Abstract
Streptococcus mitis, a normal inhabitant of the oral cavity, is a member of Viridans Group Streptococci (VGS). Generally recognized as a causative agent of invasive diseases in immunocompromised patients, S. mitis is considered to have low pathogenic potential in immunocompetent individuals. We present a rare case of sinusitis complicated by meningitis and cerebral sino-venous thrombosis (CSVT) caused by S. mitis in a previously healthy 12-year-old boy with poor oral health status. With the aim of understanding the real pathogenic role of this microorganism, an extensive review of the literature about invasive diseases due to S. mitis in pediatric patients was performed. Our data define the critical role of this microorganism in invasive infections, especially in immunocompetent children and in the presence of apparently harmful conditions such as sinusitis and caries. Attention should be paid to the choice of therapy because of VGS’s emerging antimicrobial resistance patterns.
1. Introduction
Streptococcus mitis, an important member of VGS [1], is found as part of the normal microbiota of the human skin as well as of the oropharynx, gastrointestinal, and female genital tracts [2]. Although generally considered to have a low pathogenic potential in immunocompetent individuals, VGS can cause invasive diseases such as bloodstream infections, pneumonia, endocarditis, enteritis, and meningitis in patients with immunocompromised status or other risk factors [3,4]. In the literature pediatric cases of meningitis caused by S. mitis are mainly described in patients with leukemia, lymphoma, or neutropenia, while in healthy children, meningitis and other severe conditions caused by S. mitis are considered rare [5,6].
In this report, we present a rare case of sinusitis complicated by meningitis and CSVT caused by S. mitis in a previously healthy 12-year-old boy. Starting from our case, a systematic review of the literature was performed with the aim of providing the first overview of serious infections caused by this generally saprophytic bacterium in the pediatric population (including both immunocompromised and immunocompetent individuals). Pathogenesis, clinical features, predisposing factors, therapy, and outcomes were analyzed for each case.
2. Case Report
A previously healthy 12-year-old boy was admitted to our hospital after seven days of fever, headache, and a recent onset of vomiting. At the time of admission, the patient was alert with a severe headache, diplopia, photophobia, and difficulty maintaining an upright position. A physical examination revealed a fever up to 38.5 °C, normal cardiorespiratory activity, and a painless, treatable abdomen. A mild nuchal rigidity, with pain on passive mobilization and lateral twisting of the neck, was observed in the absence of both Binda and Brudzinski signs. An intraoral examination showed poor oral health status with multiple destructive caries in the upper arch (Figure 1).
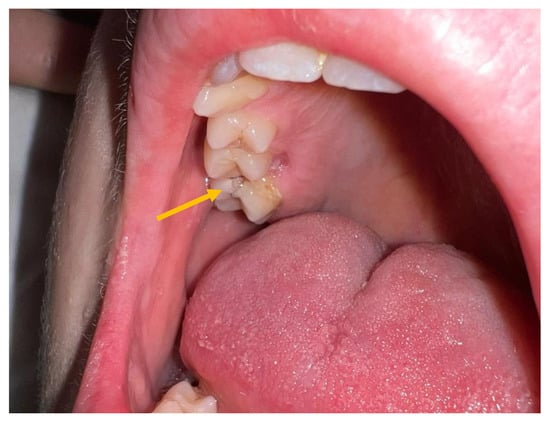
Figure 1.
Oral cavity of the patient at the time of admission. Presence of destructive caries in the upper dental arch.
Laboratory findings revealed mild anemia and neutrophilia (Table 1). Blood cultures were negative.

Table 1.
Laboratory findings.
A CT-scan showed an enlargement of the sub-tentorial ventricular system associated with sinusitis of the sphenoid, frontal, and maxillary right sinuses.
After a funduscopic examination, a lumbar puncture was performed for suspected meningitis, showing a moderately cloudy appearance of the cerebrospinal fluid with 640 white blood cells (90% polymorphonuclear), a total protein content of 0.3 g/L, and glucose of 57 mg/dL. Microbiological analyses of the CSF were negative.
In the suspect of culture-negative bacterial meningitis, a broad-spectrum empirical antibiotic therapy with ceftriaxone 50 mg/kg/12 h, vancomycin 20 mg/kg/8 h, and metronidazole 10 mg/kg/8 h was started in association with steroid therapy (dexamethasone 4 mg/6 h).
Following a few days of treatment, the patient no longer presented with fever, neck rigidity, headache, or diplopia.
A subsequently performed Contrast MRI showed radiological findings of sinusitis of both sphenoid sinuses, the right maxillary and frontal sinus, and ipsilateral ethmoidal cells, as well as meningeal inflammation and partial thrombosis of the internal jugular veins. After administration of contrast medium, a filling defect of the cavernous sinuses was also reported (Figure 2).
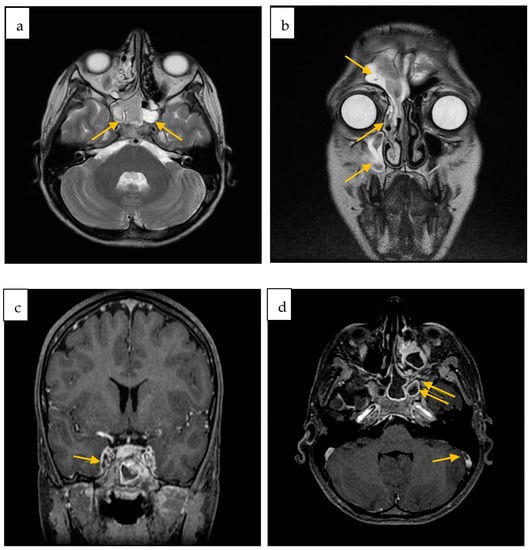
Figure 2.
Contrast-enhanced brain MRI axial (a) and coronal (b) FSE T2w sinusitis of both sphenoid sinuses (a), right maxillary and frontal sinus, and ipsilateral ethmoidal cells (b); (c) Inhomogeneous opacification of the left cavernous sinus; (d) Defect of opacification of the right sigmoid sinus (arrow) as for partial thrombosis. Right sphenoid sinus and maxillary sinus sinusitis (double arrow).
Therefore, anticoagulant therapy with enoxaparin was started.
To exclude the cardiac localization of infection, Echocardiography and color Doppler were performed with negative results.
Orthopantomography showed apical dental granulomas and caries of the upper teeth.
After the ENT consultation, functional endoscopic sinus surgery was performed, and a moderate amount of purulent exudate was drained. The culture of the exudate was positive for S. mitis, resistant to amoxicillin, penicillin, and cefuroxime.
Based on the antibiogram, vancomycin administration was discontinued while ceftriaxone and metronidazole were maintained.
Genetic causes of predisposition to thrombosis, as well as any possible cause of immunodeficiency, were ruled out by performing an HIV test, a study of lymphocyte subpopulations, and a hematological evaluation.
On the twentieth day of hospitalization, a second MRI showed a partial regression of the sinusitis and a general improvement of the previously reported brain lesions. It also revealed filling defects in both the internal jugular veins and the right maxillary sinusitis (Figure 3).
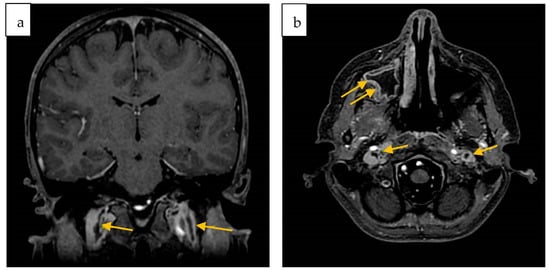
Figure 3.
Second contrast-enhanced brain MRI. Coronal (a) and Axial (b) 3D FSPGR defect of opacification of both jugular veins (arrows); (b) right maxillary sinusitis (double arrows).
The boy was finally discharged in good clinical condition after 37 days of hospitalization. A control MRI performed two months after the discharge showed an almost complete resolution of the thrombosis previously described and a noticeable reduction in the thickening of the mucous membrane of the paranasal sinuses (Figure 4).
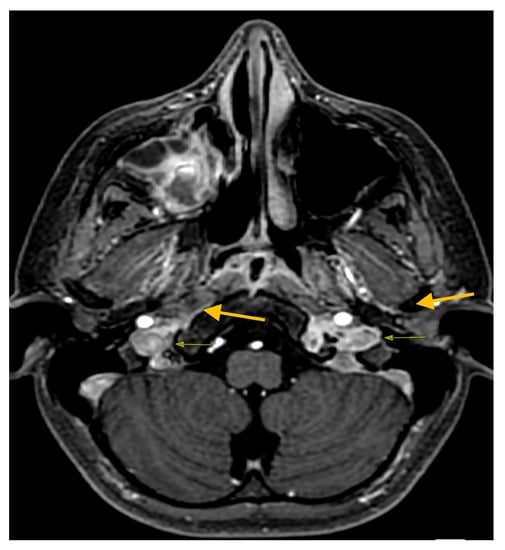
Figure 4.
Control MRI post discharge axial 3D FSPGR resolution of jugular vein thrombosis.
The boy is currently in outpatient follow-up.
3. Material and Methods
A systematic review of the literature was performed on PubMed and Scopus electronic databases by submitting the query (mitis AND ((baby[Title/Abstract]) OR (child*[Title/Abstract]) OR (pediatr*[Title/Abstract]) OR (paediatr*[Title/Abstract])), with the aim of identifying all cases of invasive disease caused by S. mitis in pediatric age reported by 31 October 2022. No filters or language restrictions were applied to the results. Furthermore, all of the listed references were hand-searched, and a citation tracker was used to identify any other relevant papers. All articles involving patients aged 0–18 years with invasive diseases requiring hospitalization (such as bloodstream infection or endocarditis), in which the only identified pathogen was S. mitis, were considered eligible for inclusion in our review. Papers without the full text available or lacking adequate information were excluded.
The selected articles were reviewed by two independent authors and judged on their relevance to the subject of the study. The following data were evaluated for each case: age, sex, comorbidities, risk factors, disease associated with S. mitis infection, source of isolation of the organism, therapy, and outcome.
This systematic review was performed in accordance with the PRISMA protocol (Reporting Items for Systematic Reviews and Meta-Analyses) [7] after systematic review registration at PROSPERO (the systematic review registration statement code is CRD42022366226).
4. Results
A total of 618 potentially eligible articles were found and screened. A total of 542 articles were excluded because they were not inherent with the topic of our research; 15 more were excluded because they concerned adult patients; and 38 could not be examined due to the inaccessibility of the complete article and/or lack of adequate information.
A total of 23 studies were selected for inclusion in the systematic review [2,4,5,6,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26], reporting 94 pediatric cases of invasive disease caused by S. mitis. Most of the articles were single case reports, while seven were case series. A flow diagram illustrating this selection process is presented in Figure 5.
Figure 5.
PRISMA study flow diagram: flow diagram of study identification, screening, eligibility, and included studies.
Clinical features, risk factors, diagnosis, therapy, and outcome of 95 patients (including our case) are reported in Table 2.

Table 2.
Reported cases of invasive disease caused by S. mitis.
The most frequent invasive conditions due to S. mitis identified by our review are represented in the graphic below (Figure 6).
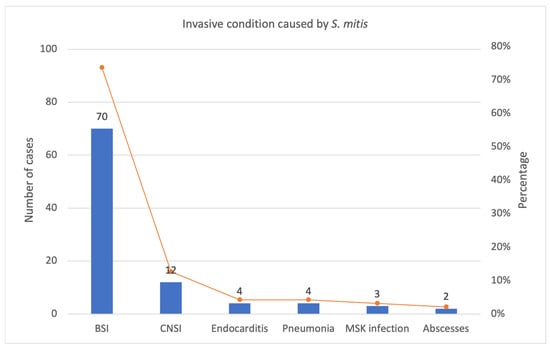
Figure 6.
Most frequent invasive condition caused by S. mitis in our review: number of cases and percentage. BSI: bloodstream infections, CNSI: central nervous system infections, MSK: musculoskeletal.
Out of the 56 patients whose gender was indicated, 33 (59%) were male and 23 (41%) were female.
The mean age was six and a half years (range: 2 days to 18 years). Eight children were under one year old; the youngest ones were two girls who became ill two days after a physiological birth [17,25].
A total of 78 of 95 (82%) patients were immunocompromised. Out of them, 56 children had a hematological disease, while 22 had an unspecified oncological condition [12].
Hence, approximately 18% of the patients had not a known immunocompromised status, but they still developed a serious condition related to a microorganism normally considered a saprophyte with low pathogenic potential. Among these children, nine had clinical features potentially predisposing to invasive infection:
(1) a 14-years-old girl with Gorham-Stout syndrome with osteolysis skull base and CSF leak had S. mitis meningitis [4];
(2–3) an eight-years-old [21] and a one-year-old [23] girl with a ventricular septal defect were diagnosed with endocarditis;
(4) a nine-day-old baby born with hydrocephalus, and thus requiring the placement of a ventriculo-peritoneal shunt a few hours after birth, had meningitis [26];
(5–9) Five children, mean age nine and a halfyears, with chronic heart disease were diagnosed with endocarditis due to S. mitis [27].
A total of eight of 95 patients, including our case, were not immunocompromised and had no apparent risk factors.
The outcome was favorable in 88/95 cases. Out of seven deaths, two were due to other causes: a 15-year-old boy with acute myeloid leukemia (AML) died of a contemporary invasive pulmonary aspergillosis [15], and a 17-years-old patient with acute lymphatic leukemia (ALL) died of the underlying disease [19].
5. Discussion
S. mitis is included in the Viridans Streptococci Group, and in particular in the Mitis group, together with other saprophytes streptococci such as S. gordonii, S. oralis, and about 20 other related species, normal inhabitants of the oral cavity, respiratory tract, gastrointestinal tract, and human skin [27,28].
Despite their traditionally low pathogenicity, these bacteria are actually identified among the most common causes of bacteremia, as well as toxic shock syndrome, pneumonia, abscesses, endocarditis, and meningitis in both children and adults with immunocompromised status [19,29,30,31,32,33].
Among VGS species, S. mitis is the dominant commensal of the oral cavity, and compared to other VGS species, it often causes clinically serious infections in immunocompromised individuals [3], suggesting that S. mitis strains have inherently virulent properties.
The genetic analysis performed by Shelburne et al. [3] made it possible to identify a close genetic correlation between S. mitis and S. pneumoniae, one of the most frequent microbial killers worldwide. Other studies have shown that some genes encoding virulence factors of S. pneumoniae are part of the genome of certain strains of S. mitis [34,35] and that some representatives of the Mitis group may be mistakenly identified as S. pneumoniae [36,37,38,39,40,41,42]. This phenomenon is due to the common evolutionary origin of these organisms as well as the homologous recombination and horizontal gene transfer mechanisms between streptococcal species residing in the same ecological niche [43].
Among others virulence factors, for example, S. pneumoniae possess choline containing teichoic acids, which are the anchor structure of choline binding proteins (CBPs). CBPs have an important function in murein metabolism and host- pathogen interactions [44], and they have been occasionally described in some isolates of S. mitis [44,45,46].
Such a “genetic closeness” could be responsible for the progressive increasing in virulence showed by these bacteria [47,48,49].
According to our analysis, invasive infections caused by S. mitis concern, as expected, predominantly immunocompromised patients with neutropenia. However, clinically severe disease also occurs in a significant number of children with competent immune systems, representing about 20% of the published pediatric cases.
As reported in the results, 8 cases concerning serious invasive infections caused by S. mitis in previously healthy children with no immunocompromised status nor evident risk factors, are described in literature.
In our case, the only potential predisposing factors can be identified in the poor oral hygiene of the patient and in the presence of some caries, being S. mitis described as a major pathogen involved in the destruction of childhood dental enamel [50,51,52,53]. The presence of destructive caries associated with apical granulomas of the roots of the upper dental arch might have presumably allowed S. mitis to invade the paranasal sinuses, causing a severe sinusitis with intracranial complications. Comparable to our case, Yiş et al. [22] reported two cases of meningitis in a previously healthy six-years-old boy and eight-years-old girl, in which the respective sources of infection were probably poor oral hygiene with multiple caries and inflammation of the maxillary sinus.
To date, the overall incidence of neurological complications of sinusitis, as well as their outcomes, have greatly improved thanks to the wide availability of antibiotics, although the mortality rate is still reported to oscillate between 5% and 27% [28], while long-term neurological morbidities such as hemiparesis, aphasia, and epilepsy occur in 13%-35% of survivors [54,55].
Our patient was 12-years-old, confirming the trend for which risks of complications from suppurative sinusitis would increase in pediatric patients above the age of six because of certain anatomical conditions [56,57,58].
The most common complications of sinusitis are orbital and include preseptal or periorbital cellulitis, subperiosteal abscess and optic neuritis [59].
Less frequent intracranial complications include meningitis, epidural abscesses, subdural empyema, intracerebral abscesses and cavernous or sagittal sinus thrombosis [60].
The clinical characteristics of our patient almost immediately suggested the diagnosis of meningitis, presenting fever, vomiting, neck rigidity and visual disturbances.
While among adults meningitis caused by S. mitis mostly occurs after invasive procedures like neurosurgical interventions or spinal anesthesia [61,62,63], our review shows that in children the predisposing conditions to S. mitis-related meningitis are the early age [16], the immunodeficiency and underlying pathologies which can provide a potential portal for systemic blood infection (such as Gorham-Stout syndrome with loss of CSF [4] and hydrocephalus with ventriculoperitoneal shunt [24]). In addition, as our case suggests, poor oral hygiene or infection of the paranasal sinuses appear to be risk conditions for developing intracranial complications caused by this bacterium. In one case, early-onset meningitis was described in a full-term baby with no obvious predisposing factors [25].
Cerebral white matter involvement during meningitis caused by S. mitis has been rarely described. In the two cases of pediatric meningitis reported by Yiş et al. [2,22], brain swelling, diffuse periventricular and white matter hyperintensity, on T2 FLAIR sequences were documented on brain MRI. In the case of our little patient, the MRI showed, in addition to sinusitis, multiple areas of hyperintensity on FLAIR sequences in the cerebellar and cortical areas. Furthermore, the MRI showed a filling defect in both jugular veins, cavernous sinuses, and the right sigmoid sinus, thus suggesting partial venous thrombosis.
CSVT is defined by thrombosis of the superficial (cortical veins, superior sagittal sinus, transverse sinus, sigmoid sinus, and jugular vein) or deep (inferior sagittal sinus, internal cerebral veins, vein of Galen, straight sinus) venous system, and it is an extremely rare complication of upper respiratory tract infections [64]. It is associated with high mortality and morbidity, and it is mostly described in older children, adolescents, and young adults. Severe long-term sequelae are reported in up to 48% of children [65].
No cases of S. mitis-related sinusitis complicated by meningitis or CSVT have been previously reported in the literature.
Early and adequate antibiotic therapy is essential in the management of invasive diseases caused by S. mitis.
In the case of our patient, empiric antibiotic therapy with vancomycin and ceftriaxone was administered as recommended by the guidelines for the management of bacterial meningitis in children [66,67,68]. All patients diagnosed with S. mitis-related meningitis described in the literature received early empiric antibiotic therapy. The most commonly used regimens involved the association of a beta-lactam antibiotic (penicillin, ticarcillin, nafcillin, or ampicillin) with an aminoglycoside (gentamicin, tobramycin, or netilmycin) or the association of a cephalosporin with vancomycin. There were no significant prognostic differences between the two empiric antibiotic regimens. Therapy was subsequently adjusted to the susceptibility of cerebral fluid cultures. The treatment duration was 2–3 weeks for most of the cases. Only one case of death has been reported in a six-year-old girl with ALL [6], while two patients reported sequelae, and one child required a tracheostomy for long-term mechanical ventilation.
A significant reduction in the antimicrobial susceptibility of VGS has been observed over the last few years [69], especially in the pediatric population [70], where Penicillin derivates are often used in clinical practices [71] and prophylaxis [72,73]. Our patient recovered without sequelae, despite the fact that the S. mitis isolated was resistant to amoxicillin, penicillin, and cefuroxime. The antimicrobial susceptibility of these bacteria, besides their ability to cause subtle life-threatening diseases, should be carefully monitored because, as stated before, they appear to be able to both acquire resistance genes from neighboring oral microbes and donate resistance genes to more pathogenic streptococci [74,75]. The existence of these “more virulent” bacterial strains could explain how, in some cases, these bacteria are able to cause serious diseases not only in immunocompromised patients but also in healthy patients.
We find it important to shed light on S. mitis as a new emerging pathogen in pediatrics. Their probably underestimated role in invasive infections, even in immunocompetent children, could be complicated by their antimicrobial resistance patterns, which justifies the need for more specific and widely shared knowledge.
Furthermore, besides the known risk factors, more attention should be given to apparently less harmful conditions such as sinusitis and neglected oral care, which become relevant for invasive S. mitis infections in children. Good oral hygiene, optimal management of sinusitis, and careful clinical evaluation of a child with nonspecific symptoms who has recently undergone dental procedures, even after antibiotic prophylaxis, can thus prevent the spread of S. mitis and the consequential development of life-threatening diseases.
Therefore, being aware of the potential antimicrobial resistance of these microorganisms is essential for the clinician to ensure a correct diagnosis and timely, effective therapy.
Author Contributions
Conceptualization, C.C., A.C. (Antonio Cascio) and V.G.; methodology, C.C. and V.G.; data curation, V.G., G.B., C.A., S.B., A.C. (Anna Condemi), L.A.C. and S.G.; validation, C.C., V.G., C.A., G.P. and A.C. (Antonio Cascio); formal analysis, C.C. and V.G.; investigation, V.G.; resources, V.G., G.B., C.A., S.B., A.C. (Anna Condemi), L.A.C., S.G. and C.G.; data curation, V.G., G.B. and C.A.; writing—original draft preparation, V.G.; writing—review and editing, C.C. and C.A.; visualization, V.G. and C.A.; supervision, C.C. and A.C. (Antonio Cascio); project administration, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
The publication costs of this article were covered by the Fund for VQR Improvement assigned to the Department of Health Promotion, Mother and Child Care, Internal Medicine, and Medical Specialties of the University of Palermo.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the absence of sensitive data, according to the legal framework in which our Institute operates.
Informed Consent Statement
Written informed consent for publication was obtained from parents.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Facklam, R. What Happened to the Streptococci: Overview of Taxonomic and Nomenclature Changes. Clin. Microbiol. Rev. 2002, 15, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Yiş, R.; Yüksel, C.N.; Derundere, U.; Yiş, U. Önceden Sağlıklı Bir Çocukta Streptococcus mitis’e Bağlı Menenjit ve Beyaz Cevher Lezyonları “Meningitis and white matter lesions due to Streptococcus mitis in a previously healthy child”. Mikrobiyol. Bul. 2011, 45, 741–745. (In Turkish) [Google Scholar]
- Shelburne, S.A.; Sahasrabhojane, P.; Saldana, M.; Yao, H.; Su, X.; Horstmann, N.; Thompson, E.; Flores, A.R. Streptococcus mitis Strains Causing Severe Clinical Disease in Cancer Patients. Emerg. Infect. Dis. 2014, 20, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Fukayama, H.; Shoji, K.; Yoshida, M.; Iijima, H.; Maekawa, T.; Ishiguro, A.; Miyairi, I. Bacterial meningitis due to the Streptococcus mitis group in children with cerebrospinal fluid leak. IDCases 2022, 27, e01406. [Google Scholar] [CrossRef] [PubMed]
- Jaing, T.-H.; Chiu, C.-H.; Hung, I.-J. Successful treatment of meningitis caused by highly-penicillin-resistant Streptococcus mitis in a leukemic child. Chang Gung Med. J. 2002, 25, 190–193. [Google Scholar] [PubMed]
- Balkundi, D.R.; Murray, D.L.; Patterson, M.J.; Gera, R.; Scott-Emuakpor, A.; Kulkarni, R. Penicillin-Resistant Streptococcus mitis as a Cause of Septicemia with Meningitis in Febrile Neutropenic Children. J. Pediatr. Hematol. 1997, 19, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ Clin. Res. Ed. 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, S.T.; Ozsurekci, Y.; Aykac, K.; Aycan, A.E.; Bıcakcigil, A.; Altun, B.; Sancak, B.; Cengiz, A.B.; Kara, A.; Ceyhan, M. Streptococcus mitis/oralis Causing Blood Stream Infections in Pediatric Patients. Jpn. J. Infect. Dis. 2019, 72, 1–6. [Google Scholar] [CrossRef]
- Blázquez-Gamero, D.; Epalza, C.; Cadenas, J.A.A.; Gero, L.C.; Calvo, C.; Rodríguez-Molino, P.; Méndez, M.; Santos, M.D.M.; Fumadó, V.; Guzmán, M.F.; et al. Fever without source as the first manifestation of SARS-CoV-2 infection in infants less than 90 days old. Eur. J. Pediatr. 2021, 180, 2099–2106. [Google Scholar] [CrossRef]
- Nomura, R.; Nakano, K.; Mäkelä, K.; Vaara, M.; Salo, E.; Alaluusua, S.; Ooshima, T. Isolation and characterization of Streptococcus mitis from blood of child with osteomyelitis. Int. J. Paediatr. Dent. 2010, 21, 192–199. [Google Scholar] [CrossRef]
- Ahmed, R.; Hassall, T.; Morland, B.; Gray, J. Viridans streptococcus Bacteremia in Children on Chemotherapy for Cancer: An Underestimated Problem. Pediatr. Hematol. Oncol. 2003, 20, 439–444. [Google Scholar] [CrossRef]
- Buldu, M.T.; Raman, R. Hip adductor pyomyositis from Streptococcus mitis in a four-year-old child. J. Clin. Orthop. Trauma 2016, 7, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Imhof, L.; Schrading, S.; Braunschweig, T.; Steinau, G.; Spillner, J.W.; Puzik, A.; Lassay, L.; Kontny, U. Abscessing Infection by Streptococcus mitis Mimicking Metastatic Lesions in a 5-Year-Old Girl with Nephroblastoma: A Case Report. J. Pediatr. Hematol. 2018, 40, e429–e431. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nagao, Y.; Endo, H.; Yamane, I.; Hirata, M.; Hatakeyama, K. An intubated 7-month-old infant with a retropharyngeal abscess and multidrug-resistant Streptococcus mitis. Clin. Case Rep. 2019, 7, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Rieske, K.; Handrick, W.; Spencker, F.-B.; Günther, E. Sepsis durch vergrünende Streptokokken bei Kindern mit malignen hämatologischen Erkrankungen. Klin. Padiatr. 1997, 209, 364–372. [Google Scholar] [CrossRef]
- Bignardi, G.E.; Isaacs, D. Neonatal Meningitis Due to Streptococcus mitis. Clin. Infect. Dis. 1989, 11, 86–88. [Google Scholar] [CrossRef]
- Tobias, J.D.; Bozeman, P.M.; Stokes, D.C. Postsepsis bradycardia in children with leukemia. Crit. Care Med. 1991, 19, 1172–1176. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Claxton, S.; Pizer, B.; Lane, S.; Cooke, R.P.; Paulus, S.; Carrol, E.D. Viridans Group Streptococcal Infections in Children After Chemotherapy or Stem Cell Transplantation. Medicine 2016, 95, e2952. [Google Scholar] [CrossRef]
- Melendez, E.L.V.; Farrell, J.J.; Hujer, A.M.; Lowery, K.S.; Sampath, R.; A Bonomo, R. Culture negative empyema in a critically ill child: An opportunity for rapid molecular diagnostics. BMC Anesthesiol. 2014, 14, 107. [Google Scholar] [CrossRef]
- Taketani, T.; Kanai, R.; Fukuda, S.; Uchida, Y.; Yasuda, K.; Mishima, S.; Suyama, T.; Kodama, R.; Yoshino, I.; Kunishi, H.; et al. Pure Red Cell Precursor Toxicity by Linezolid in a Pediatric Case. J. Pediatr. Hematol. 2009, 31, 684–686. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Jackson, M.A.; Kearns, G.L. Delayed diagnosis of penicillin-resistant Streptococcus mitis endocarditis following single-dose amoxicillin prophylaxis in a child. Clin. Pediatr. 2004, 43, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Yiş, U.; Carman, K.B.; Yiş, R.; Derundere, U. Brain magnetic resonance imaging findings suggestive of widespread white matter involvement in children with Streptococcus mitis meningitis. Turk. J. Pediatr. 2013, 54, 425–428. [Google Scholar]
- Legendre, A.; Guérin, P.; Gournay, V.; Baron, O.; Leveiller, D.; Lefevre, M. Osler endocarditis of a ventricular septal defect in a 21-month old child. Arch. Mal. Coeur Vaiss. 2000, 93, 631–634. [Google Scholar]
- Goldfarb, J.; Wormser, G.P.; Glaser, J.H. Meningitis caused by multiply antibiotic-resistant viridans streptococci. J. Pediatr. 1984, 105, 891–895. [Google Scholar] [CrossRef]
- Hellwege, H.; Ram, W.; Scherf, H.; Fock, R. Neonatal Meningitis Caused by Streptococcus mitis. Lancet 1984, 323, 743–744. [Google Scholar] [CrossRef]
- Esposito, S.; Mayer, A.; Krzysztofiak, A.; Garazzino, S.; Lipreri, R.; Galli, L.; Osimani, P.; Fossali, E.; Di Gangi, M.; Lancella, L.; et al. Infective Endocarditis in Children in Italy from 2000 to 2015. Expert Rev. Anti-Infect. Ther. 2016, 14, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Scholz, C.B.; Kilian, M. Re-evaluation of the taxonomy of the Mitis group of the genus Streptococcus based on whole genome phylogenetic analyses, and proposed reclassification of Streptococcus dentisani as Streptococcus oralis subsp. dentisani comb. nov., Streptococcus tigurinus as Streptococcus oralis subsp. tigurinus comb. nov., and Streptococcus oligofermentans as a later synonym of Streptococcus cristatus. Int. J. Syst. Evol. Microbiol. 2016, 66, 4803–4820. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.A.; Garber, D.; Hu, S.; Kamat, A. Systematic review and case report: Intracranial complications of pediatric sinusitis. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Sadowy, E.; Hryniewicz, W. Identification of Streptococcus pneumoniae and other Mitis streptococci: Importance of molecular methods. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2247–2256. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Sepkowitz, K.A. Infections Caused by Viridans Streptococci in Patients with Neutropenia. Clin. Infect. Dis. 2002, 34, 1524–1529. [Google Scholar] [CrossRef]
- Mikulska, M.; Viscoli, C.; Orasch, C.; Livermore, D.M.; Averbuch, D.; Cordonnier, C.; Akova, M. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J. Infect. 2013, 68, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J. Streptococcus mitis: Walking the line between commensalism and pathogenesis. Mol. Oral Microbiol. 2011, 26, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.D.; Sinaii, N.; Palmore, T.N. Risk Factors for Viridans Group Streptococcal Bacteremia in Neutropenic and Non-neutropenic Patients: A Single Center Case-Case-Control Study. Open Forum Infect. Dis. 2017, 5, ofx260. [Google Scholar] [CrossRef]
- Denapaite, D.; Brückner, R.; Nuhn, M.; Reichmann, P.; Henrich, B.; Maurer, P.; Schähle, Y.; Selbmann, P.; Zimmermann, W.; Wambutt, R.; et al. The Genome of Streptococcus mitis B6—What Is a Commensal? PLoS ONE 2010, 5, e9426. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Croucher, N.J.; Hiller, N.L.; Hu, F.Z.; Ehrlich, G.D.; Bentley, S.D.; García, E.; Mitchell, T.J. Comparative Genomic Analysis of Ten Streptococcus pneumoniae Temperate Bacteriophages. J. Bacteriol. 2009, 191, 4854–4862. [Google Scholar] [CrossRef] [PubMed]
- Mundy, L.S.; Janoff, E.N.; Schwebke, K.E.; Shanholtzer, C.J.; Willard, K.E. Ambiguity in the Identification of Streptococcus pneumoniae Optochin, Bile Solubility, Quellung, and the AccuProbe DNA Probe Tests. Am. J. Clin. Pathol. 1998, 109, 55–61. [Google Scholar] [CrossRef]
- Arbique, J.C.; Poyart, C.; Trieu-Cuot, P.; Quesne, G.; Carvalho, M.d.G.S.; Steigerwalt, A.G.; Morey, R.E.; Jackson, D.; Davidson, R.J.; Facklam, R.R. Accuracy of Phenotypic and Genotypic Testing for Identification of Streptococcus pneumoniae and Description of Streptococcus pseudopneumoniae sp. nov. J. Clin. Microbiol. 2004, 42, 4686–4696. [Google Scholar] [CrossRef]
- Balsalobre, L.; Hernández-Madrid, A.; Llull, D.; Martín-Galiano, A.J.; García, E.; Fenoll, A.; de la Campa, A.G. Molecular Characterization of Disease-Associated Streptococci of the Mitis Group That Are Optochin Susceptible. J. Clin. Microbiol. 2006, 44, 4163–4171. [Google Scholar] [CrossRef]
- Ikryannikova, L.; Lapin, K.; Malakhova, M.; Filimonova, A.; Ilina, E.; Dubovickaya, V.; Sidorenko, S.; Govorun, V. Misidentification of alpha-hemolytic streptococci by routine tests in clinical practice. Infect. Genet. Evol. 2011, 11, 1709–1715. [Google Scholar] [CrossRef]
- Rolo, D.; Simões, S.A.; Domenech, A.; Fenoll, A.; Liñares, J.; de Lencastre, H.; Ardanuy, C.; Sá-Leão, R. Disease isolates of Streptococcus pseudopneumoniae and non-typeable S. pneumoniae presumptively identified as atypical S. pneumoniae in Spain. PLoS ONE 2013, 8, e57047. [Google Scholar] [CrossRef]
- Simões, A.S.; Tavares, D.A.; Rolo, D.; Ardanuy, C.; Goossens, H.; Henriques-Normark, B.; Linares, J.; de Lencastre, H.; Sá-Leão, R. lytA-based identification methods can misidentify Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 2016, 85, 141–148. [Google Scholar] [CrossRef]
- Sadowy, E.; Bojarska, A.; Kuch, A.; Skoczyńska, A.; Jolley, K.A.; Maiden, M.C.J.; van Tonder, A.J.; Hammerschmidt, S.; Hryniewicz, W. Relationships among streptococci from the mitis group, misidentified as Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, L.; Toti, S.; Reguzzi, V.; Bagnoli, F.; Donati, C. A Novel Computational Method Identifies Intra- and Inter-Species Recombination Events in Staphylococcus aureus and Streptococcus pneumoniae. PLoS Comput. Biol. 2012, 8, e1002668. [Google Scholar] [CrossRef] [PubMed]
- Hakenbeck, R.; Madhour, A.; Denapaite, D.; Brückner, R. Versatility of choline metabolism and choline-binding proteins in Streptococcus pneumoniae and commensal streptococci. FEMS Microbiol. Rev. 2009, 33, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.; Nieminen, L.; Kirkham, L.-A.; Johnston, C.; Smith, A.; Mitchell, T.J. Identification of a Secreted Cholesterol-Dependent Cytolysin (Mitilysin) from Streptococcus mitis. J. Bacteriol. 2007, 189, 627–632. [Google Scholar] [CrossRef]
- Llull, D.; López, R.; García, E. Characteristic Signatures of the lytA Gene Provide a Basis for Rapid and Reliable Diagnosis of Streptococcus pneumoniae Infections. J. Clin. Microbiol. 2006, 44, 1250–1256. [Google Scholar] [CrossRef]
- Muzzi, A.; Donati, C. Population genetics and evolution of the pan-genome of Streptococcus pneumoniae. Int. J. Med. Microbiol. 2011, 301, 619–622. [Google Scholar] [CrossRef]
- Donati, C.; Hiller, N.L.; Tettelin, H.; Muzzi, A.; Croucher, N.J.; Angiuoli, S.V.; Oggioni, M.; Hotopp, J.C.D.; Hu, F.Z.; Riley, D.R.; et al. Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biol. 2010, 11, R107. [Google Scholar] [CrossRef]
- Tavares, D.A.; Handem, S.; Carvalho, R.J.; Paulo, A.C.; de Lencastre, H.; Hinds, J.; Sá-Leão, R. Identification of Streptococcus pneumoniae by a real-time PCR assay targeting SP2020. Sci. Rep. 2019, 9, 3285. [Google Scholar] [CrossRef]
- AlEraky, D.M.; Madi, M.; El Tantawi, M.; AlHumaid, J.; Fita, S.; AbdulAzeez, S.; Borgio, J.F.; Al-Harbi, F.A.; Alagl, A.S. Predominance of non-Streptococcus mutans bacteria in dental biofilm and its relation to caries progression. Saudi J. Biol. Sci. 2021, 28, 7390–7395. [Google Scholar] [CrossRef]
- Neves, B.G.; Stipp, R.N.; Bezerra, D.D.S.; Guedes, S.F.D.F.; Rodrigues, L.K.A. Quantitative analysis of biofilm bacteria according to different stages of early childhood caries. Arch. Oral Biol. 2018, 96, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J. The pathogenesis of streptococcal infections: From Tooth decay to meningitis. Nat. Rev. Genet. 2003, 1, 219–230. [Google Scholar] [CrossRef]
- Amat, F. Complications des sinusites bactériennes du grand enfant. À propos d’un cas et revue de la littérature. Arch. Pédiatrie 2010, 17, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Patadia, M.; Stankiewicz, J. Neurological Complications of Acute and Chronic Sinusitis. Curr. Neurol. Neurosci. Rep. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, T.K.; Oinas, M.; Niemelä, M.; Mäkitie, A.A.; Atula, T. Intracranial Suppurative Complications of Sinusitis. Scand. J. Surg. 2016, 105, 254–262. [Google Scholar] [CrossRef]
- Kombogiorgas, D.; Seth, R.; Athwal, R.; Modha, J.; Singh, J. Suppurative intracranial complications of sinusitis in adolescence. Single institute experience and review of literature. Br. J. Neurosurg. 2007, 21, 603–609. [Google Scholar] [CrossRef]
- Gilony, D.; Talmi, Y.P.; Bedrin, L.; Ben-Shosan, Y.; Kronenberg, J. The clinical behavior of isolated sphenoid sinusitis. Otolaryngol. Neck Surg. 2007, 136, 610–615. [Google Scholar] [CrossRef]
- Sobol, S.E.; Marchand, J.; Tewfik, T.L.; Manoukian, J.J.; Schloss, M.D. Orbital Complications of Sinusitis in Children. J. Otolaryngol. 2002, 31, 131–136. [Google Scholar] [CrossRef]
- Leung, A.K.; Kellner, J.D. Acute sinusitis in children: Diagnosis and management. J. Pediatr. Health Care 2004, 18, 72–76. [Google Scholar] [CrossRef]
- Blackmore, T.K.; Morley, H.I.; Gordon, D.L. Streptococcus mitis-induced Bacteremia and Meningitis after Spinal Anesthesia. Anesthesiology 1993, 78, 592–593. [Google Scholar] [CrossRef]
- Kilpatrick, M.E.; Girgis, N.I. Meningitis—A complication of spinal anesthesia. Anesth. Analg. 1983, 62, 513–515. [Google Scholar] [CrossRef]
- Villevieille, T.; Vincenti-Rouquette, I.; Petitjeans, F.; Koulmann, P.; Legulluche, Y.; Rousseau, J.-M.; Diraison, Y.; Brinquin, L. Streptococcus mitis-induced Meningitis After Spinal Anesthesia. Anesth. Analg. 2000, 90, 500. [Google Scholar] [CrossRef] [PubMed]
- Selvitop, O.; Poretti, A.; Huisman, T.A.; Wagner, M.W. Cerebral sinovenous thrombosis in a child with Crohn’s disease, otitis media, and meningitis. Neuroradiol. J. 2015, 28, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Bracken, J.; Barnacle, A.; Ditchfield, M. Potential pitfalls in imaging of paediatric cerebral sinovenous thrombosis. Pediatr. Radiol. 2012, 43, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Tuomanen, E.I. Molecular and Cellular Mechanisms of Pneumococcal Meningitis. Ann. N. Y. Acad. Sci. 1996, 797, 42–52. [Google Scholar] [CrossRef]
- Agrawal, S.; Nadel, S. Acute Bacterial Meningitis in Infants and Children. Pediatr. Drugs 2011, 13, 385–400. [Google Scholar] [CrossRef]
- Visintin, C.; Mugglestone, M.A.; Fields, E.J.; Jacklin, P.; Murphy, M.S.; Pollard, A.J.; on behalf of the Guideline Development Group. Management of bacterial meningitis and meningococcal septicaemia in children and young people: Summary of NICE guidance. BMJ 2010, 340, c3209. [Google Scholar] [CrossRef]
- Bruckner, L.B.; Korones, D.N.; Karnauchow, T.; Hardy, D.J.; Gigliotti, F. High incidence of penicillin resistance among α-hemolytic streptococci isolated from the blood of children with cancer. J. Pediatr. 2002, 140, 20–26. [Google Scholar] [CrossRef]
- Davidovich, N.V.; Galieva, A.S.; Davydova, N.G.; Malygina, O.G.; Kukalevskaya, N.N.; Simonova, G.V.; Bazhukova, T.A. Spectrum and resistance determinants of oral streptococci clinical isolates. Russ. Clin. Lab. Diagn. 2020, 65, 632–637. [Google Scholar] [CrossRef]
- Wald, E.R.; Applegate, K.E.; Bordley, C.; Darrow, D.H.; Glode, M.P.; Marcy, S.M.; Nelson, C.E.; Rosenfeld, R.M.; Shaikh, N.; Smith, M.J.; et al. Clinical Practice Guideline for the Diagnosis and Management of Acute Bacterial Sinusitis in Children Aged 1 to 18 Years. Pediatrics 2013, 132, e262–e280. [Google Scholar] [CrossRef]
- Wilson, W.R.; Taubert, K.A.; Gewitz, M.H.; Lockhart, P.B.; Baddour, L.M.; E Levison, M.; Bolger, A.F.; Cabell, C.H.; Takahashi, M.; Baltimore, R.S.; et al. Prevention of Infective Endocarditis. Circulation 2007, 116, 1736–1754. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.G.A.; Costa, M.L.V.A.; Silva, F.R.P.; Arcanjo, D.D.R.; Moura, L.F.A.D.; Oliveira, F.A.A.; Soares, M.J.S.; Quelemes, P.V. Amoxicillin-Resistant Streptococci Carriage in the Mouths of Children: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, P.; Que, Y.A.; Bayer, A.S. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. N. Am. 2002, 16, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Hakenbeck, R.; Balmelle, N.; Weber, B.; Gardès, C.; Keck, W.; de Saizieu, A. Mosaic Genes and Mosaic Chromosomes: Intra- and Interspecies Genomic Variation of Streptococcus pneumoniae. Infect. Immun. 2001, 69, 2477–2486. [Google Scholar] [CrossRef]
- Hannan, S.; Ready, D.; Jasni, A.S.; Rogers, M.; Pratten, J.; Roberts, A.P. Transfer of antibiotic resistance by transformation with eDNA within oral biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 345–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
