Screening for Metallo-Beta-Lactamases Using Non-Carbapenem Agents: Effective Detection of MBL-Producing Enterobacterales and Differentiation of Carbapenem-Resistant Enterobacterales
Abstract
1. Introduction
2. Results
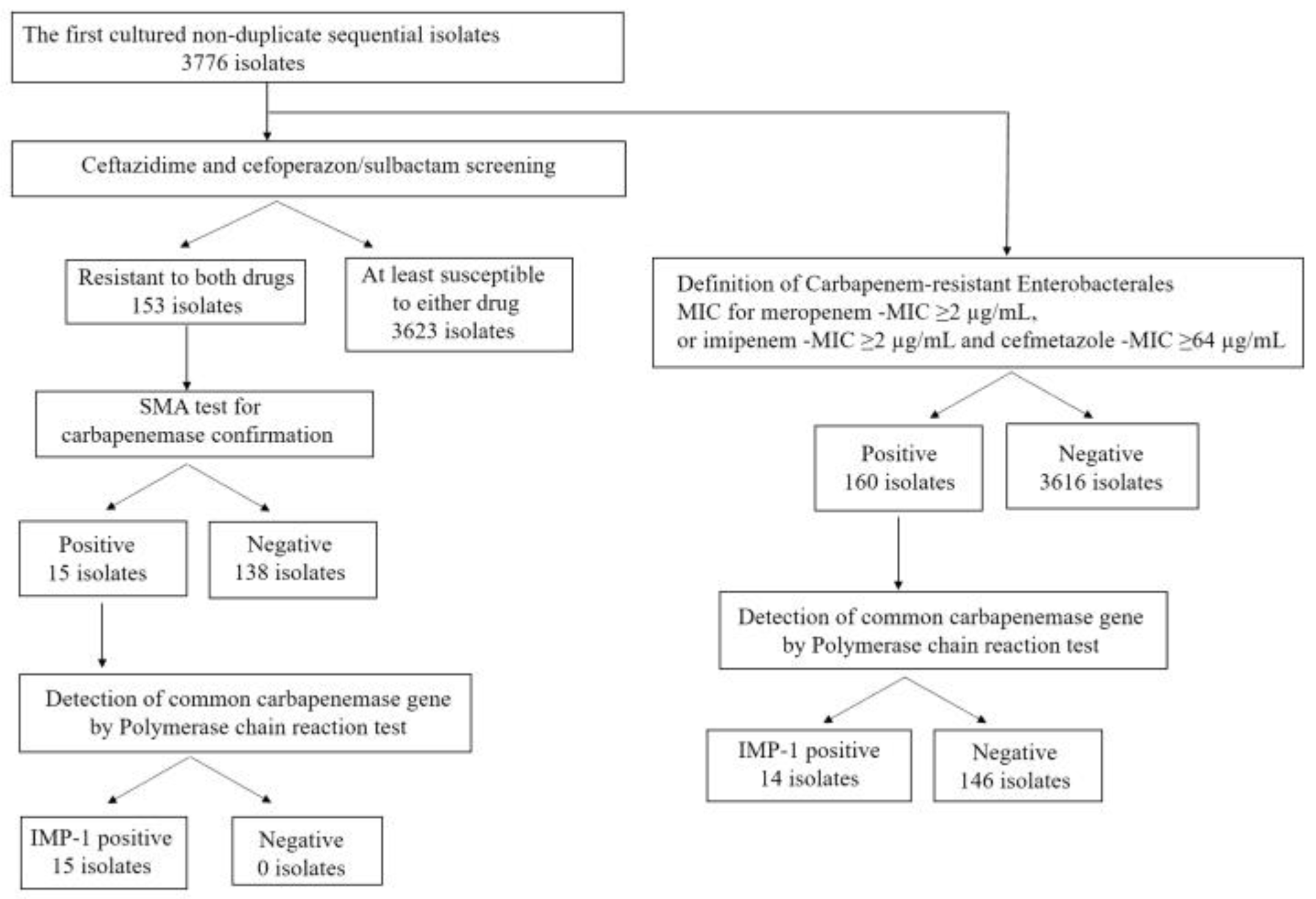
2.1. Total Bacterial Isolates and Those Screened Using Ceftazidime and Cefoperazone/Sulbactam
2.2. Enterobacterales Screened Using Ceftazidime and Cefoperazone/Sulbactam
2.3. Carbapenem-Resistant Enterobacterales
2.4. Carbapenem, Ceftazidime, and Cefoperazone/Sulbactam-Resistant Enterobacterales
3. Discussion
4. Materials and Methods
4.1. Study Design for Bacterial Isolates
4.2. Antimicrobial Susceptibility Testing
4.3. Definition and Breakpoint of Screening Antibiotics
4.4. Identification of Metallo-Beta-Lactamase
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambler, R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Osano, E.; Arakawa, Y.; Wacharotayankun, R.; Ohta, M.; Horii, T.; Ito, H.; Yoshimura, F.; Kato, N. Molecular characterization of an enterobacterial metallo beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 1994, 38, 71–78. [Google Scholar] [CrossRef]
- Mori, N.; Tada, T.; Oshiro, S.; Kuwahara-Arai, K.; Kirikae, T.; Uehara, Y. A transferrable IncL/M plasmid harboring a gene encoding IMP-1 metallo-β-lactamase in clinical isolates of Enterobacteriaceae. BMC Infect. Dis. 2021, 21, 1061. [Google Scholar] [CrossRef] [PubMed]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla (NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (Press Release). Action Needed Now to Halt Spread of Deadly Bacteria. Available online: https://www.cdc.gov/media/releases/2013/p0305_deadly_bacteria.html (accessed on 14 May 2023).
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threatsreport-508.pdf (accessed on 8 April 2023).
- Kamio, K.; Espinoza, J.L. The predominance of Klebsiella aerogenes among carbapenem-resistant Enterobacteriaceae infections in Japan. Pathogens 2022, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. 2017. Available online: http://www.eucast.org/resistance_mechanisms/ (accessed on 20 March 2023).
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100-S30; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Nishio, H.; Komatsu, M.; Shibata, N.; Shimakawa, K.; Sueyoshi, N.; Ura, T.; Satoh, K.; Toyokawa, M.; Nakamura, T.; Wada, Y.; et al. Metallo-beta-lactamase-producing gram-negative bacilli: Laboratory-based surveillance in cooperation with 13 clinical laboratories in the Kinki region of Japan. J. Clin. Microbiol. 2004, 42, 5256–5263. [Google Scholar] [CrossRef]
- Fattouh, R.; Tijet, N.; McGeer, A.; Poutanen, S.M.; Melano, R.G.; Patel, S.N. What is the appropriate meropenem MIC for screening of carbapenemase-producing Enterobacteriaceae in low-prevalence settings? Antimicrob. Agents Chemother. 2016, 60, 1556–1559. [Google Scholar] [CrossRef]
- Richter, S.; Marchaim, D. Screening for carbapenem-resistant Enterobacteriaceae: Who, when, and how? Virulence 2017, 8, 417–426. [Google Scholar] [CrossRef]
- Japanese Society of Chemotherapy; Japanese Society of Infectious Disease; Japanese Society for Infection Prevention and Control; Japanese Society for Clinical Microbiology. Joint Statement for Management of Enterobacteriaceae with Lower Susceptibility to Carbapenems: Importance of Carbapenemase-Producing Bacteria as Infection Control Targets [Japanese]. Available online: https://www.chemotherapy.or.jp/uploads/files/guideline/4gakkai2017_b.pdf (accessed on 20 March 2023).
- Pranita, D.T.; Yohei, D.; Robert, A.B.; JKristie, J.; Patricia, J.S. A Primer on AmpC β-Lactamases: Necessary Knowledge for an Increasingly Multidrug-resistant World. Clin. Infect. Dis. 2019, 69, 1446–1455. [Google Scholar] [CrossRef]
- Ma, D.Y.; Huang, H.Y.; Zou, H.; Wu, M.L.; Lin, Q.X.; Liu, B.; Huang, S.F. Carbapenem-resistant Klebsiella aerogenes clinical isolates from a teaching hospital in southwestern China: Detailed molecular epidemiology, resistance determinants, risk factors and clinical outcomes. Infect. Drug Resist. 2020, 19, 577–585. [Google Scholar] [CrossRef]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.-C. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front. Microbiol. 2019, 10, 44. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Hong, Y.-K.; Lee, H.; Ko, K.S. High prevalence of non-clonal imipenem-nonsusceptible Enterobacter spp. isolates in Korea and their association with porin down-regulation. Diagn. Microbiol. Infect. Dis. 2017, 87, 53–59. [Google Scholar] [CrossRef]
- Idrees, M.M.; Rimsha, R.; Idrees, S.A. Antimicrobial susceptibility and genetic prevalence of extended-spectrum β-lactamases in gram-negative rods isolated from clinical specimens in Pakistan. Antibiotics 2022, 12, 29. [Google Scholar] [CrossRef]
- Lin, S.Y.; Lu, P.L.; Wu, T.S.; Shie, S.-S.; Chang, F.-Y.; Yang, Y.-S.; Chiang, T.-T.; Wang, F.-D.; Ho, M.-W.; Chou, C.-H.; et al. Correlation between cefoperazone/sulbactam MIC values and clinical outcomes of Escherichia coli bacteremia. Infect. Dis. Ther. 2022, 11, 1853–1867. [Google Scholar] [CrossRef]
- Hassoun-Kheir, N.; Hussein, K.; Karram, M.; Saffuri, M.; Badaan, S.; Peleg, S.; de Kraker, M.E.; Aboelhega, W.; Warman, S.; Alon, T.; et al. Risk factors for acquisition of carbapenemase-producing versus non-carbapenemase-producing Enterobacterales: A case-control study. Clin. Microbiol. Infect. 2023, 29, 629–634. [Google Scholar] [CrossRef]
- Jong, H.L.; Il, K.B.; Chae, H.L.; Seri, J. Molecular characteristics of first IMP-4-producing Enterobacter cloacae sequence type 74 and 194 in Korea. Front. Microbiol. 2017, 8, 2343. [Google Scholar] [CrossRef]
- Latania, K.L.; Robert, A.W. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Arakawa, Y. Systematic research to overcome newly emerged multidrug-resistant bacteria. Microbiol. Immunol. 2020, 64, 231–251. [Google Scholar] [CrossRef]
- Ronat, J.B.; Oueslati, S.; Natale, A.; Kesteman, T.; Elamin, W.; Langendorf, C.; Hardy, L.; Vandenberg, O.; Naas, T. Validation of Three MicroScan® Antimicrobial Susceptibility Testing Plates Designed for Low-Resource Settings. Diagnostics 2022, 12, 2106. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Seii, K.; on behalf of the CLSI Methods Development and Standardization Working Group of the Subcommittee on Antimicrobial Susceptibility Testing. CLSI Methods Development and Standardization Working Group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Implementation of the CLSI Method for Direct Disk Diffusion Testing from Positive Blood Culture. Available online: https://clsi.org/about/blog/implementation-of-the-clsi-method-for-direct-disk-diffusion-testing-from-positive-blood-cultures/ (accessed on 12 June 2023).
- Sader, H.S.; Carvalhaes, C.G.; Streit, J.M.; Castanheira, M.; Flamm, R.K. Antimicrobial activity of cefoperazone-sulbactam tested against Gram-Negative organisms from Europe, Asia-Pacific, and Latin America. Int. J. Infect. Dis. 2020, 91, 32–37. [Google Scholar] [CrossRef] [PubMed]
| Enterobacterales Resistant to Ceftazidime and Cefoperazone/Sulbactam | n = 153 |
|---|---|
| Enterobacter cloacae | 62 |
| Klebsiella pneumoniae | 24 |
| Escherichia coli | 23 |
| Citrobacter braakii | 18 |
| Serratia marcescens | 6 |
| Klebsiella aerogenes | 4 |
| Citrobacter sp. | 3 |
| Klebsiella oxytoca | 2 |
| Morganella morganii | 2 |
| Proteus mirabilis | 2 |
| Serratia liquefaciens | 2 |
| Citrobacter werkmanii | 1 |
| Citrobacter youngae | 1 |
| Enterobacter sp. | 1 |
| Hafnia alvei | 1 |
| Yersinia enterocolitica | 1 |
| Antibiotic Minimum Inhibitory Concentration (μg/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Bacterial Strains | CRE or Non-CRE | Sample | MEM | IPM | CMZ | CAZ | CFP/SBT | CRO | FEP | LVX | TZP | GM |
| 1 | Enterobacter cloacae | CRE | Urine | >2 | 2 | >32 | >8 | >32 | >2 | >16 | 1 | ≤4 | ≤2 |
| 2 | Enterobacter cloacae | CRE | Sputum | >2 | >2 | >32 | >8 | >32 | >2 | >16 | 4 | 64 | ≤2 |
| 3 | Enterobacter cloacae | CRE | Blood | 2 | ≤0.5 | >32 | >8 | 32 | >2 | >16 | 1 | 8 | ≤2 |
| 4 | Enterobacter cloacae | CRE | Blood | >2 | >2 | >32 | >8 | >32 | >2 | 16 | 1 | 4 | ≤2 |
| 5 | Enterobacter cloacae | CRE | Urine | >2 | >2 | >32 | >8 | >32 | >2 | 16 | ≤0.12 | 16 | ≤2 |
| 6 | Enterobacter cloacae | CRE | Urine | >2 | >2 | >32 | >8 | >32 | >2 | 16 | 1 | >64 | ≤2 |
| 7 | Enterobacter cloacae | CRE | Urine | 2 | 1 | >32 | >8 | >32 | >2 | 8 | ≤0.12 | ≤4 | ≤2 |
| 8 | Enterobacter cloacae | CRE | Sputum | >2 | 1 | >32 | >8 | 32 | >2 | 8 | 1 | 16 | ≤2 |
| 9 | Enterobacter cloacae | CRE | Urine | >2 | 2 | >32 | >8 | >32 | >2 | >16 | 2 | >64 | ≤2 |
| 11 | Enterobacter cloacae | CRE | Sputum | >2 | 2 | >32 | >8 | >32 | >2 | >16 | 1 | ≤4 | ≤2 |
| 10 | Enterobacter cloacae | Non-CRE | Sputum | ≤0.25 | ≤0.5 | >32 | >8 | >32 | >2 | 8 | 1 | 16 | ≤2 |
| 12 | Klebsiella pneumoniae | CRE | Bile | >2 | >2 | >32 | >8 | >32 | >2 | >16 | 4 | 64 | ≤2 |
| 13 | Klebsiella pneumoniae | CRE | Puncture fluid | >2 | >2 | >32 | >8 | >32 | >2 | >16 | ≤0.12 | 64 | ≤2 |
| 14 | Klebsiella pneumoniae | CRE | Urine | >2 | >2 | >32 | >8 | >32 | >2 | >16 | 1 | >64 | ≤2 |
| 15 | Escherichia coli | CRE | Urine | 2 | 2 | >32 | >8 | 32 | >2 | 4 | 1 | ≤4 | ≤2 |
| Carbapenem-Resistant Enterobacterales | n = 160 |
|---|---|
| Klebsiella aerogenes | 96 |
| Enterobacter cloacae | 54 |
| Klebsiella pneumoniae | 8 |
| Escherichia coli | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takei, K.; Kanamori, H.; Nakayama, A.; Chiba, M.; Takei, Y.; Seike, I.; Kitamura, C.; Baba, H.; Oshima, K.; Tokuda, K. Screening for Metallo-Beta-Lactamases Using Non-Carbapenem Agents: Effective Detection of MBL-Producing Enterobacterales and Differentiation of Carbapenem-Resistant Enterobacterales. Antibiotics 2023, 12, 1146. https://doi.org/10.3390/antibiotics12071146
Takei K, Kanamori H, Nakayama A, Chiba M, Takei Y, Seike I, Kitamura C, Baba H, Oshima K, Tokuda K. Screening for Metallo-Beta-Lactamases Using Non-Carbapenem Agents: Effective Detection of MBL-Producing Enterobacterales and Differentiation of Carbapenem-Resistant Enterobacterales. Antibiotics. 2023; 12(7):1146. https://doi.org/10.3390/antibiotics12071146
Chicago/Turabian StyleTakei, Kentarou, Hajime Kanamori, Asami Nakayama, Mikiko Chiba, Yumiko Takei, Issei Seike, Chiho Kitamura, Hiroaki Baba, Kengo Oshima, and Koichi Tokuda. 2023. "Screening for Metallo-Beta-Lactamases Using Non-Carbapenem Agents: Effective Detection of MBL-Producing Enterobacterales and Differentiation of Carbapenem-Resistant Enterobacterales" Antibiotics 12, no. 7: 1146. https://doi.org/10.3390/antibiotics12071146
APA StyleTakei, K., Kanamori, H., Nakayama, A., Chiba, M., Takei, Y., Seike, I., Kitamura, C., Baba, H., Oshima, K., & Tokuda, K. (2023). Screening for Metallo-Beta-Lactamases Using Non-Carbapenem Agents: Effective Detection of MBL-Producing Enterobacterales and Differentiation of Carbapenem-Resistant Enterobacterales. Antibiotics, 12(7), 1146. https://doi.org/10.3390/antibiotics12071146






