Scaffolds Produced by DLC Based Micro-Stereolithography. Macromol. Symp. 2014, 346, 43–47. [Google Scholar] [CrossRef]
- Singh, R.K.; Seliktar, D.; Putnam, A.J. Capillary morphogenesis in PEG-collagen hydrogels. Biomaterials 2013, 34, 9331–9340. [Google Scholar] [CrossRef]
- Vaillard, V.A.; Trentino, A.I.; Navarro, L.; Vaillard, S.E. Fumarate-co-PEG-co-sebacate photopolymer and its evaluation as a drug release system. J. Polym. Sci. 2022, 60, 2835–2846. [Google Scholar] [CrossRef]
- Steinbach, M.; Gartz, M.; Hirsch, R. Design and characterization of 3D printable photopolymer resin containing poly(2-hydroxyethyl methacrylate) for controlled drug release. J. Drug Deliv. Sci. Technol. 2020, 59, 101850. [Google Scholar] [CrossRef]
- Alshimaysawee, S.; Fadhel Obaid, R.; Al-Gazally, M.E.; Alexis Ramirez-Coronel, A.; Bathaei, M.S. Recent Advancements in Metallic Drug-Eluting Implants. Pharmaceutics 2023, 15, 223. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating Techniques for Functional Enhancement of Metal Implants for Bone Replacement: A Review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef]
- Lyndon, J.A.; Boyd, B.J.; Birbilis, N. Metallic implant drug/device combinations for controlled drug release in orthopaedic applications. J. Control. Release Off. J. Control. Release Soc. 2014, 179, 63–75. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Jacobsen, N.R.; Loeschner, K. Distribution, metabolism, excretion, and toxicity of implanted silver: A review. Drug Chem. Toxicol. 2022, 45, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Bourell, D.L.; Babu, S.S. Metallic materials for 3D printing. Mater. Res. Soc. 2016, 41, 729–741. [Google Scholar] [CrossRef]
- Velasquez-Garcia, L.F.; Kornbluth, Y. Biomedical Applications of Metal 3D Printing. Annu. Rev. Biomed. Eng. 2021, 23, 307–338. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.A.; Mykulowycz, N.M.; Shim, J.; Fontana, R.; Schmitt, P.; Roberts, A.; Ketkaew, J.; Shao, L.; Chen, W.; Bordeenithikaseem, P.; et al. 3D printing metals like thermoplastics: Fused filament fabrication of metallic glasses. Mater. Today 2018, 21, 697–702. [Google Scholar] [CrossRef]
- Chua, K.; Khan, I.; Malhotra, R.; Zhu, D. Additive manufacturing and 3D printing of metallic biomaterials. Eng. Regen. 2021, 2, 288–299. [Google Scholar] [CrossRef]
- Jing, Z.; Zhang, T.; Xiu, P.; Cai, H.; Wei, Q.; Fan, D.; Lin, X.; Song, C.; Liu, Z. Functionalization of 3D-printed titanium alloy orthopedic implants: A literature review. Biomed. Mater. 2020, 15, 052003. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Aguilar-de-Leyva, A.; Linares, V.; Casas, M.; Caraballo, I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural Polymers for Organ 3D Bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 1–18. [Google Scholar] [CrossRef]
- Bom, S.; Santos, C.; Barros, R.; Martins, A.M.; Paradiso, P.; Claudio, R.; Pinto, P.C.; Ribeiro, H.M.; Marto, J. Effects of Starch Incorporation on the Physicochemical Properties and Release Kinetics of Alginate-Based 3D Hydrogel Patches for Topical Delivery. Pharmaceutics 2020, 12, 719. [Google Scholar] [CrossRef]
- Fan, R.; Piou, M.; Darling, E.; Cormier, D.; Sun, J.; Wan, J. Bio-printing cell-laden Matrigel-agarose constructs. J. Biomater. Appl. 2016, 31, 684–692. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, X.; Rahman, S.E.; Su, S.; Wei, J.; Ning, F.; Hu, Z.; Martinez-Zaguil, R.; Sennoune, S.R.; et al. 3D printed agar/ calcium alginate hydrogels with high shape fidelity and tailorable mechanical properties. Polymer 2021, 214, 123238. [Google Scholar] [CrossRef]
- Zou, Q.; Tian, X.; Luo, S.; Yuan, D.; Xu, S.; Yang, L.; Ma, M.; Ye, C. Agarose composite hydrogel and PVA sacrificial materials for bioprinting large-scale, personalized face-like with nutrient networks. Carbohydr. Polym. 2021, 269, 118222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Hsieh, M.F.; Fang, C.H.; Jiang, C.P.; Lin, B.; Lee, H.M. Osteochondral Regeneration Induced by TGF-beta Loaded Photo Cross-Linked Hyaluronic Acid Hydrogel Infiltrated in Fused Deposition-Manufactured Composite Scaffold of Hydroxyapatite and Poly(Ethylene Glycol)-Block-Poly(epsilon-Caprolactone). Polymers 2017, 9, 182. [Google Scholar] [CrossRef]
- Habraken, W.J.; Wolke, J.G.; Jansen, J.A. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 234–248. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regi, M. Bioceramics for drug delivery. Acta Mater. 2013, 61, 890–911. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Zafar, M.J.; Zhu, D.; Zhang, Z. 3D Printing of Bioceramics for Bone Tissue Engineering. Materials 2019, 12, 3361. [Google Scholar] [CrossRef]
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D Printing of Bioceramic Scaffolds-Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef]
- Pei, P.; Tian, Z.; Zhu, Y. 3D printed mesoporous bioactive glass/metal-organic framework scaffolds with antitubercular drug delivery. Microporous Mesoporous Mater. 2018, 272, 24–30. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, S.; Wang, Y.; Yu, Z.; Ao, H.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; et al. Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan. Acta Biomater. 2016, 46, 112–128. [Google Scholar] [CrossRef]
- Goncalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regi, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X.; Ma, B.; Zhu, H.; Huan, Z.; Ma, N.; Wu, C.; Chang, J. 3D-Printed Bioactive Ca(3)SiO(5) Bone Cement Scaffolds with Nano Surface Structure for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 5757–5767. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhai, D.; Huan, Z.; Zhu, H.; Xia, L.; Chang, J.; Wu, C. Three-Dimensional Printing of Hollow-Struts-Packed Bioceramic Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2015, 7, 24377–24383. [Google Scholar] [CrossRef] [PubMed]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Chavda, V.P.; Jogi, G.; Paiva-Santos, A.C.; Kaushik, A. Biodegradable and removable implants for controlled drug delivery and release application. Expert Opin. Drug Deliv. 2022, 19, 1177–1181. [Google Scholar] [CrossRef]
- Inzana, J.A.; Trombetta, R.P.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur. Cells Mater. 2015, 30, 232–247. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Y.; Xie, K. AgNPs-decorated 3D printed PEEK implant for infection control and bone repair. Colloids Surf. B Biointerfaces 2017, 160, 483–492. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, W.; Jiang, H.; Li, X.; An, W.; Yang, H. 3D-printed composite scaffold with anti-infection and osteogenesis potential against infected bone defects. RSC Adv. 2022, 12, 11008–11020. [Google Scholar] [CrossRef]
- Zhou, Z.; Yao, Q.; Li, L.; Zhang, X.; Wei, B.; Yuan, L.; Wang, L. Antimicrobial Activity of 3D-Printed Poly(epsilon-Caprolactone) (PCL) Composite Scaffolds Presenting Vancomycin-Loaded Polylactic Acid-Glycolic Acid (PLGA) Microspheres. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 6934–6945. [Google Scholar] [CrossRef]
- Topsakal, A.; Midha, S.; Yuca, E.; Tukay, A.; Sasmazel, H.T.; Kalaskar, D.M.; Gunduz, O. Study on the cytocompatibility, mechanical and antimicrobial properties of 3D printed composite scaffolds based on PVA/Gold nanoparticles (AuNP)/Ampicillin (AMP) for bone tissue engineering. Mater. Today Commun. 2021, 28, 1–10. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.; Zeng, L.; Wen, Z.; Xiong, Z.; Liao, Z.; Hu, Y. Biofunctionalization of 3D Printed Porous Tantalum Using a Vancomycin-Carboxymethyl Chitosan Composite Coating to Improve Osteogenesis and Antibiofilm Properties. ACS Appl. Mater. Interfaces 2022, 14, 41764–41778. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; Tang, T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018, 79, 265–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Huangfu, H.; Zhang, X.; Zhang, H.; Qin, Q.; Fua, L.; Wang, D.; Wangd, C.; Wang, L.; et al. 3D printing of bone scaffolds for treating infected mandible bone defects through adjustable dual-release of chlorhexidine and osteogenic peptide. Mater. Des. 2022, 224, 111288. [Google Scholar] [CrossRef]
- Allen, B. High-Strength, 3D-Printed Antibiotic-Eluting Spacer to Treat Periprosthetic Joint Infection. Doctoral Disseration, Duke University, Durham, NC, USA, 2020. [Google Scholar]
- Kim, T.W.B.; Lopez, O.J.; Sharkey, J.P.; Marden, K.R.; Murshed, M.R.; Ranganathan, S.I. 3D printed liner for treatment of periprosthetic joint infections. Med. Hypotheses 2017, 102, 65–68. [Google Scholar] [CrossRef]
- Guan, B.; Wang, H.; Xu, R.; Zheng, G.; Yang, J.; Liu, Z.; Cao, M.; Wu, M.; Song, J.; Li, N.; et al. Establishing Antibacterial Multilayer Films on the Surface of Direct Metal Laser Sintered Titanium Primed with Phase-Transited Lysozyme. Sci. Rep. 2016, 6, 36408. [Google Scholar] [CrossRef]
- Griseti, Q.; Jacquet, C.; Sautet, P.; Abdel, M.P.; Parratte, S.; Ollivier, M.; Argenson, J.N. Antimicrobial properties of antibiotic-loaded implants. Bone Jt. J. 2020, 102-B, 158–162. [Google Scholar] [CrossRef]
- Allen, B.; Moore, C.; Seyler, T.; Gall, K. Modulating antibiotic release from reservoirs in 3D-printed orthopedic devices to treat periprosthetic joint infection. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 2239–2249. [Google Scholar] [CrossRef]
- Maver, T.; Mastnak, T.; Mihelic, M.; Maver, U.; Finsgar, M. Clindamycin-Based 3D-Printed and Electrospun Coatings for Treatment of Implant-Related Infections. Materials 2021, 14, 1464. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Chen, K.; Wang, F.; Feng, C.; Xu, L.; Zhang, D. 3D printed chitosan-gelatine hydrogel coating on titanium alloy surface as biological fixation interface of artificial joint prosthesis. Int. J. Biol. Macromol. 2021, 182, 669–679. [Google Scholar] [CrossRef]
- Chisari, E.; Lin, F.; Fei, J.; Parvizi, J. Fungal periprosthetic joint infection: Rare but challenging problem. Chin. J. Traumatol. 2022, 25, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Petis, S.M.; Osmon, D.R.; Mabry, T.M.; Berry, D.J.; Hanssen, A.D.; Abdel, M.P. Periprosthetic Joint Infection With Fungal Pathogens. J. Arthroplast. 2018, 33, 2605–2612. [Google Scholar] [CrossRef]
- Lazic, I.; Scheele, C.; Pohlig, F.; von Eisenhart-Rothe, R.; Suren, C. Treatment options in PJI—Is two-stage still gold standard? J. Orthop. 2021, 23, 180–184. [Google Scholar] [CrossRef]
- Frank, R.M.; Cross, M.B.; Della Valle, C.J. Periprosthetic joint infection: Modern aspects of prevention, diagnosis, and treatment. J. Knee Surg. 2015, 28, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bacskay, I.; Ujhelyi, Z.; Feher, P.; Arany, P. The Evolution of the 3D-Printed Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1312. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Larraneta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Domsta, V.; Seidlitz, A. 3D-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules 2021, 26, 4066. [Google Scholar] [CrossRef]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef]
- Yuste, I.; Luciano, F.C.; Gonzalez-Burgos, E.; Lalatsa, A.; Serrano, D.R. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol. Res. 2021, 169, 105626. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, F.K.; Guerrini, L.; Gunnarsson, A.E.; Recenti, M.; Jacob, D.; Cangiano, V.; Tesfahunegn, Y.A.; Islind, A.S.; Tortorella, F.; Tsirilaki, M.; et al. CT- and MRI-Based 3D Reconstruction of Knee Joint to Assess Cartilage and Bone. Diagnostics 2022, 12, 279. [Google Scholar] [CrossRef] [PubMed]


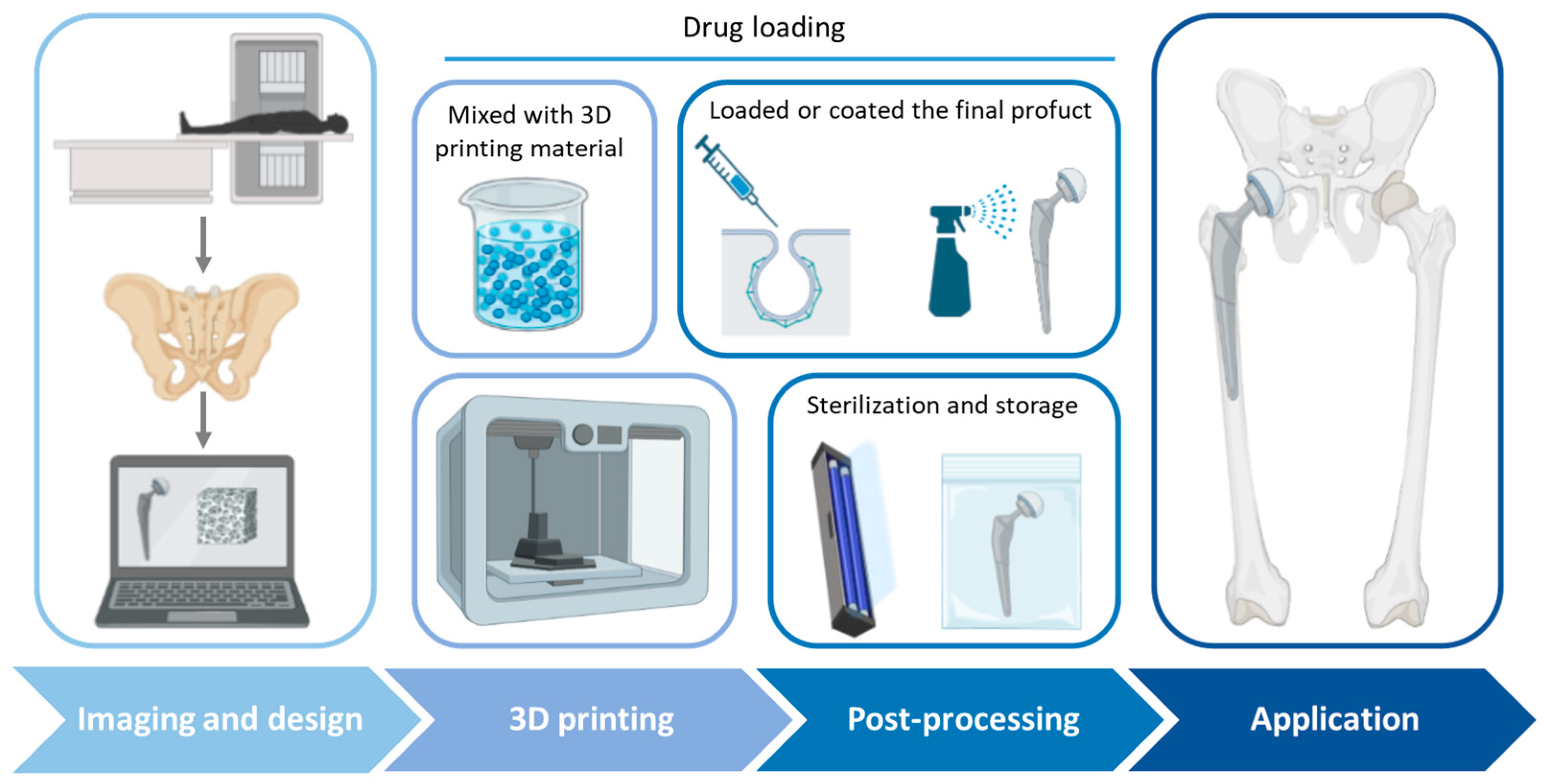
| Polymer | Printing Temperature | Printability Properties | Biological Properties | Drug Release | Ref |
|---|---|---|---|---|---|
| PCL | 55–64 °C | During the printing processes, PCL molecules maintain crystal states with low or moderate mechanical properties | Lack of natural peptide motifs that provide specific binding sites for cells | Longer degradation profile than other polymers, suitable for drug release over a year | [6,75,78] |
| PLA | 150–175 °C | High degradation temperature (325–500 °C) | Low cell affinity due to its hydrophobicity | PLA is influenced by the manipulation of its crystallinity degree and mechanical stability | [6,78] |
| PLGA | >120 °C | The glass transition temperature is reduced with a decrease in lactic acid content in the copolymer | Poor bioactivities (osteoconductive and osteoinductive capabilities) | The time required for the degradation of PLGA is related to the ratio of the monomers used in the starting materials | [75,76] |
| PGA | 220–230 °C | Higher heat distortion temperature than PLA | Improvement in cell adhesion, proliferation, migration, and differentiation for rapid tissue regeneration | The presence of functional moieties in the structural unit allows for tailored degradation rates fitting different applications | [76,79] |
| PVA | 180–228 °C | Suitable for inkjet printing and FDM | Good biodegradability and minimal adverse effects | Suitable for immediate and controlled release | [6,78] |
| PEG | 3–67 °C | Low thermal conductivity | Enhancement of cell encapsulation and it is a widely explored synthetic material for soft tissue repair | Biodegradability and release can be modified by incorporating degradable segments. | [75,77] |
| Polymer | Photo-Cross-Linked Moiety | Photoinitiator | Wavelength | Application | Ref |
|---|---|---|---|---|---|
| PEGDA | Diacrylate | LAP or PI | 365–375 nm | Local anticancer drug delivery and scaffold material | [80,85] |
| PEGDAAm | Diacrylamide | Irgacure 2959 | 365 nm | Re-endothelialization-promoting materials and cell encapsulation | [80,86] |
| Fumarate-co-PEG-co-sebacates | Fumarate | Irgacure 500 | 365 nm | Controlled drug release systems | [87] |
| pHEMA | Methacrylate | TPO | 370 nm | Controlled drug release systems | [88] |
| PVA | Methacrylate | Ru/SPS | 450 nm | New ink and scaffold material | [80,83] |
| Natural Product | 3D Printing Technique | Printing Temperature | Printability Properties | Ref |
|---|---|---|---|---|
| Alginate | PAM | Room temperature | Efficient gelation with a low percentage of material and high-quality mechanical and rheological properties | [100,104] |
| Chitosan | PAM | Room temperature | Hydrogels with optimal rheological properties, low viscosity, and a fast gelling reaction | [100,101] |
| Inkjet | ||||
| FDM (Material blends) | 182 °C (with Eudragit), 190 °C (ethyl cellulose), 200 °C (PVA), and 215 °C (PVA) | High thermoplasticity | ||
| Agarose | FDM | 55 °C (calcium alginate), 180 °C (PVA) | Low liquefaction temperature | [102,105,106,107] |
| PAM | Room temperature or 37 °C | |||
| Cellulose | FDM | 190–210 °C (With PCL or PLA) | High crystallinity, elastic modulus, good mechanical properties | [101,108] |
| Inkjet | Room temperature | |||
| EHD | ||||
| Hyaluronic acid | FDM | 65 °C (PEG and PCL) | Low shape fidelity but unsuitable to produce printable bio-inks | [102,109,110] |
| PAM | Room temperature |
| Blend Composition | Blend Ratio | 3D Printing Technique | Printing Temperature | Application | Ref |
|---|---|---|---|---|---|
| MGB/MOF | 100:0 95:5 90:10 70:30 | PAM | Room temperature | Scaffolds with antitubercular drug delivery | [116] |
| PLGA/HA | 9:1 | FDM | 150 °C | Scaffolds with antibacterial and osteoconductive properties | [117] |
| PCL/HA/carbon nanotubes | 50:45 50:0–5 | Nozzle-deposition system | Room temperature | Scaffolds for bone cell growth stimulation | [118] |
| Ca3SiO5/HPMC | 70:30 | PAM | Room temperature | Scaffolds with nano surface structure for bone regeneration | [119] |
| Ca7Si2P2O16/alginate/ pluronic F-127 | 62:3:35 | PAM | Room temperature | Hollow strut-packed bioceramic scaffolds for bone regeneration | [120] |
| Scaffold Material | Antimicrobial | Drug Loading Technique | 3D Printing Technique | 3D Printing Conditions | Ref |
|---|---|---|---|---|---|
| Calcium phosphate | Rifampin and vancomycin | Mixed in the power before printing or printed onto the scaffold | Binder jetting | Phosphoric acid-based binder solution and bed of calcium phosphate powder | [123] |
| PEEK | Ag nanoparticles | Coating | FDM | 380 °C | [124] |
| PLLA and pearl | Rifampicin and moxifloxacin | Mixed with a 3D printing material | PAM | 150 °C and 110 kPa | [125] |
| PCL and PDA | Vancomycin | Coating | FDM | Not specified | [126] |
| PVA | AuNP and/or AMP | Mixed with a 3D printing material | PAM | Room temperature and flow rate of 0.5 mL/h | [127] |
| Tantalum | Vancomycin | Coating | Not specified | Not specified | [128] |
| PLGA, HA, and HACC | None | None | PAM | 150 °C and 110 kPa | [129] |
| Β-TCP and PLGA | Chlorhexidine | Mixed with a 3D printing material | PAM | Angle of 90°; printing speed of 10–14 mm/s, and pressure of 1.5 MPa | [130] |
| Implant Material | Antimicrobials | Drug Loading Technique | 3D Printing Technique | 3D Printing Conditions | Ref |
|---|---|---|---|---|---|
| Ti-6Al-4V | - | Reservoirs and micro-channels | SLM | Argon atmosphere, 1075 nm, a constant beam spot size of 70 μm, 200 W, printing speed of up to 4000 mm/s, and layer thickness of 20 μm | [58] |
| Cobalt–chrome (Co28Cr6Mo) | Gentamicin | Syringe injection in reservoirs | SLM | Not specified | [131] |
| PLA | Tetracycline | Syringe injection in reservoirs | Not specified | Not specified | [132] |
| Ti–6Al–4V | Minocycline | Coating | DMLS | Argon atmosphere, 1054 nm, 200 W, laser scanning speed of 7 m/s, and laser spot size of 0.1 mm. | [133] |
| Titanium | Vancomycin | Soaking solution | Not specified | Not specified | [134] |
| RPU 60 | Gentamicin | Syringe injection in reservoirs | CLIP | Not specified | [135] |
| Stainless steel and Ti–6Al–4V | Clindamycin | 3D-printed coating | PAM | 0.25 mm nozzles and room temperature | [136] |
| Titanium | Chitosan and gelatine | Coating | PAM | 50 °C, pressure of 0.3 MPa, and printing speed of 3.3 mm/s | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuste, I.; Luciano, F.C.; Anaya, B.J.; Sanz-Ruiz, P.; Ribed-Sánchez, A.; González-Burgos, E.; Serrano, D.R. Engineering 3D-Printed Advanced Healthcare Materials for Periprosthetic Joint Infections. Antibiotics 2023, 12, 1229. https://doi.org/10.3390/antibiotics12081229
Yuste I, Luciano FC, Anaya BJ, Sanz-Ruiz P, Ribed-Sánchez A, González-Burgos E, Serrano DR. Engineering 3D-Printed Advanced Healthcare Materials for Periprosthetic Joint Infections. Antibiotics. 2023; 12(8):1229. https://doi.org/10.3390/antibiotics12081229
Chicago/Turabian StyleYuste, Iván, Francis C. Luciano, Brayan J. Anaya, Pablo Sanz-Ruiz, Almudena Ribed-Sánchez, Elena González-Burgos, and Dolores R. Serrano. 2023. "Engineering 3D-Printed Advanced Healthcare Materials for Periprosthetic Joint Infections" Antibiotics 12, no. 8: 1229. https://doi.org/10.3390/antibiotics12081229
APA StyleYuste, I., Luciano, F. C., Anaya, B. J., Sanz-Ruiz, P., Ribed-Sánchez, A., González-Burgos, E., & Serrano, D. R. (2023). Engineering 3D-Printed Advanced Healthcare Materials for Periprosthetic Joint Infections. Antibiotics, 12(8), 1229. https://doi.org/10.3390/antibiotics12081229







